Abstract
Objective:
To identify and compare clinical and neuroimaging predictors of primary lobar intracerebral hemorrhage (ICH) recurrence, assessing their relative contributions to recurrent ICH.
Methods:
Subjects were consecutive survivors of primary ICH drawn from a single-center prospective cohort study. Baseline clinical, imaging, and laboratory data were collected. Survivors were followed prospectively for recurrent ICH and intercurrent aspirin and warfarin use, including duration of exposure. Cox proportional hazards models were used to identify predictors of recurrence stratified by ICH location, with aspirin and warfarin exposures as time-dependent variables adjusting for potential confounders.
Results:
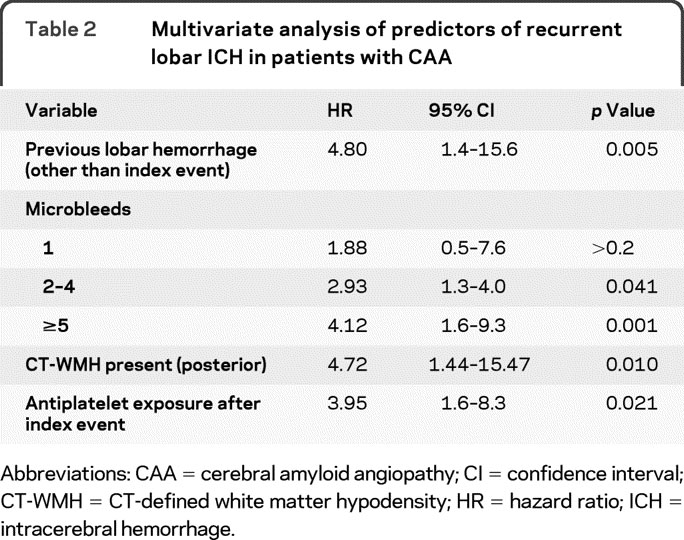
A total of 104 primary lobar ICH survivors were enrolled. Recurrence of lobar ICH was associated with previous ICH before index event (hazard ratio [HR] 7.7, 95% confidence interval [CI] 1.4–15.7), number of lobar microbleeds (HR 2.93 with 2–4 microbleeds present, 95% CI 1.3–4.0; HR = 4.12 when ≥5 microbleeds present, 95% CI 1.6–9.3), and presence of CT-defined white matter hypodensity in the posterior region (HR 4.11, 95% CI 1.01–12.2). Although aspirin after ICH was not associated with lobar ICH recurrence in univariate analyses, in multivariate analyses adjusting for baseline clinical predictors, it independently increased the risk of ICH recurrence (HR 3.95, 95% CI 1.6–8.3, p = 0.021).
Conclusions:
Recurrence of lobar ICH is associated with previous microbleeds or macrobleeds and posterior CT white matter hypodensity, which may be markers of severity for underlying cerebral amyloid angiopathy. Use of an antiplatelet agent following lobar ICH may also increase recurrence risk.
GLOSSARY
- CAA
= cerebral amyloid angiopathy;
- CI
= confidence interval;
- CT-WMH
= CT-defined white matter hypodensity;
- HR
= hazard ratio;
- ICH
= intracerebral hemorrhage;
- VIF
= variance inflation factor.
Intracerebral hemorrhage (ICH), although accounting for only 15% of acute strokes in the United States,1 carries the worst prognosis of all acute cerebrovascular diseases.2,3 Lobar ICH location (selective involvement of the cerebral cortex and underlying white matter) is associated with greater risk for recurrence than deep ICH location4–6 and is associated with different clinical features and risk factors.7,8
Nonfamilial cerebral amyloid angiopathy (CAA), caused by β-amyloid deposition in cerebral arteries and arterioles, is a major cause of lobar ICH but not deep ICH, as shown by autopsy investigations,9 as well as studies linking lobar ICH with several hallmarks of CAA, such as the APOE ε2 and ε4 alleles,5,9,10 asymptomatic microbleeds detected on gradient-echo MRI,11,12 and white matter lesions.13 Based on recent evidence, there is some suggestion that asymptomatic CAA may be highly prevalent in the elderly.14,15
Although several predictors of lobar ICH recurrence have been described, little is known regarding the relative contribution or interaction of each of these predictors. For example, while CAA appears to be a risk factor for warfarin-related ICH,16 it is unknown whether the use of antiplatelet agents or warfarin in patients with CAA increases risk of ICH. Furthermore, as lobar microbleeds are a strong predictor of recurrent ICH in patients with CAA,12 the effect of antiplatelet agents or anticoagulants on hemorrhage risk may depend on microbleed burden. In this study, we sought to identify baseline factors associated with clinically symptomatic lobar ICH recurrence and if antiplatelet agents or anticoagulants influenced recurrence rate.
