Abstract
Waldenström macroglobulinemia is a B-cell malignancy with lymphoplasmacytic infiltration in the bone marrow or lymphatic tissue and a monoclonal immunoglobulin M protein (IgM) in the serum. It is incurable with current therapy, and the decision to treat patients as well as the choice of treatment can be complex. Using a risk-adapted approach, we provide recommendations on timing and choice of therapy. Patients with smoldering or asymptomatic Waldenström macroglobulinemia and preserved hematologic function should be observed without therapy. Symptomatic patients with modest hematologic compromise, IgM-related neuropathy that requires therapy, or hemolytic anemia unresponsive to corticosteroids should receive standard doses of rituximab alone without maintenance therapy. Patients who have severe constitutional symptoms, profound hematologic compromise, symptomatic bulky disease, or hyperviscosity should be treated with the DRC (dexamethasone, rituximab, cyclophosphamide) regimen. Any patient with symptoms of hyperviscosity should first be treated with plasmapheresis. For patients who experience relapse after a response to initial therapy of more than 2 years' duration, the original therapy should be repeated. For patients who had an inadequate response to initial therapy or a response of less than 2 years' duration, an alternative agent or combination should be used. Autologous stem cell transplant should be considered in all eligible patients with relapsed disease.
DRC = dexamethasone, rituximab, cyclophosphamide; IgM = immunoglobulin M protein; IPSSWM = International Prognostic Staging System for Waldenström Macroglobulinemia; MGUS = monoclonal gammopathy of undetermined significance; mSMART = Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy; WHO = World Health Organization
Waldenström macroglobulinemia is a B-cell lymphoproliferative disorder characterized by a lymphoplasmacytic infiltration in the bone marrow or lymphatic tissue and a monoclonal immunoglobulin M protein (IgM) in the serum.1,2 The overall incidence of Waldenström macroglobulinemia is approximately 5 cases per 1 million persons per year, and this disease accounts for approximately 1% to 2% of hematologic cancers.3,4 The incidence of Waldenström macroglobulinemia is highest among white people and is rare in other population groups.5 The median age at diagnosis varies between 63 and 68 years, and most patients (55%-70%) with newly diagnosed disease are men.6
Infiltration of the bone marrow and extramedullary sites by malignant B cells and elevated IgM levels account for the symptoms associated with this disease. Patients may develop constitutional symptoms, pancytopenia, organomegaly, neuropathy, and symptoms associated with immunoglobulin deposition or hyperviscosity.6,7 However, symptoms vary considerably in individual patients. Although some patients present with the aforementioned symptoms, many are asymptomatic at the time of diagnosis.
Waldenström macroglobulinemia is incurable with current therapy, and half of the patients die of disease progression; median survival is approximately 5 years.8 This disease is diagnosed in many patients at an advanced age, and thus approximately half of the patients die of causes unrelated to Waldenström macroglobulinemia. Because the disease is incurable and the clinical presentations, comorbidities, and causes of death vary substantially, the decision to treat patients and the choice of treatment can be complex. A number of consensus meetings have listed reasonable treatment options,9-11 but the physician is still faced with a difficult treatment decision in a patient with an uncommon disease. Therefore, the goal of this article is to provide a set of simple and specific recommendations based on the available evidence and, if evidence is lacking, on consensus among experienced Mayo Clinic clinicians as to when to treat patients and which treatment to use.
CLASSIFICATION OF EVIDENCE AND GRADES OF RECOMMENDATION
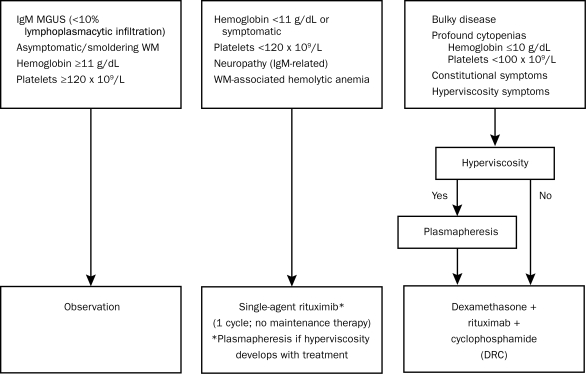
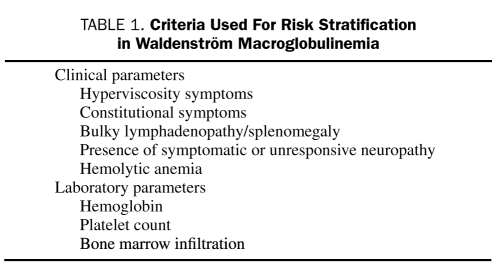
Progress has been made during the past decade in understanding the basic biology of Waldenström macroglobulinemia, in identifying factors that predict patient outcome, and in developing more effective therapies. In an attempt to use this information in a practical and evidence-based fashion, our group of 33 Mayo Clinic experts reached a consensus on who should be treated, as well as when and what therapy should be recommended. The focal point of our strategy revolves around risk stratification. Rather than promulgating any one specific prognostic system, we have focused our efforts on defining risk groups that we think should be managed differently. This approach is integral to the Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) (Figure 1; see also www.mSMART.org).12,13 The specific criteria given in Table 1 are used to classify patients into 3 distinct risk categories but are not intended to replace existing prognostic systems. Instead, these guidelines represent an attempt to offer a simplified, primarily evidence-based algorithm for making treatment decisions for patients with Waldenström macroglobulinemia.
FIGURE 1.
Mayo Clinic (Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy [mSMART]) consensus for management of newly diagnosed Waldenström macroglobulinemia (WM). MGUS = monoclonal gammopathy of undetermined significance. SI conversion factor: To convert hemoglobin values to g/L, multiply by 10.
TABLE 1.
Criteria Used For Risk Stratification in Waldenström Macroglobulinemia
The optimal management of patients with newly diagnosed Waldenström macroglobulinemia can be broadly divided into the following components. In the subsequent sections, we analyze the available evidence to support specific guidelines for each of these steps:
Confirmation of the diagnosis.
