Abstract
Aims/hypothesis
We sought to establish the extent and basis for adaptive changes in beta cell numbers in human pregnancy.
Methods
Pancreas was obtained at autopsy from women who had died while pregnant (n = 18), post-partum (n = 6) or were not pregnant at or shortly before death (controls; n = 20). Pancreases were evaluated for fractional pancreatic beta cell area, islet size and islet fraction of beta cells, beta cell replication (Ki67) and apoptosis (TUNEL), and indirect markers of beta cell neogenesis (insulin-positive cells in ducts and scattered beta cells in pancreas).
Results
The pancreatic fractional beta cell area was increased by ∼1.4-fold in human pregnancy, with no change in mean beta cell size. In pregnancy there were more small islets rather than an increase in islet size or beta cells per islet. No increase in beta cell replication or change in beta cell apoptosis was detected, but duct cells positive for insulin and scattered beta cells were increased with pregnancy.
Conclusions/interpretation
The adaptive increase in beta cell numbers in human pregnancy is not as great as in most reports in rodents. This increase in humans is achieved by increased numbers of beta cells in apparently new small islets, rather than duplication of beta cells in existing islets, which is characteristic of pregnancy in rodents.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-010-1809-6) contains supplementary material, which is available to authorised users.
Keywords: Human, Islet, Pancreas, Pregnancy
Introduction
Glucose homeostasis is regulated by insulin, which is secreted by pancreatic beta cells in a manner dependent on glucose concentration. In type 1 and 2 diabetes, insulin secretion is insufficient to maintain glycaemic control. In the former, a near complete loss of beta cell mass occurs [1], while in the latter, the deficit in beta cell mass is partial [2].
Beta cell mass has been reported to increase by two- to fivefold in rodents in response to pregnancy [3–5]. This observation has provoked interest in the signals that drive this increase in beta cell mass in the hope that the same signals might be harnessed to foster beta cell regeneration in humans with diabetes [6]. However, there are limited data on the adaptive changes in beta cell mass in humans during pregnancy.
In human pregnancy, insulin sensitivity progressively declines during the last 20 weeks of gestation [7]. In health, this pregnancy-related insulin resistance is met with a corresponding increase in insulin secretion [7–9] so that plasma glucose values remain normal or even somewhat decreased. The only available study of pancreas in humans during pregnancy reported that the fraction of pancreas occupied by beta cells increased 2.4-fold compared with non-pregnant women [10]. However, only five cases were studied and the morphological evaluation was limited.
In the present study, we sought to address the question of: (1) whether there is an adaptive increase in beta cell numbers and/or size during pregnancy in humans (and if so to what extent?); and (2) whether any increase detected in the number of beta cells in pregnancy is achieved predominantly through replication of existing beta cells or through formation of beta cells from sources independent of beta cell replication (beta cell neogenesis).
Methods
Autopsy cases
Human pancreatic tissue was obtained at autopsy from 18 women who had died while pregnant, six women who died post-partum, and 20 women who were not pregnant at death (Tables 1, 2 and 3). Potential tissue providers were identified by retrospective analysis of the Mayo Clinic autopsy database. For inclusion in the study, a full autopsy had to have been conducted within 24 h of death and pancreatic tissue of adequate size and quality stored. Exclusion criteria were if pancreatic tissue had undergone autolysis or showed evidence of acute pancreatitis. None of the deceased selected had had a history of diabetes or any other diseases affecting the pancreas. Their characteristics and diagnoses leading to death are presented in Tables 1, 2 and 3. Institutional Review Board approval was obtained from the Mayo Clinic and University of California Los Angeles (UCLA). Since death in pregnancy is fortunately now rare, we accessed samples obtained at autopsy from the late 1940s to early 2000s. The control tissue from women who were not pregnant at death was obtained from pancreas secured at autopsy over the same period. Death in the young pregnant and non-pregnant women included in this study typically followed a sudden catastrophic event (for example road traffic accident). As such the medical record did not provide a sufficiently robust family history of diabetes to document with the pancreatic morphometric findings. Also, fasting blood glucose values in health before the final catastrophic illness were unavailable. Most women had not attended the Mayo Clinic previously, but were brought to the medical centre for treatment of the final illness and autopsy. Therefore our presumption that the women did not have diabetes was based on the absence of a prior history of diabetes or diabetes in their final illness, rather than on a fasting evaluation in health. Since the controls were selected to be of similar age, to have also died a sudden death and to be matched for year of procurement of the pregnant individuals, in health ambulatory fasting blood glucose values were also unavailable in most of these cases.
Table 1.