METHODS
Patient recruitment.
Subjects were drawn from an ongoing longitudinal cohort study of primary lobar ICH as previously described.5 All subjects were recruited among consecutive patients age ≥55 years admitted to the Massachusetts General Hospital from July 1994 to March 2006 with lobar ICH, qualifying for a diagnosis of probable or possible CAA. Lobar ICH was defined as selective involvement of cerebral cortex and underlying white matter. All patients who survived at least 90 days postindex ICH and underwent CT scan were considered eligible for analysis. Subjects were excluded if MRI scans were unavailable for microbleed counting (64 patients); these 64 subjects did not differ from analyzed individuals in their baseline characteristics (all p values >0.10).
We also report results from a similar-sized cohort of deep ICH (defined as ICH involving the basal ganglia, thalamus, or brainstem) for comparison of recurrence rates. This comparison cohort includes 104 survivors of deep ICH, recruited using similar methods over the same time period. The limited number of recurrences in this second cohort prevented us from identifying predictors of deep ICH recurrence.
Clinical data collected and recorded at the time of index presentation included demographic information, history of hypertension, diabetes mellitus, coronary artery disease, previous symptomatic lobar ICH, or ischemic stroke.5
Standard protocol approvals, registrations, and patient consents.
This study was performed with approval of the institutional review board of the Massachusetts General Hospital. All participating subjects or legal guardians provided written informed consent for participation in this study.
CT-defined ICH volume and white matter hypodensity.
All admission CT scans were reviewed by study investigators blinded to clinical and genetic data to confirm ICH location. Volume of ICH was calculated as previously described.17 CT-defined white matter hypodensity (CT-WMH) was defined and assessed as in a prior study.13 In brief, an experienced rater blinded to clinical and genetic data assessed the presence of white matter hypodensity in the anterior and posterior brain regions.18 Our group has previously shown a high interrater reproducibility for this method (weighted κ = 0.89).13
MRI detection of lobar microbleeds.
MRI with axial gradient-echo images (repetition time 750/echo time 50/5-mm to 6-mm slice thickness/1-mm interslice gap) was performed as previously described12,13 using a 1.5-T magnet in lobar ICH survivors with available MRI scans (104 subjects). Cortical (lobar) hemorrhages were classified as microbleeds according to their size (<5 mm in diameter). All MRI analyses were performed and recorded without knowledge of clinical or genetic information. Only MRI scans obtained within 90 days from the index ICH were considered for analysis.
APOE genotype.
DNA was extracted from blood samples and APOE genotype was determined in lobar ICH survivors.5 DNA samples were available from 96 of the 104 lobar ICH survivors. Subjects without DNA samples declined consent for genotyping or were not available for blood draw during their hospitalization.
Follow-up.
Patients and their caregivers were interviewed by telephone at 3 months post-ICH, 6 months post-ICH, and every 6 months thereafter.13 Information collected in the follow-up interviews included medication use, appearance of new neurologic symptoms, recurrent lobar ICH, and death. If new neurologic symptoms, ischemic stroke, ICH, or hospital admission were reported by the subject or caregiver, the relevant medical records and radiographic images were reviewed by a study investigator blinded to other clinical data to assess the presence or absence of recurrent ICH. Events qualifying for censoring of subjects' data included clinically symptomatic ICH confirmed by neuroimaging, death, or follow-up period reaching the predetermined deadline for prospective ascertainment (January 1, 2009).