Stratification of risk and determination of the need for treatment.
Selection of the appropriate initial therapy.
Choice of additional therapy if initial response is inadequate or the patient's disease progresses.
The levels of evidence and grades of recommendation are shown in Table 2 and are included with each specific recommendation. When specific evidence is lacking, our group reached a consensus based on our current practice patterns. Semiannually, these guidelines have been modified as new data become available; the most current guidelines are always available at www.mSMART.org.
TABLE 2.
Classification System for Levels of Evidence and Grades of Recommendations
DIAGNOSIS
Attempts to better define Waldenström macroglobulinemia have been made in recent years by the World Health Organization (WHO) Lymphoma Classification,14 the consensus group formed at the Second International Workshop on Waldenström's Macroglobulinemia,1 and Mayo Clinic.15 However, the respective definitions of the diagnostic criteria for Waldenström macroglobulinemia by these groups are not identical. All groups recognize Waldenström macroglobulinemia as a lymphoplasmacytic lymphoma associated with an IgM monoclonal protein in the serum. The WHO definition includes lymphomas other than lymphoplasmacytic lymphoma and also allows the monoclonal protein to be IgG or IgA. In contrast, the Second International Workshop on Waldenström's Macroglobulinemia restricts the diagnosis exclusively to cases with lymphoplasmacytic lymphoma and an IgM monoclonal protein. The Second International Workshop on Waldenström's Macroglobulinemia also eliminated the requirement for either a minimum amount of marrow involvement by lymphoplasmacytic lymphoma or a threshold concentration of IgM in the serum to fulfill the diagnosis and allows any detectable amount of either. In contrast, Mayo Clinic criteria require at least 10% marrow involvement by lymphoplasmacytic lymphoma in asymptomatic patients. Furthermore, in regard to pathologic features, the WHO criteria focus predominantly on nodal involvement, whereas studies at Mayo Clinic indicate that in most cases of Waldenström macroglobulinemia, the lymphoplasmacytic lymphoma is a bone marrow–based disease.
Lymphoplasmacytic lymphoma involving either the bone marrow or the extramedullary sites typically exhibits a cytologic spectrum ranging from small lymphocytes with clumped chromatin, inconspicuous nucleoli, and sparse cytoplasm to well-formed plasma cells.1,16 Frequently present are “plasmacytoid lymphocytes” having cytologic features intermediate between these 2 extremes, although the cytologic composition and the degree of plasmacytic differentiation vary from case to case. Nodal involvement is typically characterized by paracortical and hilar infiltration with frequent sparing of the subscapular and marginal sinuses. The bone marrow usually has some combination of nodular, paratrabecular, and interstitial infiltration; in approximately one-half of cases, plasma cells that contain Dutcher bodies are present.
The cytologic spectrum of lymphoplasmacytic lymphoma in Waldenström macroglobulinemia is reflected in the immunophenotypic attributes of the neoplastic cells. A monotypic lymphocytic component is almost always detected, typically with high levels of surface CD19, CD20, and immunoglobulin light chain expression.1,16 The lymphoid component typically lacks CD10. In approximately 40% of cases, the lymphocytes show some degree of CD5 expression; however, these cases usually do not show the strong expression of this antigen associated with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. By comparison, the plasmacytic component expresses the same immunoglobulin light chain as the lymphocytic component, is positive for CD138 (particularly when assessed by immunohistochemistry), and shows diminished expression of B-cell–associated antigens such as CD19, CD20, and PAX5.
Typically, the lymphoplasmacytic lymphoma cells are positive for surface IgM, but on the basis of the WHO criteria, they may express any immunoglobulin isotype. In cases with isotype switch, the phenotype of the plasma cells closely resembles that of myelomatous plasma cells with strong CD38 and CD138 co-expression and complete lack of CD19. Waldenström macroglobulinemia cells have also been shown to be CD25+, CD27+, CD75−, FMC7+, Bcl2+, and Bcl6−. Although not completely specific for lymphoplasmacytic lymphoma, del(6)(q21) is the most common genetic abnormality seen in 40% to 50% of cases.17 This genetic abnormality is rarely seen in other lymphoproliferative or plasmaproliferative malignancies.18 Other genetic abnormalities described in association with Waldenström macroglobulinemia are deletions of regions of 13q14 that include MIRN15A and MIRN16-1 and t(11;18)(q21;q21) involving API-malt1.19 However, these are inconsistently seen in Waldenström macroglobulinemia and do not appear to aid in the diagnostic criteria of this disease.
In addition, Waldenström macroglobulinemia needs to be differentiated from IgM monoclonal gammopathy of undetermined significance (MGUS), which may have a similar immunophenotype on examination of neoplastic cells but differs greatly in prevalence and prognosis. The Mayo Clinic criteria differentiate these entities on the basis of the extent of bone marrow involvement and presence or absence of symptomatic disease. The separation of IgM MGUS from Waldenström macroglobulinemia using the Mayo criteria is validated by studies of the natural history of IgM MGUS that show that patients diagnosed as having this disorder have a survival rate similar to that in the general population.20-22
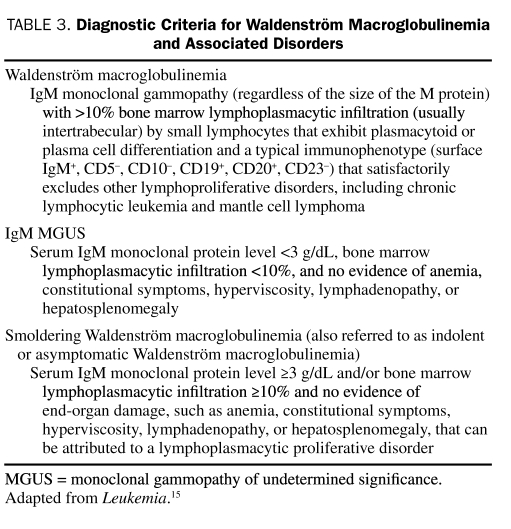
Recommendation: In practice, IgM monoclonal gammopathy (regardless of the size of the M protein) plus 10% or greater bone marrow lymphoplasmacytic infiltration (usually intertrabecular) by small lymphocytes that exhibit plasmacytoid or plasma cell differentiation and a typical immunophenotype (eg, surface IgM+, CD5−, CD10−, CD19+, CD20+, CD23−, CD79a+ and PAX5+) that satisfactorily excludes other lymphoproliferative disorders, including chronic lymphocytic leukemia and mantle cell lymphoma, is considered diagnostic of Waldenström macroglobulinemia (Table 3). Because no standard exists for the pathologic evaluation of Waldenström macroglobulinemia, the immunophenotyping approach used may vary. In all cases, at least one B-cell lineage antigen should be assessed (eg, CD19, CD20, CD79a), and the possibility of mantle cell lymphoma should be excluded by either cyclin D1 immunohistochemistry or cyclin D1:IgH fluorescence in situ hybridization analysis. Flow cytometric immunophenotyping has utility not only in establishing the presence of a monotypic B-cell disease component but also in helping to exclude the possibility of chronic lymphocytic leukemia/small lymphocytic lymphoma. Aside from excluding the diagnosis of mantle cell lymphoma, cytogenetic studies are not helpful in the diagnosis of this disease.