Clinical characteristics, pregnant women
| Woman | Gestation week at death | Age at death (years) | Pre-pregnancy BMI (kg/m2) | Beta cell area (%) | Cause of death | Fetal viability | Pregnancy history |
|---|---|---|---|---|---|---|---|
| 1 | 22 | 22 | 22.2 | 2.03 | Increased intracranial pressure due to encephalitis | Not viable | G1P0 |
| 2 | 10 | 31 | 19.9 | 2.81 | Sudden death, unexplained | Not viable | G3P0 |
| 3 | 16 | 32 | 28 | 3.44 | Acute respiratory paralysis polio | Not viable | G5P4 |
| 4 | 16 | 21 | 27.8 | 1.05 | Overdose of barbiturates | Not viable | – |
| 5 | 37 | 18 | 21.2 | 1.08 | Diffuse cerebral oedema (hypoxic encephalopathy) | Live | G1P0 |
| 6 | 15 | 19 | 23.9 | 1.14 | Trauma | Stillborn | G2P1 |
| 7 | 27 | 36 | 34.1 | 1.97 | Myocardial failure secondary to ARDS | Stillborn | G2P1 |
| 8 | 18 | 25 | 22.5 | 0.60 | Trauma | Stillborn | – |
| 9 | 34 | 26 | 18.3 | 1.57 | L temporal lobe abscess | Live | G4P3 |
| 10 | 36 | 42 | 28.0 | 3.20 | Phaeochromocytoma, hypertensive CVD | Stillborn | G14P13 |
| 11 | 40 | 42 | 23.4 | 0.61 | IPH, shock and coma | Live | G8 |
| 12 | 36 | 23 | 24 | 1.24 | Thrombotic mitral stenosis | Live | G1P1 |
| 13 | 34 | 38 | 32.3 | 1.13 | Amniotic fluid embolism | Live twins | G8 P6 |
| 14 | 15 | 31 | 25.6 | 1.01 | Systemic lupus, cardiac arrest | Not viable | – |
| 15 | 14 | 19 | 21.3 | 0.75 | Ruptured AV malformation | Not viable | G1P0 |
| 16 | 10.5 | 40 | 26.8 | 2.19 | Pulmonary hypertension | Not viable | – |
| 17 | 40 | 38 | 30.7 | 2.56 | Eclampsia | Live | G1P1 |
| 18 | 22 | 25 | 22.2 | 2.22 | Acute liver failure (fulminant HBsAg+ hepatitis) | Not viable | G2P1 |
| Mean | 24.58 | 29.33 | 25.12 | 1.70 | |||
| SEM | 2.55 | 1.98 | 1.03 | 0.21 |
ARDS, acute respiratory distress syndrome; AV, arterial venous; CVD, cardiovascular disease; HBsAg, hepatitis B surface antigen; G, gravida; IPH, intrapartum haemorrhage; P, para
Table 2.
Clinical characteristics, post-partum women
| Woman | Time of death (days PP) | Age at death (years) | Pre-pregnancy BMI (kg/m2) | Beta cell area (%) | Cause of death | Fetus viability | Previous pregnancies |
|---|---|---|---|---|---|---|---|
| 1 | 5a | 30 | 29.1 | 1.06 | Eclampsia | Live | G1P1 |
| 2 | 14b | 43 | 29.2 | 1.55 | Cor pulmonale | Live | – |
| 3 | 14b | 24 | 49.4 | 1.33 | Pulmonary thromboembolism | Live | – |
| 4 | 4b | 31 | 21.5 | 1.65 | Staphylococcus septicaemia | Live | – |
| 5 | 1b | 43 | 32.5 | 1.11 | Endometritis, pulmonary haemorrhage | Live | G12P7 |
| 6 | 21b | 23 | 19.5 | 1.81 | Toxaemia of pregnancy | Live | G3P3 |
| Mean | 32.33 | 30.20 | 1.42 | ||||
| SEM | 3.61 | 4.34 | 0.12 |
aCaesarean section at week 31; bfull term
G, gravida; P, para; PP, post-partum
Table 3.
Clinical characteristics, control women
| Woman | Age at death | BMI (kg/m2) | Beta cell area (%) | Cause of death |
|---|---|---|---|---|
| 1 | 27 | 17 | 1.19 | Ruptured saccular aneurysm, respiratory arrest |
| 2 | 29 | 18.7 | 2.05 | Motor vehicle accident, blunt force chest injury |
| 3 | 29 | 19.4 | 0.84 | Strangulation by hanging |
| 4 | 34 | 22.9 | 1.67 | Seizure disorder, anoxic brain injury following cardiopulmonary arrest |
| 5 | 19 | 22.6 | 1.19 | Motor vehicle accident, closed head injury |
| 6 | 35 | 23.7 | 1.00 | Acute liver failure |
| 7 | 31 | 19.7 | 1.46 | Acute pneumonia |
| 8 | 25 | 19.6 | 1.06 | Congenital heart disease, VSD |
| 9 | 18 | 17 | 1.48 | Congenital heart disease, VSD |
| 10 | 25 | 23.7 | 1.03 | Acute liver failure (hepatitis) |
| 11 | 26 | 20.9 | 0.77 | Motor vehicle accident, head trauma |
| 12 | 42 | 25.5 | 0.95 | Asthma and bronchopneumonia |
| 13 | 42 | 19.9 | 1.33 | Thromboembolus right cerebral artery, cerebral infarct |
| 14 | 32 | 19.7 | 1.94 | Hypertensive pulmonary vascular disease |
| 15 | 31 | 25.1 | 0.70 | Smoke inhalation |
| 16 | 22 | 22.9 | 1.27 | Motor vehicle accident |
| 17 | 18 | 17.7 | 0.93 | Aeroplane crash, spinal trauma |
| 18 | 31 | 22.9 | 0.51 | Acute meningitis |
| 19 | 37 | 27.5 | 0.99 | Strangulation |
| 20 | 33 | 38.4 | 1.35 | Respiratory arrest, MI |
| Mean | 29.30 | 22.24 | 1.19 | |
| SEM | 1.57 | 1.07 | 0.10 |
MI, myocardial infarction; VSD, ventricular septal defect
The BMI of the pregnant women was calculated from pre-pregnancy height and weight, and was comparable to that of the control group (25.1 ± 1.0 vs 22.2 ± 1.1, pregnant vs control, p = NS). The BMI was greater in the post-partum than in the control group (30.2 ± 4.3 vs 22.2 ± 1.1, post-partum vs control, p < 0.05).
