Abstract
Background:
Class I evidence for surgical effectiveness in refractory temporal lobe epilepsy (TLE) in 2001 led to an American Academy of Neurology practice parameter in 2003 recommending “referral to a surgical epilepsy center on failing appropriate trials of first-line antiepileptic drugs.” We examined whether this led to a change in referral patterns to our epilepsy center.
Methods:
We compared referral data for patients with TLE at our center for 1995 to 1998 (group 1, n = 83) and 2005 to 2008 (group 2, n = 102) to determine whether these recommendations resulted in a change in referral patterns for surgical evaluation. Patients with brain tumors, previous epilepsy surgery evaluations, or brain surgery (including epilepsy surgery) were excluded.
Results:
We did not find a difference between the groups in the duration from the diagnosis of habitual seizures to referral (17.1 ± 10.0 vs 18.6 ± 12.6 years, p = 0.39) or the age at the time of evaluation (34.1 ± 10.3 vs 37.0 ± 11.8 years, p = 0.08). However, there was a difference in the distributions of age at evaluation (p = 0.03) and the duration of pharmacotherapy (p = 0.03) between the groups, with a greater proportion of patients in group 2 with drug-resistant epilepsy both earlier and later in their treatment course. Nonepileptic seizures were referred significantly earlier than TLE in either group or when combined.
Conclusions:
Our analysis does not identify a significantly earlier referral for epilepsy surgery evaluation as recommended in the practice parameter, but suggests a hopeful trend in this direction.
GLOSSARY
- AAN
= American Academy of Neurology;
- AED
= antiepileptic drug;
- ERSET
= Early Randomized Surgical Epilepsy Trial;
- NES
= nonepileptic seizures;
- RCT
= randomized controlled trial;
- TLE
= temporal lobe epilepsy;
- VNS
= vagus nerve stimulator.

Patient Page
An estimated 20% to 40% of patients with epilepsy have drug-resistant disease,1,2 and surgical resection of an identified epileptogenic region best abolishes disabling seizures.3,4 This is important because drug-resistant epilepsy accounts for 80% of the cost of epilepsy in the United States.5 Surgical treatment for this condition has been underutilized.6 In the past, this was thought to be mostly due to lack of Class I evidence favoring surgery6 and absence of widely publicized guidelines by national bodies of referring neurologists.7 A randomized controlled trial (RCT) in 2001 demonstrated a 64% chance of seizure freedom with surgery in medically refractory temporal lobe epilepsy (TLE) compared with 8% with medical management, demonstrating the superiority of surgery over continued medical therapy.3 Based on this Class I evidence and a literature review, a practice parameter by the American Academy of Neurology (AAN) in 2003, in association with the American Epilepsy Society and the American Association of Neurological Surgeons, recommended that patients with TLE “who have failed appropriate trials of first-line antiepileptic drugs should be considered for referral to an epilepsy surgery center.”4 We examined whether these developments were associated with a change in the timing of referrals to our surgical epilepsy center. We compared referral patterns to our center for epochs 10 years apart, straddling the evidence and recommendation developments.
METHODS
Data were retrospectively collected and screened in patients admitted for video EEG monitoring to the UCLA Center for the Health Sciences from January 1995 to September 1998 and from January 2005 to September 2008. The data regarding duration of seizures were gathered from the records by an individual researcher to eliminate interobserver variation. Outcome categories based on diagnosis at discharge were divided into nonepileptic seizures (NES), TLE, and other epilepsy syndromes.
Patients included were those older than 16 years admitted for a surgical evaluation and thought to have TLE at discharge based on ictal EEG. Patients diagnosed as TLE were excluded if they had a prior epilepsy surgery evaluation, history of malignant brain tumor, previous brain surgery (including epilepsy surgery), recent onset (less than 6 months) of symptoms, or incomplete data on duration of disease. Patients with duration of seizures less than 6 months were usually self-referred for a second opinion, and none were referred as potential surgical candidates. Patients with TLE who were referred for epilepsy surgery and were found to have low-grade gliomas, cavernous angiomas, and cortical dysplasias were not excluded.