TABLE 3.
Diagnostic Criteria for Waldenström Macroglobulinemia and Associated Disorders
Level of Evidence: III
Grade of Recommendation: B
RISK STRATIFICATION AND INDICATIONS FOR INITIAL THERAPY
The prognosis of patients with Waldenström macroglobulinemia varies, with a median survival of 5 years and approximately 10% of patients still alive at 15 years.23,24 An important problem in determining survival is that Waldenström macroglobulinemia commonly has an indolent course and presents in older patients. Therefore, patients have competing causes of death, and the effect of concomitant disorders on survival has not been extensively studied. However, a recent study of the disease-specific survival in Waldenström macroglobulinemia found that it was closer to 11 years, confirming the indolent nature of the disease.25 Furthermore, at least 25% of patients are asymptomatic at presentation, and the disease is diagnosed incidentally. Fifty percent of patients who are asymptomatic at diagnosis and who are observed will not require therapy within almost 3 years.26 One in 10 patients who are managed with a watch-and-wait approach will not require therapy for 10 years.26 This highlights the importance of careful determination of the need for treatment, particularly at the onset of the disease, so that only patients who require therapy are treated.
To identify patients who need immediate therapy or who will require treatment in the short term, several studies have evaluated the impact of different clinical and laboratory variables on patient outcome. Initial studies focused on the serum IgM level but found that the level does not accurately reflect the tumor burden, assessed by marrow infiltration or prognosis in Waldenström macroglobulinemia.27 In subsequent studies, the main adverse prognostic factors were older age, presence of constitutional symptoms, anemia, low albumin serum levels, hyperviscosity, organomegaly, and high β2-microglobulin values.25 Leukopenia and thrombocytopenia were also identified as important survival predictors in most studies; however, the precise level of cytopenia with prognostic significance has varied between studies.28 Other prognostic markers currently studied are the serum free light chain,29 serum lactate dehydrogenase,30 and the serum soluble CD27.31 These prognostic data were subsequently combined to formulate the International Prognostic Staging System for Waldenström's Macroglobulinemia (IPSSWM).32 The IPSSWM is a multicenter collaborative project resulting from the analysis of a large number of previously untreated, symptomatic patients who required treatment. According to the IPSSWM, 5 adverse features (age >65 years, hemoglobin <11.5 g/dL [to convert to g/L, multiply by 10], platelet count <100 × 109/L, β2-microglobulin >3 mg/L, and serum monoclonal protein concentration >70 g/L) defined 3 risk groups with 5-year survival rates of 87%, 68%, and 36%. This staging system has been validated in patients treated with alkylating agents, nucleoside analogues, or combinations of these agents, with or without rituximab.
Most prognostic factors, however, have defined the outcome of Waldenström macroglobulinemia in patients requiring treatment. Very few studies have evaluated prognostic factors in patients who do not initially need to be treated.
This is particularly relevant in patients with IgM MGUS and smoldering Waldenström macroglobulinemia (Table 3).15,33 Although IgM MGUS and smoldering Waldenström macroglobulinemia fall within the definition of Waldenström macroglobulinemia, these entities have a very indolent clinical course, and patients do not initially require treatment. IgM MGUS is defined as a serum IgM monoclonal protein level less than 3 g/dL, bone marrow lymphoplasmacytic infiltration less than 10%, and no evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly. Patients with IgM MGUS should not be considered as having Waldenström macroglobulinemia or a malignancy.
Smoldering Waldenström macroglobulinemia (also referred to as indolent or asymptomatic Waldenström macroglobulinemia) is defined as a serum IgM monoclonal protein level of 3 g/dL or higher and/or bone marrow lymphoplasmacytic infiltration of 10% or greater and no evidence of end-organ damage, such as anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly that can be attributed to a lymphoplasmacytic disorder.
Many of these patients can be observed without therapy for prolonged periods, and some may never require therapy. Only 25% of patients with IgM MGUS will develop a symptomatic lymphoproliferative disorder within 15 years, and the cumulative probability of progression in these patients is only 1.5% per year.21 Similarly, for patients with smoldering Waldenström macroglobulinemia, the risk of progression to symptomatic Waldenström macroglobulinemia is 6% per year, and only 55% of patients with smoldering Waldenström macroglobulinemia will have progression within 5 years.33 Studies to determine prognostic factors in these patients have found that only the concentration of the serum monoclonal protein at diagnosis and the serum albumin predict progression.