Pancreatic tissue processing
At Mayo Clinic the tail of the pancreas was resected at autopsy and a block of pancreas, approximately 2.0 × 1.0 × 0.5 cm in size, along with a sample of spleen, were fixed in formaldehyde prior to being embedded in paraffin. Sections of these blocks were obtained as previously described [2]. Sequential 5-μm sections approximately 2 × 1 cm were stained as follows: (1) for insulin (peroxidase staining) and haematoxylin for light microscopy; (2) insulin, TUNEL and DAPI combined (immunofluorescence); and (3) insulin, Ki67 and DAPI combined (immunofluorescence).
For immunohistochemical staining, the following primary antibodies were used: guinea pig anti-insulin (1:200; Dako Laboratories, Carpinteria, CA, USA) and mouse Ki67 (1:200, MIB-1; Dako). Secondary antibodies labelled with Cy3 and fluorescein isothiocyanate were obtained from The Jackson Laboratories (West Grove, PA, USA) and used at dilutions of 1:100 to 1:200. For TUNEL staining, an in situ cell death detection kit (KIT AP; Roche Diagnostics, Indianapolis, IN, USA) was used.
Morphometric analysis
To determine the pancreatic fractional beta cell area, the entire pancreatic section was imaged at 40× magnification (4× objective). The ratio of the beta cell area:exocrine area was digitally quantified as previously described [2] using a software package (Image Pro Plus version 4.5.1; Media Cybernetics, Silver Springs, MD, USA). In brief, digital images were made of pancreatic lobules (excluding the interlobular connective tissue, large blood vessels and adipocytes, and thus consisting to the greatest extent of pancreatic acinar tissue and pancreatic islets). This and the other analyses below were evaluated independently by two observers (A. E. Butler and L. Cao-Minh) blinded as to the group from which the slides to be evaluated came. UCLA-based investigators remained blinded to the groups until the morphometric analysis was completed, at which time the identity of the groups was made available by R. A. Rizza from Mayo Clinic. If the inter-observer measures of any variable in any sample were more than 10%, A. E. Butler and L. Cao-Minh independently re-evaluated the slide and rescored the variable.
To determine the frequency of beta cell replication, 100 islets per section were analysed from the section stained by immunofluorescence for insulin, Ki67 and DAPI. Each islet was viewed at 200× magnification (20× objective). The total number of Ki67-positive beta cells was expressed as Ki67-positive beta cells per 100 islets.
To determine the frequency of beta cell apoptosis, 100 islets per pancreas were analysed using the section stained by immunofluorescence for insulin, TUNEL and DAPI combined. The number of apoptotic beta cells was expressed as TUNEL-positive beta cells per 100 islets.
To determine islet size and density, pancreatic sections stained for insulin (peroxidase) and haematoxylin were analysed. After assessment of the entire pancreatic section, the largest islet in the section was identified, along with ten other prominent islets. For each islet, the total islet size was measured (Image Pro Plus), followed by measurement of the insulin-positive area of each islet. The Wicksell transform [11, 12] was applied to islet radii to address the problem of estimating the real radii distribution from profile radii by means of Bach’s eigenfunctions [13, 14].
Islet density was quantified by measuring a random area of pancreas using Image Pro Plus and then counting the number of islets contained within that pancreatic area, the results being expressed as islets per mm2. An islet was defined as a cluster of four or more insulin-positive cells. Similarly, Image Pro Plus was used to quantify the total area of pancreas and then the number of individual scattered insulin-positive cells contained within that area of pancreas was counted; these results were expressed as isolated insulin-positive cells per mm2.
Insulin-positive duct cells were determined using the insulin-stained sections of pancreas (peroxidase) counterstained with haematoxylin. In each autopsy sample, 50 pancreatic ducts were identified and the total number of duct cells in those 50 ducts determined. The number of insulin-positive cells in those ducts was also determined and the result expressed as percentage of the total number of duct cells.
Islet size distribution was determined using the insulin-stained sections of pancreas (peroxidase) counterstained with haematoxylin. The first 100 to 120 islets per individual were grouped according to the number of insulin-positive cells they contained per section, i.e. one to four cells, five to nine cells, 10 to 19 cells, 20 to 49 cells and 50 or more beta cells, and the data expressed as a percentage of islets.
