Abstract
Objective:
We have shown that health-related quality of life (QOL) in children with inherited neuropathies (Charcot-Marie-Tooth disease [CMT]) is significantly reduced compared to population norms, thus establishing its utility as an outcome measure in therapeutic trials. However, the Australian ascorbic acid trial in children with CMT type 1A (CMT1A) identified no change in QOL scores despite a trend toward improvement in nerve conduction velocities in the treated group. The objective of this study was to identify clinical, electrophysiologic, and functional correlates of QOL in children with CMT1A, to guide future investigations of strategies to improve QOL and reduce disability in these patients.
Methods:
In this cross-sectional study, a series of multivariate regression models were developed to determine whether QOL scores could be explained by demographic and symptom data, standardized measures of gross motor function, foot/ankle and hand/finger involvement, electrophysiology, and gait characteristics in 70 children aged 5–16 years with CMT1A.
Results:
Independent determinants of reduced QOL in children with CMT1A, from strongest to weakest, were leg cramps, hand tremor, short step length, reduced long jump distance, ankle inflexibility, poor agility and endurance, advancing age, and foot drop. Many of the standardized clinical and electrophysiologic measures used as endpoints in clinical trials of CMT correlated poorly with QOL.
Conclusion:
QOL is negatively affected by CMT1A in children. Multivariate modeling suggests that interventions designed to improve leg cramps, tremor, agility, endurance, and ankle flexibility might have a substantial effect on QOL in children with CMT1A.
GLOSSARY
- BMI
= body mass index;
- CHQ
= Child Health Questionnaire;
- CMAP
= compound muscle action potential;
- CMT
= Charcot-Marie-Tooth disease;
- CMT1A
= Charcot-Marie-Tooth disease type 1A;
- QOL
= quality of life.
Charcot-Marie-Tooth disease (CMT) is the most common inherited neurologic disorder, affecting 1 in 2,500 people.1,2 The dominantly inherited CMT type 1A (CMT1A) causes progressive length-dependent weakness and atrophy of the distal muscles of the limbs. While the clinical features of CMT are widely recognized, data on the impact of the disease on health-related quality of life (QOL) are scarce. Using the generic Child Health Questionnaire (CHQ), we have previously shown that QOL is negatively affected by many types of CMT in childhood and adolescence.3 The recent Australian ascorbic acid trial in children with CMT1A also identified a significant reduction in QOL compared to healthy norms.4 However, although a trend toward improvement in nerve conduction velocity was seen in children receiving treatment rather than placebo, the trial showed no corresponding change in QOL scores using the CHQ.5 While this may reflect treatment failure, we cannot rule out a lack of responsiveness, i.e., sensitivity to change over time, of the generic QOL instrument. It is also possible that the reduction in QOL in children with CMT may represent physical, mental, or social consequences of the disease unrelated to electrophysiology. There is a need to understand what really affects QOL in children with CMT, and thus identify strategies to improve impairment and disability in these patients, especially given the many new treatment possibilities for this population.6–8 The aim of this study was to identify factors associated with QOL in children with CMT1A; our hypothesis was that functional measures would correlate better with QOL scores than electrophysiologic measures.
METHODS
Participants.
In this cross-sectional study, data obtained from the Australian ascorbic acid trial were utilized.5 Children aged 5–16 years with proven CMT1A, i.e., a 17p11.2 duplication including the PMP22 gene, or a confirmed duplication test in a first- or second-degree relative with a consistent clinical phenotype and confirmatory electrophysiologic testing in the child, were recruited nationally through The Children's Hospital at Westmead (Sydney, New South Wales, Australia) and Royal Children's Hospital (Melbourne, Victoria). Children were excluded if they had acute lower limb injuries, had undergone previous foot/ankle surgery, or were diagnosed with arthritis, diabetes, congenital defects, or neuromuscular disorders other than CMT1A.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional ethics review boards at each hospital (The Children's Hospital at Westmead [Ethics Approval Ref. No. 2006/056], The University of Sydney [Ethics Approval Ref. No. 9733], and Royal Children's Hospital Melbourne [Ethics Approval Ref. No. 26144A]). Informed written consent was also obtained from a parent or guardian of each participant.
QOL in pediatric CMT1A.