Because of the heterogeneity of this disease, efforts have been made to define which patients require treatment. A consensus panel convened during the Second International Workshop on Waldenström's Macroglobulinemia agreed that initiation of therapy was appropriate for patients with constitutional symptoms such as fever, night sweats, fatigue due to anemia, or weight loss. The presence of progressive, symptomatic lymphadenopathy or splenomegaly provided additional reasons to begin therapy.28 The presence of anemia with a hemoglobin value of 10 g/dL or lower or a platelet count lower than 100 × 109/L due to marrow infiltration also justified treatment. Certain complications such as hyperviscosity syndrome, symptomatic sensorimotor peripheral neuropathy, systemic amyloidosis, renal insufficiency, or symptomatic cryoglobulinemia may also be indications for therapy. It was recommended that patients with IgM MGUS and smoldering Waldenström macroglobulinemia be observed without treatment.
The recommendations for follow-up of these patients being managed with a watch-and-wait approach were that those with IgM MGUS have a serum protein electrophoresis repeated each year and that patients with asymptomatic (smoldering) macroglobulinemia be evaluated every 6 months.
Although such data identify patients who should be treated and provide prognostic information concerning their outcome, specific guidelines regarding which patients require what treatment are lacking. It is clear that some patients have very indolent disease and do not require any therapy. Others require treatment but will have an excellent outcome with very modest amounts of treatment. A third group of patients may have severe symptoms and may be at risk of potentially life-threatening complications and therefore require a more aggressive treatment approach. We have defined 3 groups of patients on the basis of presenting symptoms, prognostic factors, and laboratory parameters (Figure 1). The first group includes patients with IgM MGUS or smoldering (asymptomatic) Waldenström macroglobulinemia who have preserved hematologic function. The second group includes patients with symptomatic Waldenström macroglobulinemia who have modest hematologic compromise or immunoglobulin-associated complications. The third group includes patients with severe symptomatic Waldenström macroglobulinemia and profound hematologic compromise, bulky disease (defined as multiple sites of lymphadenopathy and/or organomegaly), or hyperviscosity.
Recommendation: A risk-adapted approach to the management of Waldenström macroglobulinemia is recommended. Patients with IgM MGUS or smoldering (asymptomatic) Waldenström macroglobulinemia and preserved hematologic function should be managed with a watch-and-wait approach.
Patients with symptomatic Waldenström macroglobulinemia and modest hematologic compromise, IgM-related neuropathy, or hemolytic anemia should receive monoclonal antibody therapy. Patients with Waldenström macroglobulinemia who have severe constitutional symptoms, profound hematologic compromise, bulky disease, or hyperviscosity should be treated with combination immunochemotherapy.
Level of Evidence: III
Grade of Recommendation: B
CHOICE OF INITIAL THERAPY
Not all patients with Waldenström macroglobulinemia warrant immediate treatment, and our proposed risk-adapted approach defines the group that should be initially observed (Figure 1). Because this disease is incurable with current treatment, the goal of therapy for Waldenström macroglobulinemia is to provide symptomatic relief and reduce the risk of organ damage. As with other indolent lymphomas, a “watch-and-wait” approach is reasonable in patients with Waldenström macroglobulinemia who do not have systemic symptoms, compromise of vital organs, bulky disease, or evident progression or transformation to a higher-grade lymphoma. To date, no evidence suggests that treatment of patients with smoldering/asymptomatic Waldenström macroglobulinemia provides a survival benefit compared with patients who begin therapy once symptoms occur.34,35 Also, no evidence shows that delaying therapy until clinical progression occurs adversely affects the subsequent response to treatment.36 Asymptomatic patients have a better quality of life without therapy, and withholding initial therapy limits their exposure to chemotherapeutic agents and possibly prevents development of drug resistance. However, the watch-and-wait strategy may have disadvantages in that patients must be monitored closely to prevent insidious complications or progression, and many patients are uncomfortable with the option of allowing their disease to progress without therapy. Nonetheless, with the watch-and-wait strategy, the 10-year survival is approximately 70% to 75%.37
Patients who require therapy are commonly treated with a monoclonal antibody alone or an antibody in combination with chemotherapy. All Waldenström macroglobulinemia cells express CD20, which enables use of the anti-CD20 monoclonal antibody rituximab. Initial studies using rituximab demonstrated improvements in cytopenias, neurologic symptoms, and the extent of bone marrow involvement.38-40 In patients with both treatment-naïve and previously treated Waldenström macroglobulinemia, rituximab has been associated with response rates ranging from 29% to 65%.40-44 Over time, it has become clear that the best response to rituximab may not be seen for many months after treatment.43 This may result in an underestimation of the activity of rituximab considering that the response end points in most clinical trials are at earlier time points. An important aspect in administering rituximab is that some patients will have a paradoxical increase in their monoclonal proteins after rituximab, the so-called rituximab flare.45 These levels may persist for up to 4 months and do not indicate treatment failure; however, plasmapheresis may be necessary to reduce hyperviscosity. Because of the lack of randomized trials comparing rituximab to other treatment approaches, it is impossible to state that rituximab is superior to other therapies. However, it is the opinion of our group that the use of single-agent rituximab is appropriate in low-risk patients with symptomatic Waldenström macroglobulinemia and modest hematologic compromise, and also in patients with IgM-related neuropathy who require treatment or who have hemolytic anemia unresponsive to corticosteroids. Whereas maintenance rituximab has been shown to prolong the time to progression in other low-grade lymphomas, the impact of maintenance rituximab therapy on time to progression has not been specifically validated in Waldenström macroglobulinemia, and we do not currently recommend maintenance therapy.