The age at intervention was taken as the time at which the habitual seizure leading to the surgery evaluation was deemed by a physician as requiring treatment. For example, a patient with staring spells since childhood and generalized seizures since age 15 years would be considered as having an age at intervention of 15 years if the staring spells were not identified as seizures until the generalized seizures developed. In those patients who had a single seizure followed by a prolonged seizure-free period before the development of regular seizures, the age at onset for the recurring seizures was taken as the time requiring treatment. This was done to measure consistently the time taken from definitive diagnosis of the habitual seizures and institution of pharmacotherapy to the time of presurgical evaluation. This is also relevant when thinking of health care costs as seizures not recognized as such do not add to increased direct health care costs. The absence of clear criteria makes it difficult to identify the onset of drug resistance retrospectively. The duration of pharmacotherapy was the time from the age at intervention to the age at evaluation. Collected data were analyzed using PASW 17 (SPSS Inc., Chicago, IL).
Standard protocol approvals.
The study was approved and the need for a written informed consent from all patients was waived by UCLA's Institutional Review Board.
RESULTS
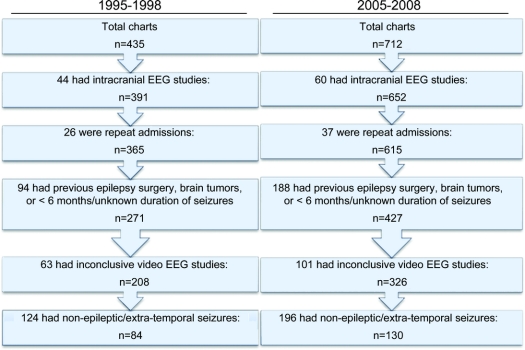
A total of 997 patients were screened for the study, including 435 patients from 1995 to 1998 (group 1), and 562 patients from 2005 to 2008 (group 2). After applying the inclusion and exclusion criteria, primary analyses were performed using 83 patients (37 males) with TLE from 1995 to 1998 and 102 patients (50 males) from 2004 to 2008 (figure 1). There was no difference in the overall age of diagnosis, duration of pharmacotherapy, or age at evaluation between 1995 and 1998 and 2005 to 2008 (table). There also was no difference in these measures for other seizure types or for patients with inconclusive results.
Figure 1 Flowchart detailing selection of patients with temporal lobe epilepsy from initial chart review
Table Age at onset of regular habitual seizures, years of seizure, and age at evaluation for temporal lobe epilepsy compared between groups 1 and 2
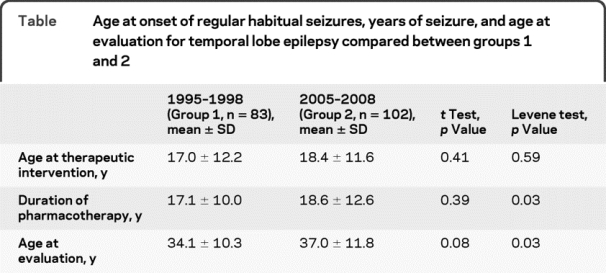
Although there was no significant difference in the mean duration of pharmacotherapy and age at referral between groups 1 and 2, the distribution of values was different (p = 0.03, Levene test for equality of variance; table). The histograms depicting the distribution of duration of pharmacotherapy for the 2 groups (figure 2) demonstrate a greater number of patients in the higher and lower percentiles in group 2 compared with group 1. These 2 extremes counterbalanced each other resulting in net measures of central tendency to appear similar in the statistical analyses.
Figure 2 Percentage of patients (x-axis) with different durations of drug therapy (in years, y-axis) for groups 1 and 2 during the 2 epochs (1995–1998 and 2005–2008)
The duration between diagnosis and referral was shorter in NES for both time periods compared with TLE (t test, p < 0.0001). In group 1, the duration in years was 6.8 ± 8.5 for NES (n = 47) and 17.1 ± 10 for TLE (n = 83), whereas in group 2, it was 7.2 ± 7.9 for NES (n = 67) and 18.6 ± 12.6 for TLE (n = 102).