The use of aspirin, warfarin, or statins was specifically questioned. Dates of initiation and discontinuation for these pharmacologic agents were determined based on subject interview and review of medical records. In 12 subjects, the exact date that aspirin or anticoagulant therapy was instituted or discontinued could not be recalled or determined from the medical records; for these cases, the date was assigned as the midpoint between successive telephone interviews.
Sixteen survivors of lobar ICH were exposed to aspirin at some point during the study period. Indications for antiplatelet therapy were ischemic heart disease (n = 7), atrial fibrillation (n = 3), prior ischemic stroke or TIA (n = 2), artificial heart valve (n = 2), and unknown (n = 2). Two of the 16 subjects were on aspirin at discharge from the index ICH and the remainder were started at a later date (median time from ICH to initiation 7.5 months, interquartile range 4.2–16.2). Median duration of aspirin exposure was 9.3 months (interquartile range 5.1–24.5). Aspirin dosage for exposed subjects ranged from 81 to 325 mg daily. Introduction of aspirin dosage as a covariate did not alter results (data not shown).
Of the 11 subjects in the cohort exposed to warfarin, 9 were on anticoagulation at discharge from index ICH and the remaining 2 started within 30 days of discharge. Median duration of warfarin exposure was 4.9 months (interquartile range 2.6–29.2). Indications for anticoagulation (3 patients had multiple indications) included atrial fibrillation (n = 8), valve replacement surgery (n = 2), pulmonary embolism (n = 1), congestive heart failure (n = 1), cryoglobulinemia (n = 1), and unknown (n = 1).
Statistical methods.
Age at index ICH was analyzed as both a continuous variable and a dichotomous variable categorized by the median age of the cohort (<75 vs ≥75). MRI lobar microbleeds counts were categorized using cutpoints (0, 1, 2–4, or ≥5) chosen to approximately divide the subjects into quartiles. APOE genotype was analyzed as a categorical variable according to the presence or absence of the ε2 or ε4 alleles with the ε3/ε3 genotype serving as a reference.13
Categorical variables were compared using Fisher exact test and continuous variables using the Mann-Whitney rank-sum or unpaired t test as appropriate. We determined univariate predictors of ICH recurrence using the Kaplan-Meier plots with significance testing by the log-rank test. Cox regression analysis was performed to calculate univariate hazard ratio (HR) as a measure of the effect size. For individuals experiencing multiple recurrent lobar ICH during follow-up, data were censored at time of first recurrence.
To determine the influence of aspirin and warfarin on ICH recurrence, we used Cox regression analysis with exposure to these drugs as time-varying covariates. The proportional hazard assumption was tested using graphical checks and Schoenfeld residuals-based tests, while model fit was assessed via Cox-Snell residuals and computing Harrell's C statistic.19 Candidate covariates included all variables showing a trend in association with recurrent lobar ICH in univariate analysis (p < 0.20) and potential predictors of recurrent lobar ICH based on prior studies (APOE genotype, previous symptomatic hemorrhage before index ICH, and number of baseline MRI microbleeds).5,12 Backward elimination of nonsignificant variables (p > 0.05) was subsequently used to generate a minimal model. Potential confounders and predictors were then reincorporated into the resulting minimal model using change-in-effect criteria: all remaining variables were individually added and retained if they improved the overall model fit (as assessed by Harrell's C) by >10%. Multicollinearity was assessed by computing variance inflation factors (VIF) for all predictors and removing all variables with VIF >5.
To assess the presence of confounding by indication, we modeled the influence of all predictors of recurrent lobar ICH on exposure to antiplatelet/warfarin (via a logistic regression analysis) or duration of exposure (via ordinal logistic regression of quartiles of duration of exposure in days, because of the non-normal distribution of data on duration of exposure).
All analyses were performed with R software v 2.8.1 (The R Foundation for Statistical Computing). All significance tests were 2-tailed.