Initially, the standard therapy for patients with Waldenström macroglobulinemia was treatment with oral alkylating agents such as chlorambucil, melphalan, or cyclophosphamide.23,46,47 Oral chlorambucil was most commonly used, and more than half of the patients treated achieved a partial response. A randomized trial of chlorambucil administered either on a daily basis at low doses or intermittently at higher doses found that both schedules were equally effective.23
Combinations of alkylating agents with or without vinca alkaloids or anthracyclines have been used,48,49 although no prospective randomized trial has compared these regimens to single-agent chlorambucil. Subsequent studies have evaluated the role of purine nucleoside analogues and found that this class of agents is effective in Waldenström macroglobulinemia.50-54 Because alkylating agents and nucleoside analogues have been shown to be effective, combinations of these agents have been tested, and response rates of 58% to 88% have been observed.55-57 The addition of rituximab to these agents has been well tolerated with little additional toxicity and appears to further increase the response rate.58-60 Recent studies using the proteasome inhibitor bortezomib in combination with rituximab have found this combination to be effective.61,62
Faced with many effective regimens and few comparative studies, a consensus panel of experts recommended that alkylating agents, nucleoside analogues, and rituximab were all reasonable choices as first-line treatment of Waldenström macroglobulinemia.9,10 These guidelines were then updated to recommend combinations such as rituximab with nucleoside analogues with or without alkylating agents or with cyclophosphamide-based therapies (eg, cyclophosphamide, doxorubicin, vincristine, and prednisone or cyclophosphamide and dexamethasone) or combinations of rituximab with thalidomide.11 However, the guidelines did not recommend a specific first-line regimen.
Because of the long natural history of Waldenström macroglobulinemia, the choice of initial treatment is critical so that agents are not used that limit future treatment options. The prior use of purine nucleoside analogues has been associated with difficulty in mobilizing stem cells and should therefore be avoided in patients who may be eligible for autologous transplant.63,64 Furthermore, a recent report has indicated that nucleoside analogue–based combinations may be associated with an increased risk of transformation or myelodysplasia.65
Alkylating agent–based regimens in combination with rituximab may be preferable as initial therapy for Waldenström macroglobulinemia. Dimopoulos et al60 reported on the use of the DRC regimen consisting of 20 mg of dexamethasone administered intravenously followed by rituximab intravenously at 375 mg/m2 on day 1 and cyclophosphamide orally at 100 mg/m2 twice daily on days 1 to 5 every 21 days for 6 months in previously untreated patients with symptomatic Waldenström macroglobulinemia. An objective response was documented in 83% of patients, including 7% with complete response, 67% with partial response, and 9% with minor responses. The 2-year progression-free survival rate was 90%, and only 9% of patients experienced grade 3 or 4 hematologic toxicity. Although there are no data from randomized clinical trials showing that the DRC regimen is superior to combinations containing purine nucleoside analogues, anthracyclines, or proteasome inhibitors, this effective regimen is associated with a modest toxicity profile and a low likelihood of limiting future stem cell collections. Therefore, the opinion of our group is that this regimen is the combination of choice as initial treatment of patients with symptomatic or bulky disease, hematologic compromise, or hyperviscosity.
In tandem with the choice of initial therapy, patients should be evaluated for symptoms of hyperviscosity, and plasmapheresis should be performed if symptomatic hyperviscosity is present (Figure 1). A 3-4 liter plasma exchange will lower plasma IgM levels by approximately 60% to 75%.66 Serum viscosity is not linearly correlated with IgM levels, so a single exchange may lower the viscosity by at least 50%.67 Multiple exchanges may be necessary, and systemic therapy should accompany plasmapheresis for cytoreduction.
Recommendation: Using the risk groups outlined above, we recommend the following.
Patients with IgM MGUS or smoldering (asymptomatic) Waldenström macroglobulinemia and preserved hematologic function should be observed without initial therapy.
Patients with symptomatic Waldenström macroglobulinemia and modest hematologic compromise, IgM-related neuropathy requiring treatment, or hemolytic anemia unresponsive to corticosteroids should receive standard doses of rituximab alone without maintenance therapy.
Patients with Waldenström macroglobulinemia who have severe constitutional symptoms, profound hematologic compromise, bulky disease, or hyperviscosity should be treated with the DRC regimen. Any patient with symptoms of hyperviscosity should first undergo plasmapheresis.
Level of Evidence: III
Grade of Recommendation: B
TREATMENT OF RELAPSED PATIENTS
Because of the indolent but incurable nature of Waldenström macroglobulinemia, patients will inevitably experience relapse after initial therapy and require further treatment. Numerous treatment combinations have been tested in the relapsed setting, but comparative trials to identify the optimal treatment approach have not been performed.
The choice of salvage therapy may depend on the specific first-line treatment used, the quality and duration of response to initial therapy, as well as other variables such as tolerance of initial therapy and candidacy for stem cell transplant. The consensus recommendations from the Fourth International Workshop on Waldenström's Macroglobulinemia suggested the reuse of a first-line single agent or combination if the patient achieved a response that lasted for at least 12 months.11 For patients who had short remissions or resistance to a first-line regimen, the recommendation was to use agents of a different class either alone or in combination. The panel specifically highlighted the potential roles of fludarabine, bortezomib, and alemtuzumab. Additionally, recent data have suggested that bendamustine, either alone or in combination with rituximab, may be effective in patients with relapsed disease.68
The consensus panel from the Fourth International Workshop on Waldenström's Macroglobulinemia also noted that autologous or allogeneic stem cell transplant should be considered in patients with high-risk disease.11 High-dose therapy followed by autologous stem cell transplant has been performed in a growing number of patients with Waldenström macroglobulinemia.69,70 A variety of preparative regimens have been used, such as high-dose melphalan, BEAM (carmustine, etoposide, cytarabine, and melphalan), or cyclophosphamide with or without total body irradiation.