DISCUSSION
Drug-resistant TLE is the classic surgically remediable epilepsy syndrome.8 However, only a small percentage of potential surgical candidates are being referred to surgical epilepsy centers.9 Furthermore, among patients with drug-resistant epilepsy referred to surgical centers, there is a significant delay of 18 to 23 years between the onset of habitual seizures and surgical referral or surgery, which seems to be consistent over time.3,10–14 The optimal timing for surgery in drug-resistant TLE is unclear,6 but earlier surgery is potentially important to avoid irreversible adverse consequences of epilepsy. In the past, the underutilization of surgery for TLE in the United States was thought to be due to lack of Class I evidence and national recommendations.4,7,15
We compared patient groups with TLE referred to our center before (group 1) and after (group 2) such Class I evidence3 and national recommendations4 were published. We observed that the overall duration of pharmacotherapy was not significantly different between these patient groups (17.1 vs 18.6 years). Our measure of the duration of pharmacotherapy, as calculated from the onset of definitive diagnosis with intervention to the initial presurgical evaluation, is less than the duration of disease in previous surgical series of TLE, which were measured from the time of the first seizure to the surgery and were reported to be between 18 and 23 years.3,10–14 A large multicenter study among patients with TLE who underwent surgery reported the latency from the time of the first seizure to the time of surgical evaluation to be 22 years.16 Other studies have measured the latency from the first habitual seizure to the initial visit at the epilepsy center15 or to the time of inpatient evaluation17 to be approximately 18 years. We measured the duration of pharmacotherapy rather than the duration of disease because it more directly reflects the time for the referring physicians to consider surgery rather than further drug trials. Given the nonuniform course of TLE, the most appropriate measure for delay before surgery would be the duration since resistance to medical treatment,18 but this could not be calculated from our retrospective data. The duration from the time of failure of the second antiepileptic drug (AED) to surgery has previously been measured to be approximately 12 to 14 years.19
Although the overall mean age at evaluation and the duration of pharmacotherapy was not significantly different between the groups, their distribution was significantly different. This was due to a larger proportion of patients in group 2 being referred earlier and later in the course of their treatment compared with group 1 (figure 2). The presence of patients with delay greater than 40 years in group 2, but not group 1, perhaps related to a recent tendency to operate on older patients20 or a greater awareness among older patients of a surgical option, likely counterbalanced the effect of some earlier referrals resulting in no net difference in the overall results between the groups. The number of early referrals, however, is still small, and it is worth noting that this increase was potentially influenced by the fact that UCLA was actively recruiting patients with TLE who had recently developed drug resistance for the Early Randomized Surgical Epilepsy Trial (ERSET)21 up until June 2005. Nevertheless, this finding gives hope that this positive change may have resulted from an increased awareness among referring physicians or the patients themselves about the benefits of early surgical treatment for TLE as a consequence of the national recommendations. Another study looking at the duration of TLE from the onset of nonfebrile seizures to surgery between 1996 to 2007 also failed to detect an overall difference in duration, but stratified analysis of durations between time periods was not similarly analyzed.14
That the mean duration of pharmacotherapy is still more than 17 years at the point of surgical referral suggests that despite the Class I evidence and the practice parameter, there is a persistent lack of understanding on the part of referring physicians regarding the safety and efficacy of epilepsy surgery and the referral criteria for early surgery in TLE.22 Data from the National Association of Epilepsy Centers (Robert J. Gumnit, MD, President, written communication, November 2, 2008) indicate the rate of referral remains unchanged since the publication of the RCT3 and a practice parameter.4 In a survey published in 2008, a third of general neurologists who refer patients for epilepsy surgery evaluation believed that there were “serious complications” from epilepsy surgery22 despite publications reporting low rates of permanent neurologic deficits (3%) and cognitive deficits (6%, half of which resolved in 2 months).4 These complications are well below the morbidity associated with continued seizures.23
Early surgery helps to avoid the adverse consequences of persisting seizures. Continuing seizures are associated with increased risk of mortality,23 physical injuries,24 cognitive dysfunction,23 and lower quality of life.25 Improved self-reported quality of life has consistently been associated with improved postsurgical seizure control,3,26 and vocational and social rehabilitation is more difficult after a patient has settled into a disabled lifestyle.27 There is also evidence to suggest that at least some forms of TLE are progressive and that outcome with respect to seizures is better when surgical intervention is early.28
It is likely that the development and marketing of several new AEDs, as well as the vagus nerve stimulator (VNS), in recent years has engaged more attention among practicing physicians compared with surgery, which has no comparable marketing program, leading to more prolonged drug or device trials before considering surgery. Our interesting finding that the duration of pharmacotherapy at the time of evaluation is significantly lower for NES than for TLE suggests that primary care physicians and general neurologists are more likely to look to tertiary referral centers for help with diagnosis than with treatment. The duration of pharmacotherapy before referral in NES of 7.0 (± 8.1) years in our patients is consistent with previous reports.29,30
A major reason for the delay in surgical referrals is undoubtedly the ambiguity in defining drug resistance in epilepsy.31 Most epileptologists currently define drug resistance as inefficacy of 2 AEDs, and this view has been supported by a recent Commission Report of the International League Against Epilepsy.32 However, a majority of neurologists surveyed in the past year defined medically refractory epilepsy as the failure of 3 monotherapy and 2 polytherapy AED trials.22 A significant percentage believed that all approved AEDs should fail (19%) or VNS failure should occur (15%) before declaring a patient medication refractory.22 To compound the ambiguity, recent literature suggests that as many as 15% to 20% of patients whose seizures do not respond to 2 AEDs will become seizure free with further trials,33,34 although conditions commonly considered for surgery in TLE, including hippocampal sclerosis, are less likely to respond to further medications.34 Management of the heterogeneous etiologies constituting drug-resistant TLE necessitates substantial knowledge of the underlying factors and familiarity with the individual AEDs, and medication trials beyond 2 or 3 AEDs are ideally performed by an epilepsy subspecialist.33 Primary care physicians and general neurologists should optimally refer all patients with persistent seizures that impair school, work, or interpersonal relationships, after appropriate trials with a few AEDs, to an epilepsy center for additional evaluation31 and therapeutic considerations including, but not limited to, surgery.