RESULTS
Baseline characteristics of the lobar ICH cohort according to antiplatelet use are detailed in table 1. All patients with lobar ICH fulfilled the diagnosis of probable or possible CAA by the Boston criteria.20 During a median follow-up time of 34.3 months (interquartile range 15.1–57.6, total time at risk 353.2 years), we observed 29 recurrent lobar ICH. Recurrent hemorrhage was more common in survivors of lobar ICH as compared to deep ICH survivors in the comparison cohort (cumulative 2-year recurrence rate 15.7% vs 3.4%, p = 0.011) and was consistent with a previous study.
Table 1 Characteristics of CAA survivors of lobar ICH
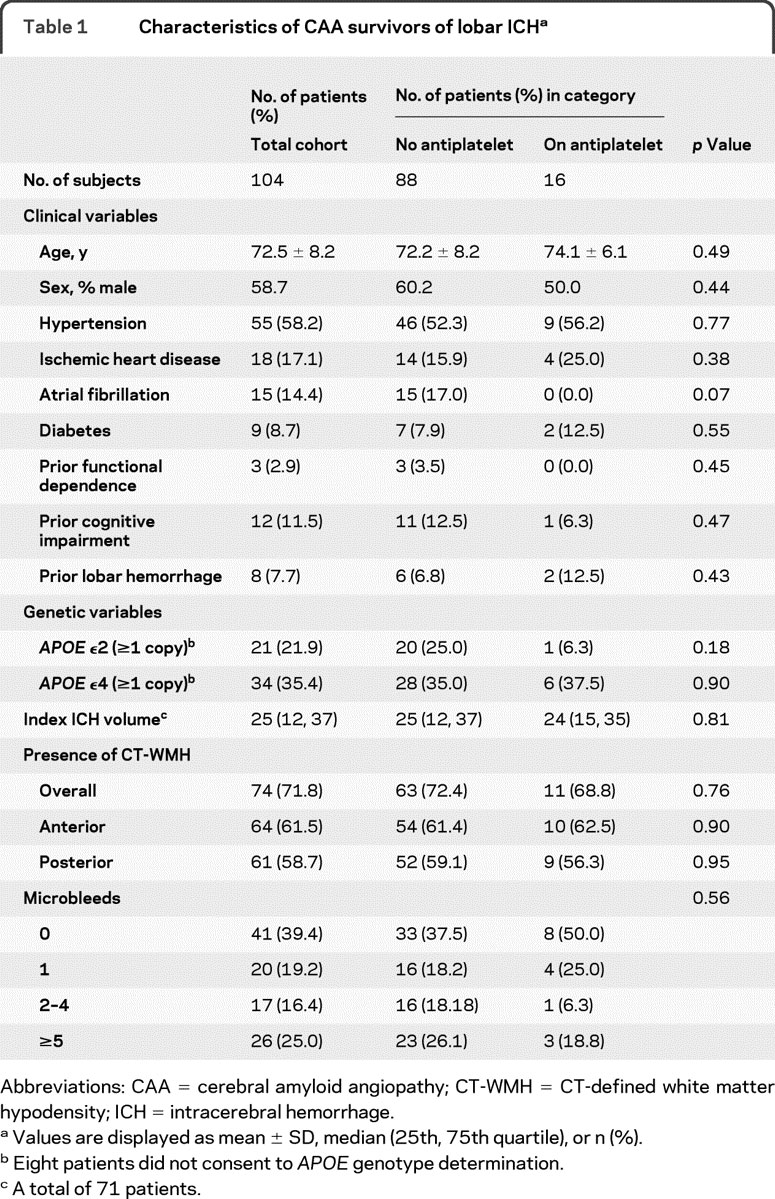
Lobar microbleed counts were associated with both the presence of CT-WMH (p = 0.015) and posterior CT-WMH (p = 0.0001). No association was identified between anterior CT-WMH and number of microbleeds (p = 0.17).