Autologous stem cell transplants have been relatively well tolerated with a low rate of nonrelapse mortality and high response rate even in patients whose disease was refractory to several regimens of standard chemotherapy. Furthermore, long-lasting complete responses have been observed. In the largest series of 158 adult patients reported to the European Group for Blood and Marrow Transplantation,71 half of the patients remained in remission at 5 years after an autologous stem cell transplant, and the nonrelapse mortality rate was 3.8%. The progression-free survival and overall survival rates at 5 years were 39.7% and 68.5%, respectively, but were significantly influenced by the number of previous therapies and whether patients' disease was refractory to therapy at the time of transplant. The authors concluded that autologous stem cell transplant is feasible, particularly in younger patients, but should not be offered to patients with chemoresistant disease. In contrast, allogeneic stem cell transplant has a much higher risk (40%) of nonrelapse mortality and should not be considered outside the context of a clinical trial.72
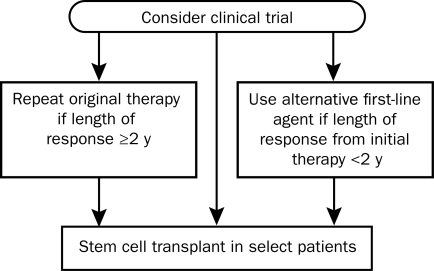
Recommendation: Because there is no standard approach to the management of patients with relapsed Waldenström macroglobulinemia, it is our view that these patients should always be considered for participation in clinical trials. Two further issues need to be considered—whether the patient had a durable response to frontline therapy and whether the patient is a candidate for an autologous stem cell transplant. Our approach (Figure 2) is to consider all patients for participation in a clinical trial either as definitive therapy for their disease or as preparative therapy before transplant. For patients who are ineligible or unwilling to enter a clinical trial, the choice of therapy is determined by their response to frontline treatment. Because responses to initial therapies are often delayed and can occur 12 months or more after treatment is initiated, we recommend using a 2-year cutoff to determine treatment. For patients whose response has lasted more than 2 years, the original therapy can be repeated. For patients who have an inadequate response to initial therapy or a response lasting less than 2 years, an alternative agent or combination should be used. An autologous stem cell transplant should be considered in all eligible patients with relapsed disease.
FIGURE 2.
Mayo Clinic (Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy [mSMART]) consensus for management of relapsed Waldenström macroglobulinemia.
Level of Evidence: III
Grade of Recommendation: B
CONCLUSIONS
Waldenström macroglobulinemia is a rare disease, and practicing hematologists and oncologists may infrequently treat such patients. Patients may present with a spectrum of clinical findings, and many patients do not require treatment initially. When patients do require therapy, it is important that therapies are selected that do not adversely impact future treatment options. To provide a simple risk-adapted approach to managing patients with Waldenström macroglobulinemia, we have outlined the approach of 33 hematologists from a single institution.
These recommendations are regularly modified as new data become available. The most current guidelines are always available at www.mSMART.org.
Supplementary Material
Footnotes
An earlier version of this article appeared Online First.
REFERENCES
- 1.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30(2):110-115 [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP. Diagnosis and management of Waldenström's macroglobulinemia. J Clin Oncol. 2005;23(7):1564-1577 [DOI] [PubMed] [Google Scholar]
- 3.Herrinton LJ, Weiss NS. Incidence of Waldenström's macroglobulinemia. Blood. 1993;82(10):3148-3150 [PubMed] [Google Scholar]
- 4.Groves FD, Travis LB, Devesa SS, Ries LA, Fraumeni JF., Jr Waldenström's macroglobulinemia: incidence patterns in the United States, 1988-1994. Cancer. 1998;82(6):1078-1081 [PubMed] [Google Scholar]
- 5.Benjamin M, Reddy S, Brawley OW. Myeloma and race: a review of the literature. Cancer Metastasis Rev. 2003;22(1):87-93 [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M. Waldenström's macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18(1):214-226 [DOI] [PubMed] [Google Scholar]
- 7.Vijay A, Gertz MA. Waldenström macroglobulinemia. Blood. 2007;109(12):5096-5103 [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Galani E, Matsouka C. Waldenström's macroglobulinemia. Hematol Oncol Clin North Am. 1999;13(6):1351-1366 [DOI] [PubMed] [Google Scholar]
- 9.Gertz MA, Anagnostopoulos A, Anderson K, et al. Treatment recommendations in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30(2):121-126 [DOI] [PubMed] [Google Scholar]
- 10.Treon SP, Gertz MA, Dimopoulos M, et al. Update on treatment recommendations from the Third International Workshop on Waldenström's Macroglobulinemia. Blood. 2006;107(9):3442-3446 [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Gertz MA, Kastritis E, et al. Update on treatment recommendations from the Fourth International Workshop on Waldenström's Macroglobulinemia. J Clin Oncol. 2009;27(1):120-126 [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82(3):323-341 [DOI] [PubMed] [Google Scholar]
- 13.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84(12):1095-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2 4th ed.Geneva, Switzerland: International Agency for Research on Cancer (IARC); 2008:441 [Google Scholar]
- 15.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morice WG, Chen D, Kurtin PJ, Hanson CA, McPhail ED. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenström's macroglobulinemia. Mod Pathol. 2009;22(6):807-816 [DOI] [PubMed] [Google Scholar]
- 17.Schop RF, Kuehl WM, Van Wier SA, et al. Waldenström macroglobulinemia neoplastic cells lack immunoglobulin heavy chain locus translocations but have frequent 6q deletions. Blood. 2002;100(8):2996-3001 [DOI] [PubMed] [Google Scholar]