This study should be interpreted considering the limitation of the data being derived from a single surgical epilepsy center with several fixed referral sources in the community. Attempting to examine changes within 5 years of the AAN practice parameter may have precluded statistical detection of longer term trends in evolution. A multi-center examination with more years of data may be helpful in better delineating the study findings. Such studies comparing recent to historical data will be essentially constrained by the limitations of being retrospective in nature.
Epilepsy surgery provides the best outcome in drug-resistant TLE, and early surgery is recommended to avoid irreversible disability.4 Although our data suggest a trend toward earlier referral for TLE surgery at our center since the publication of the AAN practice parameter,4 an average delay of approximately 18 years from initial therapeutic intervention to surgical evaluation remains unacceptably long. Treating patients with epilepsy who continue to have disabling seizures impacting physical, psychological, or social health after adequate trials of 2 or 3 AEDs is complex and challenging. Such patients stand to gain substantial benefit from referral to the specialized services provided by epilepsy centers.31
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Zulfi Haneef, MD.
DISCLOSURE
Dr. Haneef receives research support from the Epilepsy Foundation. Dr. Stern serves as an Associate Editor for MedLink Neurology; receives royalties from the publication of Atlas of EEG Patterns (Lippincott Williams & Wilkins, 2008–2010); has received speaker honoraria from UCB and Lundbeck, Inc.; has serves as a consultant for Sepracor Inc., UCB, and Cyberonics, Inc.; and receives research support from the NIH (NINDS K23, NS044936 [PI]) and from the Fidelity Charitable Trust. Ms. Dewar serves on the Professional Advisory Board of the Epilepsy Foundation of America. Dr. Engel has received funding for travel or speaker honoraria from Eisai Inc., UCB, Johnson & Johnson, Novartis, Best Doctors, and S&S HealthCare Strategies; serves on the editorial boards of Epilepsia, Epileptic Disorders, Brain Stimulation, Experimental Neurology, and WFN Seminars in Clinical Neurology, and as an Associate Editor of MedLink; serves as a consultant for Acorda Therapeutics Inc.; holds patents re: Magnetonanoparticle for MRI for which he has received royalty and license fee payments from Epinano, Inc.; estimates that 20% of his clinical effort is spent on presurgical evaluation for epilepsy; and receives research support from the NIH (NINDS P01 NS002808 [PI] and NINDS R37 NS033310 [PI]), the Epilepsy Foundation, and from private donors.
Address correspondence and reprint requests to Dr. Jerome Engel, David Geffen School of Medicine at UCLA, 710 Westwood Plaza, Los Angeles, CA 90095-1769 engel@ucla.edu
Editorial, page 678
Disclosure: Author disclosures are provided at the end of the article.
Received November 25, 2009. Accepted in final form March 22, 2010.