To measure the whole-cell diameter of beta cells, insulin-stained sections of pancreas (peroxidase) counterstained with haematoxylin were used. Five islets per individual selected at random were photographed at 400× magnification on an inverted system microscope (Olympus I×70; Olympus, Melville, NY, USA). These islets were then examined to identify five representative beta cells in each. Selection criteria included a circular shape (similar dimensions in all directions) and the appearance to the observer that the cell had been sectioned through its maximum diameter. For determination of the mean cell diameter, five distances between two adjacent beta cell nuclei (including one of the nuclei) were measured in each of the five islets. The mean individual beta cell area was calculated from the measured mean cell diameter in each autopsy case, assuming a spherical shape of beta cells.
Statistical analysis
Data are presented as means ± SE. Statistical calculations were carried out using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). We sought to address two specific questions a priori in the present studies: first, is there an adaptive increase in beta cell numbers and/or size during pregnancy in humans, and if so to what extent? Second, is any increase in beta cell numbers detected achieved predominantly through replication of existing beta cells or through formation of beta cells from sources independent of beta cell replication (beta cell neogenesis)? To address these questions we used the non-paired Student’s t test with p < 0.05 taken to represent a significant difference. The six women who had died post-partum were identified unexpectedly and included because of the rarity of available data. As a second and separate analysis we sought to establish whether there was a retained adaptive increase in fractional pancreatic beta cell area in the post-partum state. Since this was a separate analysis the t test was also employed for this question, recognising the limitations of using any statistical analysis with just six cases.
Results
Fractional beta cell area
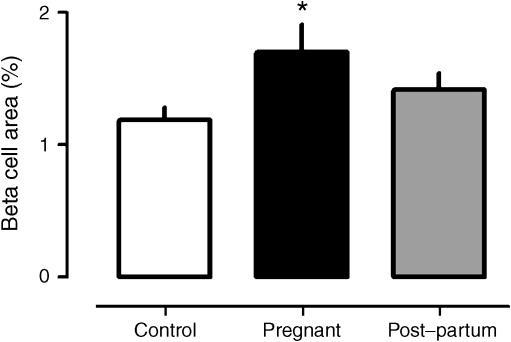
The pancreatic fractional area positive for insulin was increased 1.4-fold in the pregnant compared with control women (1.7 ± 0.2% vs 1.2 ± 0.1 pregnant vs control, p < 0.05) (Fig. 1). The pancreatic fractional beta cell area in the post-partum women was higher than in the controls (1.4 ± 0.1%), but not significantly so. This comparison should also be considered in the context that BMI was higher in the post-partum than in the non-pregnant women.
Fig. 1.
The mean fractional pancreatic beta cell area in non-pregnant controls, and in women who were pregnant or in the post-partum state. During pregnancy the mean fractional beta cell area was 1.4-fold increased (*p < 0.05) compared with the controls
Islet size and density
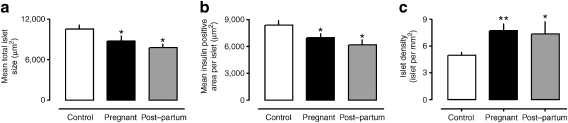
The measured mean islet radius was decreased in pregnancy (45.0 ± 24.6 vs 52.6 ± 28.1 μm, pregnancy vs controls) and remained decreased in the post-partum women (34.4 ± 20.0 μm). The frequency distribution of the profile radii and the probability distributions of islet radii after Wicksell transform are provided in Electronic supplementary material (ESM) Fig. 1. The mean islet cross-sectional area was decreased in pregnancy (8.9 ± 0.8 vs 11.1 ± 0.9 μm2 × 103, p < 0.05, pregnant vs control) (Fig. 2a). There was also a decrease in the mean insulin-positive area per islet in pregnancy (7.0 ± 0.5 vs 8.4 ± 0.5 μm2 × 103, p < 0.05 pregnant vs control) (Fig. 2b). The mean islet cross-sectional area (7.8 ± 0.6 vs 11.1 ± 0.9 μm2 × 103, p < 0.05, post-partum vs control) and insulin area per islet (6.2 ± 0.6 vs 8.4 ± 0.5 μm2 × 103, p < 0.05 post-partum vs control) remained less than that of controls in the post-partum women (Fig. 2a,b).
Fig. 2.