The Child Health Questionnaire (CHQ), a well-developed broadly based generic measure of health status in children, was used to explore the parent-reported health-related QOL of all children with CMT1A. The CHQ was developed to understand the everyday functioning and well-being of children and their families.9 It measures health status in 12 domains, including physical functioning, impact of emotion/behavior on social roles, impact of physical ability on social roles, bodily pain, general behavior, mental health, self-esteem, general health, emotional impact on parent, time impact on parent, family limitation in activities, and family cohesion. These are then grouped and can be reported as composite psychosocial and physical scores. The CHQ has undergone rigorous development and evaluation since 1990 and is regarded as a reliable, well-validated, and comprehensive assessment tool for use across a diverse group of children with and without chronic medical conditions.10–12 The CHQ is considered a gold standard of pediatric QOL research. The Australian authorized adaptation of the 50-item parent form (CHQ PF50), which provides population-based normative data for 5,414 Australian children, was used to measure QOL in children with CMT1A (CHQ License Number 4029).11,13
Determinants of QOL in CMT1A.
Demographic and subjective data collected included age, gender, height, weight, body mass index (BMI), presence of leg cramps, and hand tremor. Standardized measures of gross motor function (balance, agility, power-long jump, endurance), foot and ankle involvement (strength of inversion, eversion, dorsiflexion, and plantarflexion; foot structure, ankle flexibility, foot drop), hand and finger dexterity and strength (9-hole peg test, grip and pinch strength), electrophysiology (motor conduction velocity, compound muscle action potential amplitude, distal motor latency of the median nerve), and gait characteristics (speed, cadence, step time, step length, stride length, base of support) were obtained using reliable and valid instruments, described previously.5,14–16
Statistical analysis.
Study sample size calculations have been previously described.5 Descriptive statistics were calculated to characterize the study sample in SPSS version 17.0 (Chicago, IL). Data were subsequently analyzed from one limb only (dominant limb) to satisfy the independence requirements for statistical analysis.17 Normality of data distribution was assessed using the Kolmogorov-Smirnov test with Lilliefors significance correction, and the appropriate parametric or nonparametric tests subsequently employed. Published norms were used to describe the impact of CMT1A on QOL.11 A series of multivariate regression models were developed to determine whether changes in QOL could be explained by 7 categories of standardized measures including demographic and subjective symptom information, gross motor function, foot/ankle and hand/finger involvement, electrophysiology, and gait characteristics in children with CMT1A. First, measures related to growth and development were scaled to account for differences in age and physical body size in accordance with established nondimensional normalization principles.18 Second, Pearson correlation coefficients were computed to examine associations between the 7 categories of standardized measures with QOL domains. Third, all measures identified as significantly associated with QOL domains were entered simultaneously into a stepwise multiple regression model that was reduced to a most parsimonious model to yield a set of variables that best predict (and can be regarded as independent determinants of) each outcome.19 Only the most strongly associated variables and physiologically plausible factors were entered into the model. To avoid multicollinearity, only one variable from highly correlated (r >0.7) variables (such as agility and long jump; dorsiflexion strength and plantarflexion, inversion, eversion strength; finger pinch strength and grip strength; step length and other temporospatial variables) was included. β Weights for all variables entered into the regression model were examined to ensure they made meaningful contributions to each QOL domain. The standardized β weights provided an indication of the relative importance of the various CMT1A characteristics entered in the model, to explain the variance in individual and composite CHQ QOL domains.
RESULTS
Participants.
Details of individuals recruited at each stage of the ascorbic acid trial have been reported elsewhere.5 Our series included 70 children (40 boys, 30 girls) aged 5–16 years (mean age 9.1, SD 3.0 years; mean height 1.37 m, SD 0.18, range 1.08–1.83 m; mean weight 33.8 kg, SD 14.9, range 18.1–84.1kg) from 46 families diagnosed with CMT1A (44 with confirmed 17p11.2 duplication of PMP22 gene; 24 with confirmed 17p11.2 duplication in first-degree relative and consistent phenotype/electrophysiology; 2 with confirmed 17p11.2 duplication in second-degree relative and consistent phenotype/electrophysiology). Comorbidities were reported in 13 children (4 with asthma, 3 with attention-deficit hyperactivity disorder, 3 with Asperger syndrome, 1 with epilepsy, 1 with eczema, 1 with arrhythmia, 1 with bladder dysfunction, 1 with oppositional defiant disorder, 1 with migraine, and 1 with nystagmus of the palate).
QOL in pediatric CMT1A.