In univariate analysis, significant predictors of lobar ICH recurrence included previous hemorrhage before the index event (HR 9.8; 95% CI 3.31–28.93; p < 0.0001), presence of CT-WMH (HR 3.7; 95% CI 1.27–11.1; p = 0.01), and the presence of posterior CT-WMH (HR 5.7; 95% CI 2.16–15.3; p = 0.001), presence of 2 to 4 MRI-detected microbleeds at baseline (HR 3.27 compared to 0 microbleeds; 95% CI 1.14–1.39; p = 0.028), or presence of ≥5 microbleeds at baseline (HR 5.25 compared to 0 microbleeds; 95% CI 2.00–13.8; p = 0.001).
The use of aspirin after ICH was not associated with lobar ICH recurrence in univariate analyses (HR 1.72; p = 0.2). There was, however, a nonsignificant trend for patients with higher numbers of microbleeds to be less likely to receive aspirin (table 1). No baseline variable was associated with exposure to antithrombotic therapy (p < 0.05).
A multivariable Cox regression model was constructed to include all variables with a univariate p value <0.2 or previously suspected to influence the risk of hemorrhage recurrence (APOE genotype, warfarin exposure, statin exposure). The model demonstrated that previous ICH, posterior CT-WMH, the presence of ≥2 microbleeds, and antiplatelet use are all independently associated with increased risk of recurrent lobar ICH (figure). No effect was evident for warfarin or statin exposure. The final multivariate model, including only significant predictors of lobar ICH recurrence, is detailed in table 2.
Figure Aspirin and recurrent lobar intracerebral hemorrhage
Modified Kaplan-Meier plot of the effect of aspirin use on recurrent lobar intracerebral hemorrhage in patients with lobar intracerebral hemorrhage, adjusting for baseline clinical and imaging characteristics. Because antiplatelet use varied over time, the graphic display of the antiplatelet stratum does not include follow-up time during which the individual was not exposed to antiplatelet. AP = acetylsalicylic acid/antiplatelet intercurrent use.
Table 2 Multivariate analysis of predictors of recurrent lobar ICH in patients with CAA
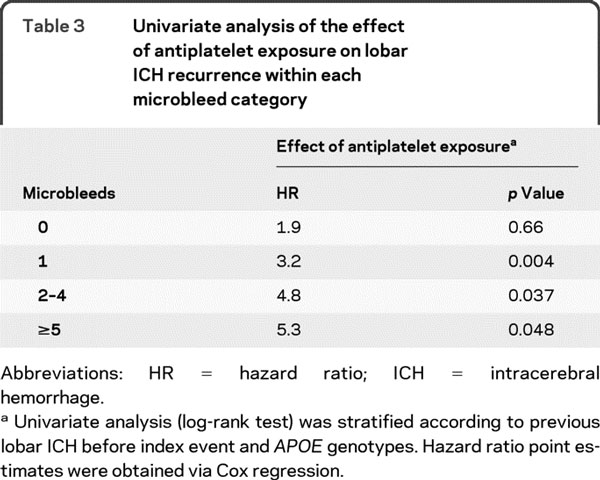
To explore whether the risk of aspirin varied according to the number of baseline microbleeds, the antithrombotic drug-associated HR for recurrence in each microbleed category was determined (table 3). There was an aspirin-associated increase in HR for lobar ICH recurrence in those with 0, 1, 2–4, or ≥5 microbleeds (table 3).
Table 3 Univariate analysis of the effect of antiplatelet exposure on lobar ICH recurrence within each microbleed category
DISCUSSION
The major finding from this prospective cohort study is that lobar ICH is associated with a high rate of recurrent hemorrhage and that severity of white matter disease and cerebral microbleeds independently increase this risk. The use of antithrombotic agents in CAA after lobar ICH may also increase this risk when the analysis is controlled for baseline microbleed count.
Although the association between aspirin exposure, microbleeds, and symptomatic ICH was previously reported in case-control studies,21,22 prospective data on the influence of aspirin on lobar ICH recurrence have been lacking. This analysis of prospectively followed patients with CAA associates aspirin use with recurrent hemorrhage when adjusting for baseline predictors of recurrent ICH.