- 18.Schop RF, Van Wier SA, Xu R, et al. 6q deletion discriminates Waldenström macroglobulinemia from IgM monoclonal gammopathy of undetermined significance. Cancer Genet Cytogenet. 2006;169(2):150-153 [DOI] [PubMed] [Google Scholar]
- 19.Braggio E, Keats JJ, Leleu X, et al. Identification of copy number abnormalities and inactivating mutations in two negative regulators of nuclear factor-κB signaling pathways in Waldenström's macroglobulinemia. Cancer Res. 2009;69(8):3579-3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102(10):3759-3764 [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Rajkumar SV, Therneau TM, Larson DR, Plevak MF, Melton LJ., III Prognostic factors and predictors of outcome of immunoglobulin M monoclonal gammopathy of undetermined significance. Clin Lymphoma. 2005;5(4):257-260 [DOI] [PubMed] [Google Scholar]
- 22.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenström's macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23(21):4662-4668 [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Greipp PR, Gertz MA, et al. Waldenström's macroglobulinaemia: a prospective study comparing daily with intermittent oral chlorambucil. Br J Haematol. 2000;108(4):737-742 [DOI] [PubMed] [Google Scholar]
- 24.Morel P, Monconduit M, Jacomy D, et al. Patients with the description of a new scoring system and its validation on 253 other patients. Blood. 2000;96(3):852-858 [PubMed] [Google Scholar]
- 25.Ghobrial IM, Fonseca R, Gertz MA, et al. Prognostic model for disease-specific and overall mortality in newly diagnosed symptomatic patients with Waldenström macroglobulinaemia. Br J Haematol. 2006;133(2):158-164 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Sanz R, Montoto S, Torrequebrada A, et al. Waldenström macroglobulinaemia: presenting features and outcome in a series with 217 cases. Br J Haematol. 2001;115(3):575-582 [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos M, Gika D, Zervas K, et al. The international staging system for multiple myeloma is applicable in symptomatic Waldenström's macroglobulinemia. Leuk Lymphoma. 2004;45(9):1809-1813 [DOI] [PubMed] [Google Scholar]
- 28.Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30(2):116-120 [DOI] [PubMed] [Google Scholar]
- 29.Leleu X, Moreau AS, Weller E, et al. Serum immunoglobulin free light chain correlates with tumor burden markers in Waldenström macroglobulinemia. Leuk Lymphoma. 2008;49(6):1104-1107 [DOI] [PubMed] [Google Scholar]
- 30.Kastritis E, Zervas K, Repoussis P, et al. Prognostication in young and old patients with Waldenström's macroglobulinemia: importance of the International Prognostic Scoring System and of serum lactate dehydrogenase. Clin Lymphoma Myeloma. 2009;9(1):50-52 [DOI] [PubMed] [Google Scholar]
- 31.Ho AW, Hatjiharissi E, Ciccarelli BT, et al. CD27-CD70 interactions in the pathogenesis of Waldenström macroglobulinemia. Blood. 2008;112(12):4683-4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenström macroglobulinemia. Blood. 2009;113(18):4163-4170 [DOI] [PubMed] [Google Scholar]
- 33.Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9(1):17-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexanian R, Weber D, Delasalle K, Cabanillas F, Dimopoulos M. Asymptomatic Waldenström's macroglobulinemia. Semin Oncol. 2003;30(2):206-210 [DOI] [PubMed] [Google Scholar]
- 35.Gobbi PG, Baldini L, Broglia C, et al. Prognostic validation of the international classification of immunoglobulin M gammopathies: a survival advantage for patients with immunoglobulin M monoclonal gammopathy of undetermined significance? Clin Cancer Res. 2005;11(5):1786-1790 [DOI] [PubMed] [Google Scholar]
- 36.Vitolo U, Ferreri AJ, Montoto S. Lymphoplasmacytic lymphoma-Waldenström's macroglobulinemia. Crit Rev Oncol Hematol. 2008;67(2):172-185 [DOI] [PubMed] [Google Scholar]
- 37.O'Brien ME, Easterbrook P, Powell J, et al. The natural history of low grade non-Hodgkin's lymphoma and the impact of a no initial treatment policy on survival. Q J Med. 1991;80(292):651-660 [PubMed] [Google Scholar]
- 38.Weide R, Heymanns J, Koppler H. Induction of complete haematological remission after monotherapy with anti-CD20 monoclonal antibody (RITUXIMAB) in a patient with alkylating agent resistant Waldenström's macroglobulinaemia. Leuk Lymphoma. 1999;36(1-2):203-206 [DOI] [PubMed] [Google Scholar]
- 39.Weide R, Heymanns J, Koppler H. The polyneuropathy associated with Waldenström's macroglobulinaemia can be treated effectively with chemotherapy and the anti-CD20 monoclonal antibody rituximab. Br J Haematol. 2000;109(4):838-841 [DOI] [PubMed] [Google Scholar]
- 40.Treon SP, Agus TB, Link B, et al. CD20-directed antibody-mediated immunotherapy induces responses and facilitates hematologic recovery in patients with Waldenström's macroglobulinemia. J Immunother. 2001;24(3):272-279 [PubMed] [Google Scholar]
- 41.Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18(2):317-324 [DOI] [PubMed] [Google Scholar]
- 42.Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström's macroglobulinemia with rituximab. J Clin Oncol. 2002;20(9):2327-2333 [DOI] [PubMed] [Google Scholar]
- 43.Treon SP, Emmanouilides C, Kimby E, et al. Extended rituximab therapy in Waldenstrom's macroglobulinemia. Ann Oncol. 2005;16(1):132-138 [DOI] [PubMed] [Google Scholar]
- 44.Gertz MA, Rue M, Blood E, Kaminer LS, Vesole DH, Greipp PR. Multicenter phase 2 trial of rituximab for Waldenström macroglobulinemia (WM): an Eastern Cooperative Oncology Group Study (E3A98). Leuk Lymphoma. 2004;45(10):2047-2055 [DOI] [PubMed] [Google Scholar]
- 45.Ghobrial IM, Fonseca R, Greipp PR, et al. Initial immunoglobulin M ‘flare’ after rituximab therapy in patients diagnosed with Waldenström macroglobulinemia: an Eastern Cooperative Oncology Group Study. Cancer. 2004;101(11):2593-2598 [DOI] [PubMed] [Google Scholar]
