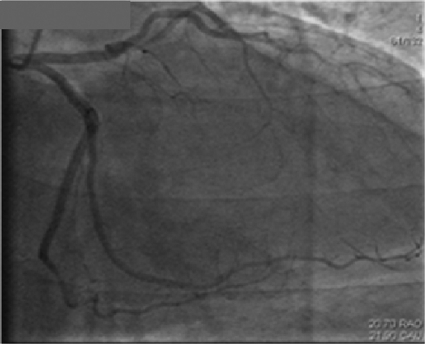
To the Editor: A 61-year-old morbidly obese man (body mass index, 46 [calculated as the weight in kilograms divided by height in meters squared]) with a history of arterial hypertension, chronic obstructive pulmonary disease, and obstructive sleep apnea was admitted for radical resection of a squamous cell carcinoma of the skin overlying the right zygoma and temporal area extending into the auricle. The patient's postoperative course was uncomplicated until development of substernal chest pain associated with dyspnea and anterolateral ST-segment elevation on electrocardiography (performed while he was in bed) after he had engaged in an argument on postoperative day 14. Cardiac catheterization revealed no obstructive coronary artery disease (Figure 1). Serial electrocardiograms showed persistent ST-segment elevation, but serum troponin levels were negative. A transthoracic echocardiogram demonstrated moderately decreased left ventricular function with an ejection fraction of 35% and apical akinesis (Figure 2), suggesting stress-induced cardiomyopathy (SCM). Given the hypothesis that sex hormones play a modulating role in SCM,1 sex hormone levels were measured. His serum estrone value was elevated at 7.5 ng/dL, serum estradiol was normal at 27 pg/mL, and free testosterone was depressed at 5.7 ng/dL. He was treated with oral lisinopril, 2.5 mg/d, and oral carvedilol, 12.5 mg twice daily. Echocardiography performed 3 weeks after the patient was discharged from the hospital revealed a normal left ventricular ejection fraction of 65% to 70%, without wall motion abnormalities.
FIGURE 1.
Angiogram revealing clean coronary arteries.
FIGURE 2.
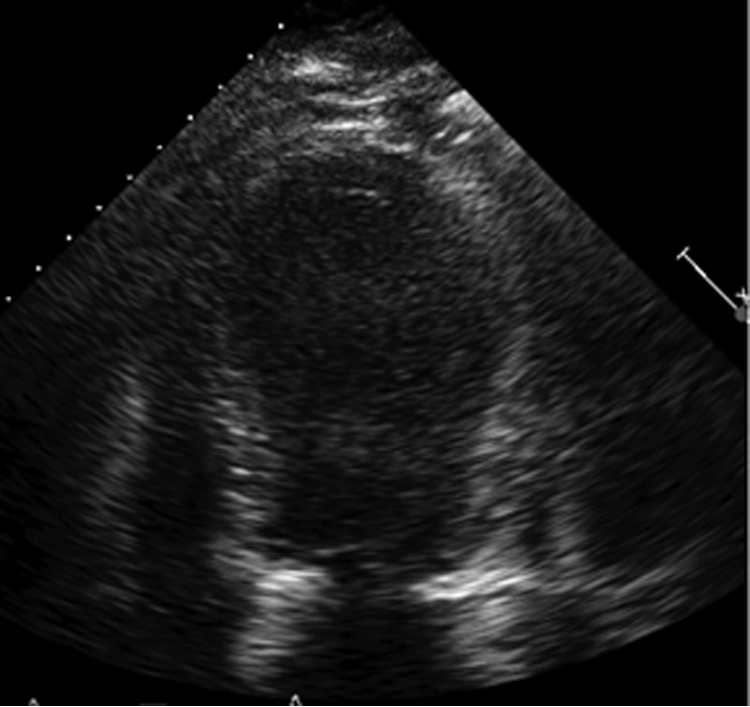
Transthoracic echocardiogram revealing apical ballooning shortly after chest pain episode.
In recent years, several studies have suggested that SCM is associated with increased catecholamine and sympathetic activity.1 The sex and age predominance of SCM, which seems to occur mainly in postmenopausal women, suggests that estrogens may adversely modulate sympathetic activity and therefore increase susceptibility to SCM. Conversely, the lower incidence of SCM in healthy men suggests that androgens may play a protective regulatory role in the pathophysiology of SCM. The protective role of androgens may be lost in obese men because of altered sex hormone balance with decreased testosterone levels.
These clinical considerations are supported by several experimental and clinical studies. Male rat arteries showed greater vasoconstriction in response to adrenergic nerve stimulation compared with female rat arteries. Estradiol reduces the response to vasoconstrictors in both male and female arteries.2 In postmenopausal women, attenuation of sympathetic nerve discharge may be induced by administration of transdermal estrogen.3 Estrogen also down-regulates β1-adrenergic receptor expression.4
Testosterone has been reported to cause a direct and rapid vasodilatory effect in coronary arteries.5 Intracoronary administration of testosterone, at physiological concentrations, dilates coronary arteries and increases coronary blood flow in men with established coronary artery disease.6 Testosterone is thought to induce vasodilatation by blocking a membrane-associated calcium channel.7 Efflux of potassium through channels in vascular smooth muscle is another possible mechanism of the vasodilatory effects of testosterone.8
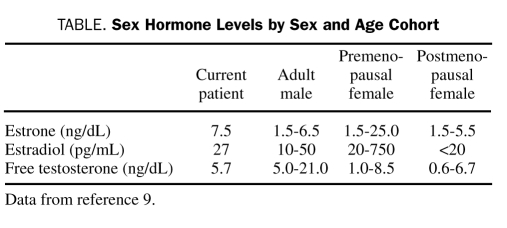
In postmenopausal women, estradiol and estrone levels are closer to those of healthy adult men than those of premenopausal women (Table). The lower levels of estrogen in the absence of testosterone may explain the greater vulnerability of postmenopausal women.
TABLE.
Sex Hormone Levels by Sex and Age Cohort
Our patient's free testosterone level was at the lower limit of normal, and his estrone level was above the limits of normal for an adult male. This sex hormone profile in a morbidly obese man likely results from aromatization of androgens to estrogens. Also, several studies have linked obesity to endothelial dysfunction. Therefore, it is plausible that this patient was particularly vulnerable to catecholamine-induced vasospasm in the absence of the protective effects of high estrogen or normal testosterone levels.
References
- 1.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Villalon AL, Buchholz JN, Krause DN, et al. Sex differences in the effects of 17 beta-estradiol on vascular adrenergic responses. Eur J Pharmacol. 1996;314(3):339-345 [DOI] [PubMed] [Google Scholar]
- 3.Vongpatanasin W, Tuncel M, Mansour Y, et al. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103(24):2903-2908 [DOI] [PubMed] [Google Scholar]
- 4.Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of beta 1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72(16):1813-1824 [DOI] [PubMed] [Google Scholar]
- 5.Yue P, Chatterjee K, Beale C, et al. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91(4):1154-1160 [DOI] [PubMed] [Google Scholar]
- 6.Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100(16):1690-1696 [DOI] [PubMed] [Google Scholar]
- 7.Jones RD, English KM, Pugh PJ, et al. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol. 2002;39(6):814-823 [DOI] [PubMed] [Google Scholar]
- 8.Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol. 2001;91(6):2742-2750 [DOI] [PubMed] [Google Scholar]
- 9.Gardner DG, Shoback D, eds. Greenspan's Basic & Clinical Endocrinology. 8th ed.New York, NY: McGraw Hill; 2007. [Google Scholar]