Several mechanisms might be implicated in the effect of aspirin exposure in patients with CAA. Recently published data on a cross-sectional population study of microbleeds prevalence suggest that exposure to aspirin may be associated with increased prevalence of lobar microbleeds, one of the hallmarks of CAA.23 Antiplatelet agents might therefore increase the risk of recurrent ICH by substantially increasing the number of microbleeds at risk for conversion into clinically manifest macrobleeds. Antiplatelet therapy might also act on preexisting and new spontaneous microbleeds by increasing the risk of evolution into clinically symptomatic ICH.
Although our analysis was not adequately powered to explore this mechanism in regard to warfarin, anticoagulation therapy might also influence ICH recurrence based on microbleed burden. Limited evidence supporting this hypothesis has been recently provided in a small case-control series.24 Additional studies with larger numbers of ICH survivors taking warfarin will be needed to better determine the risk of anticoagulation in ICH survivors.
We identified the presence of CT-WMH in posterior brain regions as a predictor of lobar ICH recurrence. Although previous evidence suggests that the presence of CT-WMH correlates with lobar ICH recurrence,13 in that study we did not examine the location of CT-WMH. Here we extend those results by showing that the location as well as extent of white matter damage might be relevant in predicting ICH recurrence in CAA. These results contrast somewhat with a voxel-wise MRI-based analysis of white matter lesions, which found no major differences in location between patients with CAA, AD, and normal aging.25
Posterior CT-WMH may be a stronger marker of underlying CAA than overall WMH burden. Consistent with this hypothesis, the correlation of number of microbleeds with posterior CT-WMH was stronger than with overall CT-WMH. Microbleeds in patients with CAA have been shown to have a posterior predominance.26 Further studies are required to determine the relationship between CT-WMH and MRI-based measures to better determine whether white matter damage may also have a posterior predominance in patients with CAA.
We did not identify APOE alleles ε2 and ε4 as risk factors for lobar ICH recurrence, but we observed mild multicollinearity with other predictors in our Cox model. This can be explained by the association of APOE alleles with presence of lobar microbleeds14,15 and white matter disease.27 The extremely high predictive power of these imaging variables is likely to obscure the relatively weaker effects of correlated APOE alleles.
Our results have potential implications for patients with CAA at high risk for ischemic cardiovascular and cerebrovascular diseases. Previous studies have suggested that antiplatelet therapy might be used in selected ICH survivors without a substantial increase in the risk of rebleeding.6,28 These studies, however, were not limited to patients with CAA and complete data regarding the cerebral microbleeds were not available. Our results suggest that there may be an increased risk of recurrent ICH in patients with CAA treated with aspirin. However, baseline predictors of ICH recurrence (microbleeds in particular) likely play an important role in the overall risk of hemorrhage recurrence in these patients. As data continue to accumulate on predicting future risk of ICH, clinicians should cautiously weigh the risks and benefits of antiplatelet treatment in individuals diagnosed with CAA.
This study has limitations. Although we were able to develop a powerful predictive model for lobar ICH recurrence (Harrell's C = 0.84 for lobar ICH), predictive performance evaluation in a separate cohort is required. In this study, all subjects were exposed to aspirin as the antiplatelet agent. The effect of nonaspirin antiplatelet agents or the combination of such agents cannot be assessed using these results. Although our results could suggest that the risk of recurrent ICH with aspirin use in CAA is moderated by microbleed burden, we had insufficient statistical power to answer this question definitively by testing for an interaction. Patients with CAA who did not undergo MRI were excluded from the current study. However, as we did not find any difference in baseline characteristics between subjects in the study and excluded subjects, it is unlikely to introduce significant bias. Finally, aspirin use was not randomly assigned, but rather resulted from each individual patient and physician decisions. Indeed, the effect of antiplatelet therapy on ICH recurrence became apparent only when controlling for some of the baseline factors that may have influenced this decision.