REFERENCES
- 1.Sander J. Some aspects of prognosis in the epilepsies: a review. Epilepsia 1993;34:1007–1016. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 4.Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy—report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–547. [DOI] [PubMed] [Google Scholar]
- 5.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 6.Engel J Jr. The timing of surgical intervention for mesial temporal lobe epilepsy: a plan for a randomized clinical trial. Arch Neurol 1999;56:1338–1341. [DOI] [PubMed] [Google Scholar]
- 7.NIHC. National Institutes of Health Consensus Conference: surgery for epilepsy. JAMA 1990;264:729–733. [PubMed] [Google Scholar]
- 8.Engel J Jr. Surgery for seizures. N Engl J Med 1996;334:647–653. [DOI] [PubMed] [Google Scholar]
- 9.Engel J Jr, Shewmon DA. Overview: who should be considered a surgical candidate? In: Engel J Jr Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993. [Google Scholar]
- 10.Cascino GD, Trenerry MR, So EL, et al. Routine EEG and temporal lobe epilepsy: relation to long-term EEG monitoring, quantitative MRI, and operative outcome. Epilepsia 1996;37:651–656. [DOI] [PubMed] [Google Scholar]
- 11.Sperling MR, O'Connor MJ, Saykin AJ, Plummer C. Temporal lobectomy for refractory epilepsy. JAMA 1996;276:470–475. [PubMed] [Google Scholar]
- 12.Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia 2002;43:170–174. [DOI] [PubMed] [Google Scholar]
- 13.Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol 1996;40:446–450. [DOI] [PubMed] [Google Scholar]
- 14.Choi H, Carlino R, Heiman G, Hauser WA, Gilliam FG. Evaluation of duration of epilepsy prior to temporal lobe epilepsy surgery during the past two decades. Epilepsy Res 2009;86:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benbadis SR, Heriaud L, Tatum WI, Vale FL. Epilepsy surgery, delays and referral patterns: are all your epilepsy patients controlled? Seizure 2003;12:167–170. [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Langfitt J, Shinnar S, et al. How long does it take for partial epilepsy to become intractable? Neurology 2003;60:186–190. [DOI] [PubMed] [Google Scholar]
- 17.Haut SR, Shinnar S, Moshe SL. Seizure clustering: risks and outcomes. Epilepsia 2005;46:146–149. [DOI] [PubMed] [Google Scholar]
- 18.Berg AT. Understanding the delay before epilepsy surgery: who develops intractable focal epilepsy and when? CNS Spectr 2004;9:136. [DOI] [PubMed] [Google Scholar]
- 19.Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology 2007;68:1290–1298. [DOI] [PubMed] [Google Scholar]
- 20.Grivas A, Schramm J, Kral T, et al. Surgical treatment for refractory temporal lobe epilepsy in the elderly: seizure outcome and neuropsychological sequels compared with a younger cohort. Epilepsia 2006;47:1364–1372. [DOI] [PubMed] [Google Scholar]
- 21.Engel J Jr. The goal of epilepsy therapy: no seizures, no side effects, as soon as possible. CNS Spectr 2004;9:95–97. [DOI] [PubMed] [Google Scholar]
- 22.Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists' views on epilepsy surgery and medically refractory epilepsy. Epilepsy Behav 2008;13:96–101. [DOI] [PubMed] [Google Scholar]
- 23.Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr 2004;9:106–109. [DOI] [PubMed] [Google Scholar]
- 24.Buck D, Baker GA, Jacoby A, Smith DF, Chadwick DW. Patients' experiences of injury as a result of epilepsy. Epilepsia 1997;38:439–444. [DOI] [PubMed] [Google Scholar]
- 25.Kellett MW, Smith DF, Baker GA, Chadwick DW. Quality of life after epilepsy surgery. J Neurol Neurosurg Psychiatry 1997;63:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLachlan RS, Rose KJ, Derry PA, Bonnar C, Blume WT, Girvin JP. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol 1997;41:482–489. [DOI] [PubMed] [Google Scholar]
- 27.Langfitt JT, Wiebe S. Early surgical treatment for epilepsy. Curr Opin Neurol 2008;21:179. [DOI] [PubMed] [Google Scholar]
- 28.Jeong SW, Lee SK, Kim KK, Kim H, Kim JY, Chung CK. Prognostic factors in anterior temporal lobe resections for mesial temporal lobe epilepsy: multivariate analysis. Epilepsia 1999;40:1735–1739. [DOI] [PubMed] [Google Scholar]
- 29.Reuber M, Fernandez G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58:493–495. [DOI] [PubMed] [Google Scholar]
- 30.Kuyk J, Siffels MC, Bakvis P, Swinkels WAM. Psychological treatment of patients with psychogenic non-epileptic seizures: an outcome study. Seizure 2008;17:595–603. [DOI] [PubMed] [Google Scholar]
- 31.Engel J Jr. Surgical treatment for epilepsy: too little, too late? JAMA 2008;300:2548–2550. [DOI] [PubMed] [Google Scholar]
- 32.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia Epub 2009 Nov 3. [DOI] [PubMed]
- 33.Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol 2007;62:375–381. [DOI] [PubMed] [Google Scholar]
- 34.Liimatainen SP, Raitanen JA, Ylinen AM, Peltola MA, Peltola JT. The benefit of active drug trials is dependent on aetiology in refractory focal epilepsy. J Neurol Neurosurg Psychiatry 2008;79:808–812. [DOI] [PubMed] [Google Scholar]