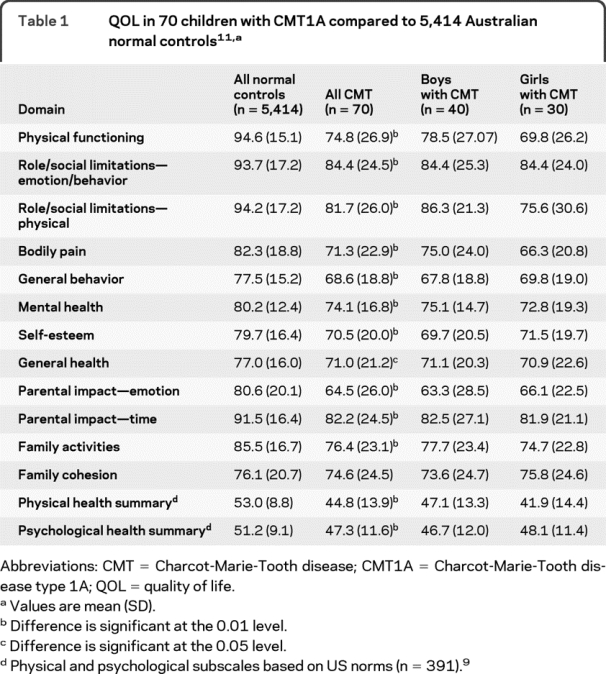
Children with CMT1A demonstrated lower mean CHQ scores than age-matched population norms11 in all 12 domains of the CHQ except one (family cohesion). Mean physical and psychological composite scores in children with CMT1A were also significantly reduced compared to US population norms9; this reduction was greater for the physical composite score (mean reduction of 15.5%) than for the psychological composite score (mean reduction of 7.6%). In contrast, in the 13 children with comorbidities, the reduction in the psychological composites score was greater (29%) than the reduction in the physical composite score (12%). Mean CHQ scores were similar for both genders in children with CMT1A (p > 0.05). A summary of the QOL scores is provided in table 1.
Table 1 QOL in 70 children with CMT1A compared to 5,414 Australian normal controls11
Determinants of QOL in pediatric CMT1A.
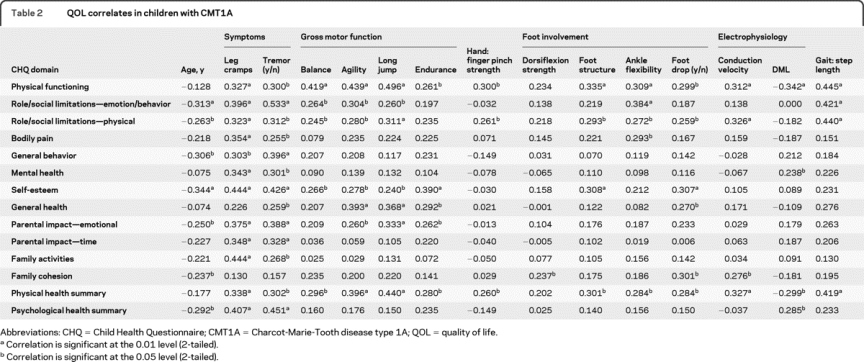
A Pearson correlation coefficient matrix was generated to examine clinical markers of pediatric CMT1A and their relationship with individual CHQ QOL domains. The 7 categories of clinical measures were reduced for modeling, consisting of demographic information (gender, age, BMI), subjective symptoms (leg cramps, hand tremor), gross motor function (balance, agility, long jump, endurance), foot/ankle involvement (dorsiflexion strength, foot structure, ankle flexibility, foot drop), hand/finger involvement (finger pinch strength, hand dexterity), electrophysiology (conduction velocity, distal motor latency, distal compound muscle action potential [CMAP] amplitude), and gait characteristics (step length). Variables that showed significant correlations are presented in table 2. Among the 7 categories, subjective symptoms (leg cramps and hand tremor) showed significant correlations with almost all CHQ QOL domains. Measures of gross motor function correlated with 4 to 6 of the 12 CHQ domains, and as expected, correlated significantly with the physical composite summary score. Other significant correlations with the individual domains of the CHQ were hand/finger involvement (1–2 domains), foot/ankle involvement (1–5 domains), electrophysiology (1–3 domains), and gait characteristics (3 domains). Of the 7 categories, 4 variables (gender, BMI, hand dexterity, and distal CMAP amplitude) did not correlate significantly with any of the CHQ QOL domains.