Mean islet size (a), mean area per islet positive for insulin (b) and mean islet density (c) in non-pregnant controls, and in women who were pregnant or in the post-partum state; values determined by evaluating ten prominent islets per woman. During pregnancy and post-partum the mean islet size and the mean insulin area per islet were decreased (*p < 0.05) compared with non-pregnant controls. Islet density (c) was increased (**p < 0.01 pregnant; *p < 0.05 post-partum)
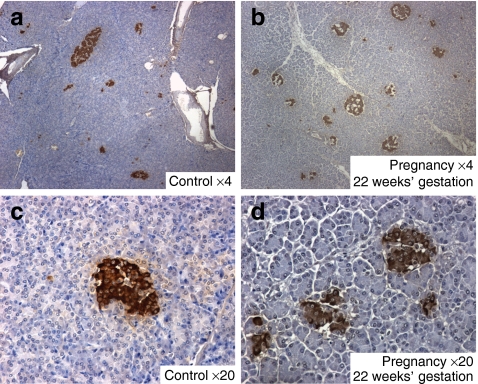
Since the overall fractional area of the pancreas that was positive for insulin was increased, while the mean insulin area per islet was decreased in pregnancy, we anticipated that in pregnancy islet density would be increased. This was confirmed (7.7 ± 0.8 vs 5.1 ± 0.3 islets per mm2, p < 0.01, pregnant vs control) (Fig. 2c). Islet density remained increased in the post-partum women (7.4 ± 1.4 vs 5.1 ± 0.3 islets per mm2, p < 0.05, post-partum vs control) (Fig. 2c). Comparison of pancreas sections from pregnant with those from control women revealed that this increased islet density was composed primarily of islets of comparable or smaller size than those in non-pregnant women (Fig. 3).
Fig. 3.
Sections of pancreas from control women (a, c) and pregnant women at 20 weeks (b) and 22 weeks (d) of gestation. Images were generated at low power (4×) (a, b) and at higher power (20×) (c, d), with samples stained for insulin (brown) and counterstained by haematoxylin. While a range of islet sizes is apparent on cross-section in both cases, the abundance of small islets is increased in the pregnant women
Since the finding that, on average, islets were smaller in human pregnancy was the opposite of that observed in pregnant rodents, we wondered whether some islets increased in size during human pregnancy, but were not identifiable by measurement of mean islet size? To address this, we measured the total islet area and islet fractional area in the largest islet per section per tissue source. The cross-sectional area of the largest islet identified per section was not increased in pregnancy (55.6 ± 6.8 vs 54.8 ± 5.5 μm2 × 103; pregnant vs control, p = NS). Likewise there was no increase in the mean insulin-positive cross-sectional area in the largest islet per section in pregnancy compared with controls (42.0 ± 4.4 vs 38.8 ± 3.6 μm2 × 103, p = NS). In the post-partum women, the total islet cross-sectional area of the largest islet was comparable to controls (60.8 ± 11.1 μm2 × 103, p = NS vs controls), as was the mean insulin-positive cross-sectional area of the largest islet (40.3 ± 5.3 μm2 × 103, p = NS vs controls).
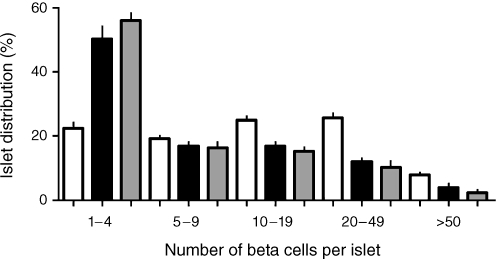
To evaluate more directly the impression that there were more, but smaller islets in pregnancy, we examined the abundance of islets by number of beta cells per islet as seen on cross section. The resulting islet size frequency distribution reveals a shift towards smaller islets in pregnant and post-partum women compared with controls (Fig. 4, ESM Fig. 1), although the majority of beta cells were present in intermediate-sized islets.
Fig. 4.
The frequency distribution of beta cells per islet on section in non-pregnant controls (white bars), and in women who were pregnant (black bars) or in the post-partum state (grey bars). There was a marked shift towards small islets in pregnancy and in the post-partum state compared with the non-pregnant state. This shift is the opposite to that observed in pregnant rodents, in which more abundant large islets have been detected
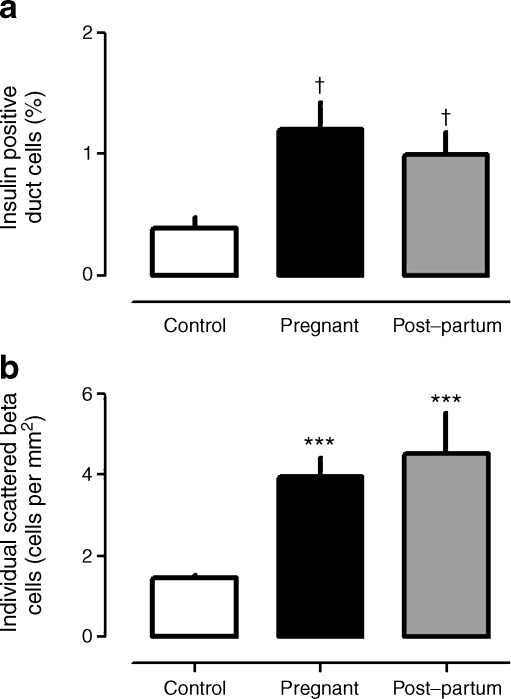
Insulin-positive duct cells and isolated beta cells
The percentage of pancreatic duct cells immunoreactive for insulin was increased in pancreases from pregnant and post-partum women compared with non-pregnant controls (pregnant vs post-partum vs control: 1.2 ± 0.2 vs 1.0 ± 0.2 vs 0.4 ± 0.1%, p < 0.005 pregnant vs control, p < 0.01 post-partum vs control) (Fig. 5a). Likewise, the frequency of isolated insulin-positive cells in the exocrine pancreas was comparably increased in pregnant and post-partum women compared with control (pregnant vs post-partum vs control 4.0 ± 0.5 vs 4.5 ± 1.0 vs 1.5 ± 0.1 individual insulin-positive cells per mm2, p < 0.001 control vs pregnant and post-partum [Fig. 5b]).