DISCLOSURE
Dr. Biffi has received research support from the American Heart Association-Bugher Foundation. A. Halpin, Dr. Towfighi, A. Gilson, and Dr. Busl report no disclosures. Dr. Rost has received research support from the NIH/NINDS (1K23NS064052-01A1 [PI]), the National Stroke Association, and the American Stroke Association-Bugher Foundation. Dr. Smith has received speaker honoraria from the Canadian Consortium on Dementia; has served on speakers' bureaus for QuantiaMD and BMJ Best Practice; and has received/receives research support from the NIH (5R01NS062028-03 [co-PI], NIH 5K23NS046327-05 [PI]), the Canadian Institutes for Health Research, the Canadian Stroke Network, and the Hotchkiss Brain. Dr. Greenberg serves on scientific advisory boards for Roche and Alzheimer's Immunotherapy; serves on the editorial boards of Neurology®, Stroke, Cerebrovascular Disease, and the Journal of Alzheimer's Disease and Other Dementias; has received speaker honoraria from Merck Serono, Esteve, and Medtronics, Inc.; and has received/receives research support from the NIH (R01AG026484 [PI], K24NS056207 [PI], R01AG021084 [coinvestigator], R01NS042147 [PI], U54NS057405, 2007–2012 [coinvestigator]), and the Alzheimer's Association. Dr. Rosand serves on the editorial board of Stroke and receives research support from the NIH (5R01NS059727-02 [PI], 3RO1NS059727-01A1S1 [PI]) and the American Heart Association. Dr. Viswanathan receives research support from the NIH/NIA (2P50AG005134-268382 [coinvestigator]).
Address correspondence and reprint requests to Dr. Anand Viswanathan, Hemorrhagic Stroke Research Program, Massachusetts General Hospital Stroke Research Center, Harvard Medical School, 175 Cambridge Street, Suite 300, Boston, MA 02114 aviswanathan1@partners.org
Study funding: Supported by NIH 5K23NS046327-05 and 5R01AG026484-04 (Massachusetts General Hospital).
Disclosure: Author disclosures are provided at the end of the article.
Received September 2, 2009. Accepted in final form May 11, 2010.
REFERENCES
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007;38:2001–2023. [DOI] [PubMed] [Google Scholar]
- 3.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med 2000;342:240–245. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66:206–209. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan A, Greenberg SM. Intracerebral hemorrhage. In: Handbook of Clinical Neurology 2008;93:767–790. [DOI] [PubMed]
- 8.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke 2002;33:1190–1195. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke 1996;27:1333–1337. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon 2 and vasculopathy in cerebral amyloid angiopathy. Neurology 1998;50:961–965. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology 1996;46:1751–1754. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 13.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004;63:1606–1612. [DOI] [PubMed] [Google Scholar]
- 14.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 15.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosand J, Hylek EM, O'Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology 2000;55:947–951. [DOI] [PubMed] [Google Scholar]
- 17.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 18.van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatry 1990;53:1080–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Malden, MA: Wiley-Interscience; 2008. [Google Scholar]
- 20.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 21.Wong KS, Mok V, Lam WW, et al. Aspirin-associated intracerebral hemorrhage: clinical and radiologic features. Neurology 2000;54:2298–2301. [DOI] [PubMed] [Google Scholar]
- 22.Wong KS, Chan YL, Liu JY, Gao S, Lam WW. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003;60:511–513. [DOI] [PubMed] [Google Scholar]
- 23.Vernooij MW, Haag MD, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol 2009;66:714–720. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology 2009;72:171–176. [DOI] [PubMed] [Google Scholar]
- 25.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 2008;39:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol 2005;58:459–462. [DOI] [PubMed] [Google Scholar]
- 27.Lunetta KL, Erlich PM, Cuenco KT, et al. Heritability of magnetic resonance imaging (MRI) traits in Alzheimer disease cases and their siblings in the MIRAGE study. Alzheimer Dis Assoc Disord 2007;21:85–91. [DOI] [PubMed] [Google Scholar]
- 28.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716. [DOI] [PubMed] [Google Scholar]