Table 2 QOL correlates in children with CMT1A
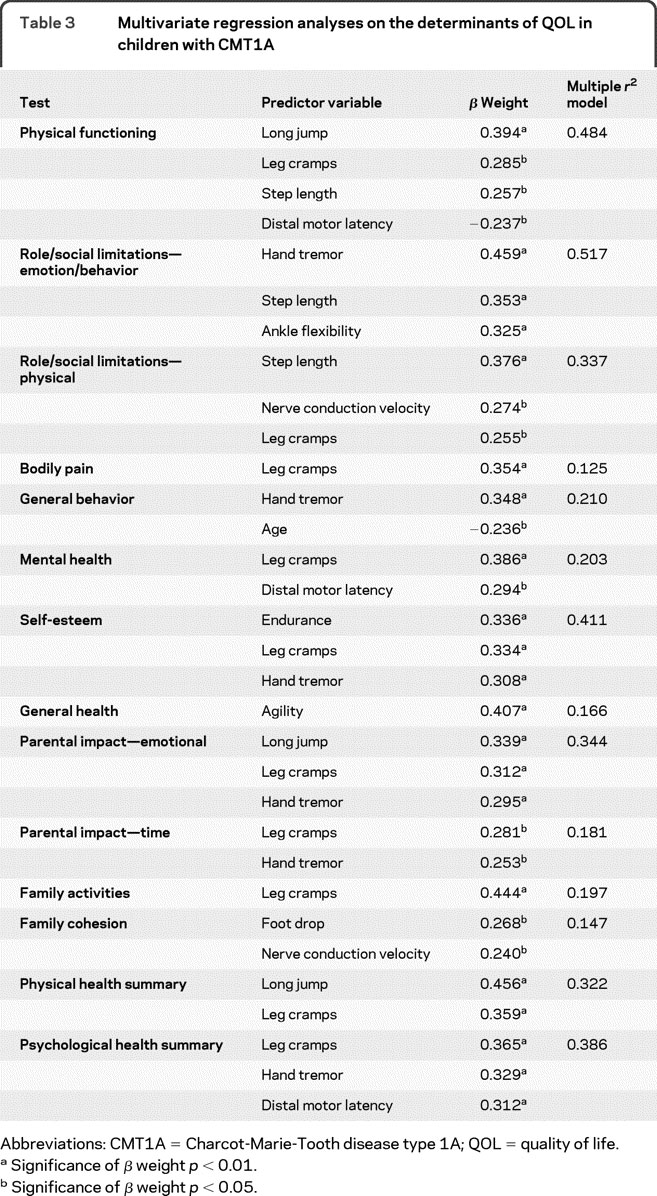
The results of the multivariate regression analyses for each of the 12 domains of the CHQ, as well as the 2 summary scores (physical and psychological) of the CHQ, are presented in table 3. One or more of the standardized measures were found to be a significant independent predictor for each of the CHQ domains. In the models for the 2 summary scores, long jump distance (power) and leg cramps were independent predictors of the physical composite score, and leg cramps, hand tremor, and distal motor latency were independent predictors of the psychological health composite score. Within individual CHQ domains, leg cramp was an independent determinant of 8 out of 12 QOL domains; hand tremor was an independent determinant of 5 out of 12 QOL domains. Of the electrophysiologic measures, distal motor latency and nerve conduction velocity were found to be significant independent predictors for 3 out of the 12 CHQ domains.
Table 3 Multivariate regression analyses on the determinants of QOL in children with CMT1A
DISCUSSION
There is a clear need for randomized controlled therapeutic trials in the peripheral neuropathies,20 especially in children, in whom disease progression, clinical responses, and need for ongoing treatment may vastly differ from those of adults.21,22 This need is especially evident in children with CMT, where the long-term impact during critical developmental stages may result in a high disease burden by adulthood. It is clear that QOL in children with CMT is significantly reduced compared to healthy children.3,4 This study shows that the physical aspects of QOL are significantly more reduced than the psychological aspects of QOL in children with CMT, suggesting that physical signs and symptoms are more relevant to an affected child's overall QOL. Interestingly, parents of children with comorbidities report even lower psychological QOL scores, presumably due to the burden of multiple diseases states. Both findings seem to negate the disability paradox seen in certain disorders, where a high disease burden does not correspond to low scores in QOL.23–25 However, longitudinal studies are required to see if the lower QOL scores continue to worsen, or stabilize and improve, as patients set new physical, mental, and social goals to adjust for emerging physical disabilities. Generalization of these results to a broader population of children with inherited neuropathy should be approached with caution, as the characteristics of children enrolled in a clinical trial may differ from those of children who do not participate in similar trials.