- 46.Annibali O, Petrucci MT, Martini V, et al. Treatment of 72 newly diagnosed Waldenström macroglobulinemia cases with oral melphalan, cyclophosphamide, and prednisone: results and cost analysis. Cancer. 2005;103(3):582-587 [DOI] [PubMed] [Google Scholar]
- 47.Petrucci MT, Avvisati G, Tribalto M, Giovangrossi P, Mandelli F. Waldenström's macroglobulinaemia: results of a combined oral treatment in 34 newly diagnosed patients. J Intern Med. 1989;226(6):443-447 [DOI] [PubMed] [Google Scholar]
- 48.Case DC, Jr, Ervin TJ, Boyd MA, Redfield DL. Waldenström's macroglobulinemia: long-term results with the M-2 protocol. Cancer Invest. 1991;9(1):1-7 [DOI] [PubMed] [Google Scholar]
- 49.Leblond V, Levy V, Maloisel F, et al. Multicenter, randomized comparative trial of fludarabine and the combination of cyclophosphamide-doxorubicin-prednisone in 92 patients with Waldenström macroglobulinemia in first relapse or with primary refractory disease. Blood. 2001;98(9):2640-2644 [DOI] [PubMed] [Google Scholar]
- 50.Dimopoulos MA, Weber DM, Kantarjian H, Keating M, Alexanian R. 2Chlorodeoxyadenosine therapy of patients with Waldenström macroglobulinemia previously treated with fludarabine. Ann Oncol. 1994;5(3):288-289 [DOI] [PubMed] [Google Scholar]
- 51.Foran JM, Rohatiner AZ, Coiffier B, et al. Multicenter phase II study of fludarabine phosphate for patients with newly diagnosed lymphoplasmacytoid lymphoma, Waldenström's macroglobulinemia, and mantle-cell lymphoma. J Clin Oncol. 1999;17(2):546-553 [DOI] [PubMed] [Google Scholar]
- 52.Thalhammer-Scherrer R, Geissler K, Schwarzinger I, et al. Fludarabine therapy in Waldenström's macroglobulinemia. Ann Hematol. 2000;79(10):556-559 [DOI] [PubMed] [Google Scholar]
- 53.Dhodapkar MV, Jacobson JL, Gertz MA, et al. Prognostic factors and response to fludarabine therapy in patients with Waldenström macroglobulinemia: results of United States intergroup trial (Southwest Oncology Group S9003). Blood. 2001;98(1):41-48 [DOI] [PubMed] [Google Scholar]
- 54.Hellmann A, Lewandowski K, Zaucha JM, Bieniaszewska M, Halaburda K, Robak T. Effect of a 2-hour infusion of 2-chlorodeoxyadenosine in the treatment of refractory or previously untreated Waldenström's macroglobulinemia. Eur J Haematol. 1999;63(1):35-41 [DOI] [PubMed] [Google Scholar]
- 55.Tamburini J, Levy V, Chaleteix C, et al. Fludarabine plus cyclophosphamide in Waldenström's macroglobulinemia: results in 49 patients. Leukemia. 2005;19(10):1831-1834 [DOI] [PubMed] [Google Scholar]
- 56.Laurencet FM, Zulian GB, Guetty-Alberto M, Iten PA, Betticher DC, Alberto P. Cladribine with cyclophosphamide and prednisone in the management of low-grade lymphoproliferative malignancies. Br J Cancer. 1999;79(7-8):1215-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Den Neste E, Louviaux I, Michaux JL, et al. Phase I/II study of 2-chloro-2′-deoxyadenosine with cyclophosphamide in patients with pretreated B cell chronic lymphocytic leukemia and indolent non-Hodgkin's lymphoma. Leukemia. 2000;14(6):1136-1142 [DOI] [PubMed] [Google Scholar]
- 58.Treon SP, Branagan AR, Ioakimidis L, et al. Long-term outcomes to fludarabine and rituximab in Waldenström macroglobulinemia. Blood. 2009;113(16):3673-3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tam CS, Wolf M, Prince HM, et al. Fludarabine, cyclophosphamide, and rituximab for the treatment of patients with chronic lymphocytic leukemia or indolent non-Hodgkin lymphoma. Cancer. 2006;106(11):2412-2420 [DOI] [PubMed] [Google Scholar]
- 60.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25(22):3344-3349 [DOI] [PubMed] [Google Scholar]
- 61.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27(23):3830-3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghobrial IM, Hong F, Padmanabhan S, et al. Phase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory Waldenström macroglobulinemia. J Clin Oncol. 2010;28(8):1422-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tournilhac O, Cazin B, Lepretre S, et al. Impact of frontline fludarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemia. Blood. 2004;103(1):363-365 [DOI] [PubMed] [Google Scholar]
- 64.Desikan R, Dhodapkar M, Siegel D, et al. High-dose therapy with autologous haemopoietic stem cell support for Waldenström's macroglobulinaemia. Br J Haematol. 1999;105(4):993-996 [DOI] [PubMed] [Google Scholar]
- 65.Leleu X, Soumerai J, Roccaro A, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol. 2009;27(2):250-255 [DOI] [PubMed] [Google Scholar]
- 66.Kaplan AA. Therapeutic apheresis for the renal complications of multiple myeloma and the dysglobulinemias. Ther Apher. 2001;5(3):171-175 [PubMed] [Google Scholar]
- 67.Avnstorp C, Nielsen H, Drachmann O, Hippe E. Plasmapheresis in hyperviscosity syndrome. Acta Med Scand. 1985;217(1):133-137 [DOI] [PubMed] [Google Scholar]
- 68.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(15):3383-3389 [DOI] [PubMed] [Google Scholar]
- 69.Gilleece MH, Pearce R, Linch DC, et al. The outcome of haemopoietic stem cell transplantation in the treatment of lymphoplasmacytic lymphoma in the UK: a British Society Bone Marrow Transplantation study. Hematology. 2008;13(2):119-127 [DOI] [PubMed] [Google Scholar]
- 70.Caravita T, Siniscalchi A, Tendas A, et al. High-dose therapy with autologous PBSC transplantation in the front-line treatment of Waldenstrom's macroglobulinemia. Bone Marrow Transplant. 2009;43(7):587-588 [DOI] [PubMed] [Google Scholar]
- 71.Kyriakou C, Canals C, Sibon D, et al. High-dose therapy and autologous stem-cell transplantation in Waldenström macroglobulinemia: the lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28(13):2227-2232 [DOI] [PubMed] [Google Scholar]
- 72.Anagnostopoulos A, Hari PN, Perez WS, et al. Autologous or allogeneic stem cell transplantation in patients with Waldenström's macroglobulinemia. Biol Blood Marrow Transplant. 2006;12(8):845-854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.