Fig. 5.
The mean percentage of pancreatic duct cells positive for insulin (a) and the abundance of single scattered beta cells (b) in pregnant controls, and in women who were pregnant or in the post-partum state. These indices, which have been used previously as indirect measures of beta cell neogenesis, were both increased with pregnancy, remaining so post-partum. ***p < 0.001 vs control; † p < 0.005 vs control
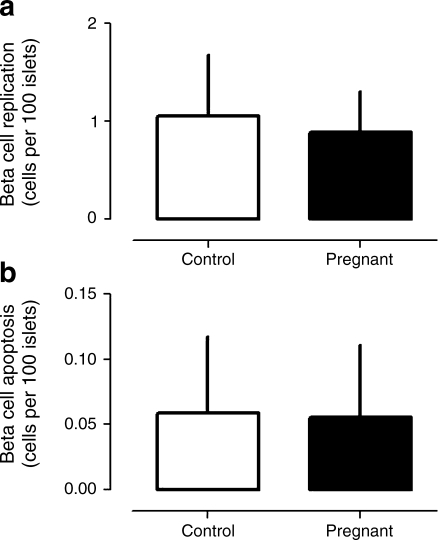
Beta cell replication and apoptosis
By way of positive control, replication detected by Ki67 immunostaining and apoptosis detected by TUNEL were comparably abundant in sections of spleen from control, pregnant and post-partum women (ESM Fig. 2). In contrast, beta cell replication and beta cell apoptosis were rarely detected. The frequency of beta cell replication was not increased in pregnancy (0.89 ± 0.42 vs 0.95 ± 0.59 Ki67-positive beta cells per 100 islets, p = NS pregnant vs control) (Fig. 6a). There was also no change in the frequency of beta cell apoptosis in pregnancy (0.06 ± 0.06 vs 0.05 ± 0.06 TUNEL-positive beta cells per 100 islets, p = NS pregnant vs control) (Fig. 6b). Because of the low frequency of replication and apoptosis, and the relatively small number of women in the post-partum group, the data were insufficient to be meaningful.
Fig. 6.
The mean frequency of beta cell replication by Ki67 (a) and apoptosis by TUNEL (b) in non-pregnant controls and in pregnant women. As previously reported in humans, both indices are infrequent implying relatively slow beta cell turnover. No detectable change in either was observed in pregnancy. Given the low frequency of these measures and the limited pancreatic tissue available per woman, data were insufficient to allow reliable documentation of frequency of these variables in the six post-partum women
Beta cell hyperplasia vs hypertrophy
To establish if the ∼50% increase in pancreatic insulin-positive fractional beta cell area in pregnancy was due to increased beta cell size or number, we compared mean beta cell size by evaluating the mean cross-sectional area per beta cell in each group. Beta cells were not enlarged in pregnancy as judged by mean cross-sectional area of individual beta cells (71.2 ± 4.6 vs 68.2 ± 3.3 μm2, p = NS pregnant vs control). Interestingly, however, beta cells were on average larger in the post-partum period than during pregnancy or in the non-pregnant women (80.4 ± 6.7 vs 68.2 ± 3.3 μm2, p < 0.05 post-partum vs control).
Discussion
The present studies are in agreement with the one previous report on human pancreas obtained at autopsy in pregnant humans, which indicated an adaptive increase in beta cell numbers [10].
Not surprisingly, given the limitations of autopsy studies, the present report and that of Van Assche and colleagues differed somewhat in the extent to which the numbers of beta cells increased in response to pregnancy. While we report an approximately 1.4-fold increase in fractional beta cell area in pregnant vs non-pregnant women, Van Assche and colleagues reported an approximately 2.4-fold increase in beta cell fractional area. They also reported an expansion in islet size and beta cells per islets in pregnancy, which we did not observe. Several differences between the studies may account for this. Van Assche and colleagues measured the fractional endocrine and beta cell area with Ivic’s Victoria blue acid fuchsin staining, whereas we used insulin immunohistochemistry to define beta cells. While we were able to include 18 pregnant, six post-partum and 20 non-pregnant control women, Van Assche and colleagues evaluated five pregnant and five non-pregnant women. It is also possible that underlying diseases leading to death in the individuals included in both studies might have influenced the fractional beta cell area. In the present study, we were also able to examine beta cell replication and apoptosis. In contrast to rodent studies [3–5], we did not find an increase in beta cell replication during pregnancy in humans.