Out of the 7 categories of standardized measures, it was striking to note that the largest number of significant correlations with CHQ QOL domains was seen with the subjective symptoms: leg cramps and hand tremor. This held true even in multiple regression models for the physical and psychological composite scores. These results suggest that therapy targeting leg cramps or tremor will have a substantial effect on physical and mental aspects of QOL in children with CMT1A. Of note, the CHQ is a proxy measure; parents fill it out on behalf of their children. While parents and their perception of the child's QOL play a crucial role in the medical decisions made for the child with CMT, it is possible that the impact of subjective symptoms (tremor, cramps) on QOL may have been estimated differently by the children themselves.
It was surprising and disappointing to observe that standardized assessments of function, including balance and strength, as well as electrophysiologic measures did not correlate well individually or in the regression models with many of the CHQ QOL domains. Given that the physical composite score is affected more than the psychological composite score in children with CMT1A, this raises the concern that the questions in the CHQ may be targeting aspects of physical function and disease severity that are not assessed by our standardized clinical measures. Development of more functionally relevant outcome measures, for clinical trials of rehabilitative, pharmacologic, and surgical interventions in children with CMT, might be warranted. Another possibility is that the CHQ lacks sensitivity to discriminate between children with a range of neuropathic disability and disease severity. For instance, several studies have shown that axonal loss is a determinant of worsening physical disability in neuropathies,26–28 yet no relationship was observed between QOL and CMAP amplitudes in the median motor nerve in our study. The very first electrophysiologic abnormality in CMT1A is prolongation of the distal motor latency, then loss of nerve conduction velocity, followed ultimately by loss of CMAP amplitude.29 Therefore, our findings may reflect that significant axon loss had not yet occurred in the child. It is also possible that the generic QOL instrument, the CHQ, lacks sensitivity to longitudinal changes in disease severity in pediatric inherited neuropathy. Disease-specific outcome measures capture domains relevant to a specified condition, thus increasing content validity, sensitivity, and specificity.30 A disease-specific, pediatric CMT QOL outcome measure would therefore have more relevance in clinical trials, where greater sensitivity to change with disease-specific interventions needs to be demonstrated.31
Outcome measures such as QOL in CMT can reflect the clinical course as well as the patient's perspective of the disease, and can be useful as endpoints in clinical neuromuscular trials. This study provides evidence that physical aspects of QOL are significantly reduced in children with CMT1A. Interventions designed to improve leg cramps, tremor, walking ability, endurance, power, agility, and ankle flexibility might have a substantial effect on QOL in children with CMT1A.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Joshua Burns.
DISCLOSURE
Dr. Burns has received speaker honoraria from Keystone Education and has received research support from The University of Sydney Research and Development Scheme (K2701 U3332), National Health and Medical Research Council of Australia (NHMRC 336705), New South Wales Podiatrists Registration Board, Australian Podiatry Education & Research Foundation, Charcot-Marie-Tooth Association of Australia, and the Australian-American Fulbright Commission. Dr. Ramchandren and Dr. Ryan report no disclosures. Dr. Shy serves on the speakers' bureau for and has received funding for travel and speaker honoraria from Athena Diagnostics, Inc.; serves on the editorial board of the Journal of the Peripheral Nervous System; and receives research support from the NIH (NINDS U54-NS065712-01 [PI]), the Charcot Marie Tooth Association, and the Muscular Dystrophy Association. Dr. Ouvrier reports no disclosures.
Address correspondence and reprint requests to Dr. Sindhu Ramchandren, Department of Neurology, Wayne State University-Detroit Medical Center, 4201 St. Antoine, UHC 8D, Detroit, MI 48201 sramchan@med.wayne.edu
Disclosure: Author disclosures are provided at the end of the article.
Received December 15, 2009. Accepted in final form May 11, 2010.