The absence of a documented increase in beta cell replication during pregnancy in humans cannot simply be attributable to use of autopsy tissue, since the spleen from women studied was examined as positive control and abundant specific nuclear Ki67 staining was readily detected in those sections. By the same approach, moreover, we were readily able to detect beta cell replication in infants [15]. In rodents, the increase in beta cell replication during pregnancy occurs during a relatively short period at mid gestation [5]. It is plausible that the same occurs in human pregnancy, but that we had no women of the appropriate gestation period to catch that window. However, if the increase in beta cell numbers in human pregnancy is predominantly achieved by replication of existing beta cells, then the increase in beta cell mass could be expected to be accompanied by an increase in mean islet size and mean number of beta cells per islet, as apparent in rodents during pregnancy [3–5]. In contrast to rodents, we found no increase in mean islet size or in numbers of beta cells per islet; indeed, we found the contrary.
One possible explanation for the smaller increase in beta cell numbers in humans compared with rodents, as well as for the absence of increased beta cell replication in response to pregnancy comes from recent studies showing that beta cell epigenetic changes during ageing markedly decrease the capacity for beta cell replication [16–19]. To date, most rodent studies have been performed at an age that precedes these epigenetic changes. It is as yet unknown to what extent beta cell mass adaptively increases in rodents in response to pregnancy after the epigenetic changes that limit beta cell replication have occurred.
Since the increase in beta cell numbers observed in human pregnancy resulted from an increase in density of islets, it is plausible that the adaptive increase in beta cells in humans is achieved by formation of new islets or islet neogenesis. The notion of islet and/or beta cell neogenesis after birth is controversial. Lineage studies in rodents have been interpreted as showing that replication of existing beta cells is the only potential source of new beta cells [20]. A variety of approaches independent of lineage tracing have been used to support the concept of beta cell formation occurring independently of beta cell duplication [2, 3, 21–25]. We used two of these in the present manuscript, namely determination of (1) the presence and extent of beta cells in exocrine ducts and (2) the abundance of single beta cells scattered in exocrine tissue; in both we detected increases during pregnancy. Recent lineage tracing approaches have also been used to support the concept of beta cell neogenesis in rodents, but these obviously cannot be applied in humans [26, 27].
Gestational diabetes is due to an insufficient adaptive increase in insulin secretion in response to the insulin resistance that occurs in pregnancy [28]. In health, the increased requirement for insulin secretion in pregnancy is approximately twofold [9], exceeding the increment in beta cell numbers observed here. These data imply that in health the adaptive increase in insulin secretion in pregnant humans is achieved in part by an increased work load (insulin synthesis and secretory burden) per beta cell. Since beta cell failure in type 2 diabetes is best predicted by beta cell work load [29] and is characterised by endoplasmic stress [30, 31], it is not surprising that gestational diabetes occurs in those genetically predisposed to type 2 diabetes. Several studies have reported increased detectable C-peptide during pregnancy in patients with type 1 diabetes [32, 33]. This might be due to new beta cell formation in the setting of relative immunosuppression during pregnancy [34] or, possibly, to enhanced beta cell function in residual beta cells with intensified glycaemic control.
In the present studies, the fractional beta cell area increase in pregnancy was established early (Table 1), even though the most prominent levels of insulin resistance in pregnancy do not develop until the third trimester [7, 8]. These findings are intriguing since it has previously been shown that the increment in first- and second-phase insulin release in response to glucose during pregnancy is present early in pregnancy (first trimester), while the insulin resistance that develops in pregnancy occurs later (third trimester) [7]. Taking these findings together, this implies that the signal that provokes the early increase in glucose-induced insulin secretion in human pregnancy may do so at least in part through fostering an increase in beta cell formation. It also implies that the factor(s) driving the expansion of beta cell numbers in human pregnancy are independent of the major decline in insulin sensitivity that occurs in the third trimester. Interestingly, as evaluated in patients with type 1 diabetes, insulin requirements increase early in pregnancy (up to week 9) [35]—an increase that, though not as marked as that occurring from week 18 to term, coincides with the increment in beta cell numbers in humans during pregnancy as observed in the present study. Since mechanisms that signal an expansion of beta cell mass in pregnancy are of interest as a potential means to promote beta cell regeneration in diabetes, it would appear that in humans such signals would best be identified in the first trimester.
Beta cell mass can theoretically increase because of an increase in new cell formation or a decrease in cell death (prolonged beta cell survival). In healthy humans, the frequency of beta cell apoptosis is very low [3] presumably reflecting a relatively long beta cell life span [36]. Thus there is little scope for adaptive changes in beta cell apoptosis to increase beta cell mass during pregnancy. We did not document any decrease in the low frequency of beta cell apoptosis in human pregnancy.
However, we did note a decrease in fractional beta cell area in the few post-partum women studied compared with pregnant women, although this decrease was not completely to the baseline present in controls. Interestingly, the post-partum women included in the present study were more obese than either the pregnant or control women. Since obesity is associated with an increase in beta cell mass in humans [37], the failure of pancreatic fractional beta cell area to return to pre-pregnant levels in our post-partum women may have been due to the insulin resistance/inflammation associated with the obesity. The post-partum women retained the pattern of having more (but small) islets than did non-pregnant women, implying (1) that these putative new islets are not immediately and selectively lost after birth; or (2) that they formed partly in response to obesity. Given the smaller size of the post-partum group, and the low frequency of beta cell replication and apoptosis in human pancreas, we had insufficient numbers to establish a meaningful record of these variables in this group. While we did not observe any difference in mean beta cell size in human pregnancy, beta cells, interestingly, were on average larger in the post-partum period, perhaps reflecting the accumulation of granules with a decreased secretory burden per beta cell, since beta cell numbers did not undergo as rapid a decrease in humans as previously reported in rodents after pregnancy [4].
In summary, we report that there is an adaptive increase in beta cell numbers in human pregnancy, although it is more limited in extent than that in rodents. Also, the pattern of increase differs from that in rodents, with increased numbers of islets in human pregnancy in contrast to an increase in islet size due to the increased beta cell replication observed in rodent pregnancy. Indirect evidence in human pregnancy supports the notion that at least some beta cell formation arises from sources other than beta cell replication (beta cell and/or islet neogenesis). While our studies support the concept that study of enhanced beta cell numbers in pregnancy may help develop strategies to promote beta cell regeneration in diabetes, they suggest that caution should be exercised when assuming that the mechanisms inducing increased beta cell replication during pregnancy in rodents would also effectively induce an expansion of beta cell mass in humans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 39 kb)
(PDF 37 kb)
Acknowledgements
The present studies were supported by funding from the United States Public Health Services National Institute of Health grant (DK059579, DK061539, DK077967) and the Larry Hillblom Foundation (2007-D-003-NET). We gratefully acknowledge R. Basu and C. Nordyke at Mayo Clinic and Medical College for their assistance in procuring the pancreas samples. The authors are grateful to T. Buchanan at USC, and to the reviewers, for valuable suggestions.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviation
- UCLA
University of California, Los Angeles
References
- 1.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/en.130.3.1459. [DOI] [PubMed] [Google Scholar]
- 4.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/en.136.12.5461. [DOI] [PubMed] [Google Scholar]
- 5.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 6.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162:1008–1014. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 9.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jc.86.2.568. [DOI] [PubMed] [Google Scholar]
- 10.Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol. 1978;85:818–820. doi: 10.1111/j.1471-0528.1978.tb15835.x. [DOI] [PubMed] [Google Scholar]
- 11.Wicksell SD. The corpuscle problem. A mathematical study of a biometric problem. Biometrika. 1925;17:84–99. [Google Scholar]
- 12.Wicksell SD. The corpuscle problem II. Biometrika. 1926;18:151–172. [Google Scholar]
- 13.Bach G. Uber die Grossenverteilung von Kugelschnitten in durchsichtigen Schnitten endlicher Dicke. Z Wiss Mikroskopie. 1959;64:265–270. [Google Scholar]
- 14.Keiding N, Jensen ST. Maximum likelihood estimation of the size distribution of liver cell nuclei from the observed distribution in a plane section. Biometrics. 1972;28:813–829. doi: 10.2307/2528765. [DOI] [PubMed] [Google Scholar]
- 15.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta cell proliferation restricts the capacity of beta cell regeneration in mice. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin MM, Kushner JA. Adaptive beta cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009;23:906–911. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 21.Bouwens L, Wang RN, De Blay E, Pipeleers DG, Kloppel G. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes. 1994;43:1279–1283. doi: 10.2337/diabetes.43.11.1279. [DOI] [PubMed] [Google Scholar]
- 22.Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 23.Jetton TL, Everill B, Lausier J, et al. Enhanced beta-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab. 2008;294:E679–E687. doi: 10.1152/ajpendo.00569.2007. [DOI] [PubMed] [Google Scholar]
- 24.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diabetes.44.3.249. [DOI] [PubMed] [Google Scholar]
- 25.Saisho Y, Manesso E, Gurlo T, et al. Development of factors to convert frequency to rate for beta-cell replication and apoptosis quantified by time-lapse video microscopy and immunohistochemistry. Am J Physiol Endocrinol Metab. 2009;296:E89–E96. doi: 10.1152/ajpendo.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, D'Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(Suppl 2):S105–S111. doi: 10.2337/dc07-s201. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 30.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 31.Huang CJ, Lin CY, Haataja L, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 32.Ilic S, Jovanovic L, Wollitzer AO. Is the paradoxical first trimester drop in insulin requirement due to an increase in C-peptide concentration in pregnant type I diabetic women? Diabetologia. 2000;43:1329–1330. doi: 10.1007/s001250051530. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen LR, Rehfeld JF, Pedersen-Bjergaard U, Damm P, Mathiesen ER. Pregnancy-induced rise in serum C-peptide concentrations in women with type 1 diabetes. Diabetes Care. 2009;32:1052–1057. doi: 10.2337/dc08-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia. 2010;53:446–451. doi: 10.1007/s00125-009-1633-z. [DOI] [PubMed] [Google Scholar]
- 36.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 37.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 39 kb)
(PDF 37 kb)