REFERENCES
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth disease. Clin Genet 1974;6:98–118. [DOI] [PubMed] [Google Scholar]
- 2.Martyn CN, Hughes RAC. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry 1997;62:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns J. Ryan MM, Ouvrier RA. Quality of life in children with Charcot-Marie-Tooth disease. J Child Neurol 2010;25:343–347. [DOI] [PubMed] [Google Scholar]
- 4.Ramchandren S, Shy ME, Finkel RS. Quality of life in children with CMT type1A. Lancet Neurol 2009;8:880–881. [DOI] [PubMed] [Google Scholar]
- 5.Burns J, Ouvrier RA, Yiu EM, et al. Ascorbic acid for Charcot-Marie-Tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol 2009;8:537–544. [DOI] [PubMed] [Google Scholar]
- 6.Passage E, Norreel JC, Noack-Fraissignes P, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med 2004;10:396–401. [DOI] [PubMed] [Google Scholar]
- 7.Khajavi M, Shiga K, Wiszniewski W, et al. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am J Hum Genet 2007;81:438–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer zu Horste G, Prukop T, Liebetanz D, Mobius W, Nave KA, Sereda MW. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann Neurol 2007;61:61–72. [DOI] [PubMed] [Google Scholar]
- 9.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ) User's Manual, second ed. Boston, MA: HealthAct; 1999. [Google Scholar]
- 10.Landgraf J, Maunsell E, Speechley K, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res 1998;7:433–445. [DOI] [PubMed] [Google Scholar]
- 11.Waters E, Salmon L, Wake M. The parent-form Child Health Questionnaire in Australia: comparison of reliability, validity, structure, and norms. J Pediatr Psychol 2000;25:381–391. [DOI] [PubMed] [Google Scholar]
- 12.Rentz AM, Matza LS, Secnik K, Swensen A, Revicki DA. Psychometric validation of the child health questionnaire (CHQ) in a sample of children and adolescents with attention-deficit/hyperactivity disorder. Qual Life Res 2005;14:719–734. [DOI] [PubMed] [Google Scholar]
- 13.Waters E, Salmon L, Wake M, Wright M, Hesketh K. Australian Authorised Adaptation of the Child Health Questionnaire. Melbourne, Victoria: HealthAct; 1999. [Google Scholar]
- 14.Burns J, Ryan MM, Ouvrier RA. Evolution of foot and ankle manifestations in children with CMT1A. Muscle Nerve 2009;39:158–166. [DOI] [PubMed] [Google Scholar]
- 15.Burns J, Bray P, Cross L, North KN, Ryan MM, Ouvrier RA. Hand involvement in children with Charcot-Marie-Tooth disease type 1A. Neuromuscul Disord 2008;18:970–973. [DOI] [PubMed] [Google Scholar]
- 16.Yiu E, Burns J, Ryan MM, Ouvrier RA. Neurophysiologic abnormalities in children with Charcot-Marie-Tooth disease type 1A. J Peripher Nerv Syst 2008;13:236–241. [DOI] [PubMed] [Google Scholar]
- 17.Sutton AJ, Muir KR, Jones AC. Two knees or one person: data analysis strategies for paired joints or organs. Ann Rheum Dis 1997;56:401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stansfield BW, Hillman SJ, Hazlewood ME, et al. Normalisation of gait data in children. Gait Posture 2003;17:81–87. [DOI] [PubMed] [Google Scholar]
- 19.Tabachnick BG, Fidell LS. Using Multivariate Statistics, 5th ed. Boston: Pearson/Allyn & Bacon; 2007. [Google Scholar]
- 20.Hughes RA. Treating nerves: a call to arms. J Peripher Nerv Syst 2008;13:105–111. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803–811. [DOI] [PubMed] [Google Scholar]
- 22.Klassen TP, Hartling L, Craig JC, Offringa M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med 2008;5:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutner JS, Nowels DE, Kassner CT, Houser J, Bryant LL, Main DS. Confirmation of the “disability paradox” among hospice patients: preservation of quality of life despite physical ailments and psychosocial concerns. Palliat Support Care 2003;1:231–237. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med 1999;48:977–988. [DOI] [PubMed] [Google Scholar]
- 25.Lundqvist C, Siosteen A, Blomstrand C, Lind B, Sullivan M. Spinal cord injuries: clinical, functional, and emotional status. Spine 1991;16:78–83. [PubMed] [Google Scholar]
- 26.Krajewski KM, Lewis RA, Fuerst DR, et al. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain 2000;123:1516–1527. [DOI] [PubMed] [Google Scholar]
- 27.Sancho S, Magyar JP, Aguzzi A, Suter U. Distal axonopathy in peripheral nerves of PMP22-mutant mice. Brain 1999;122:1563–1577. [DOI] [PubMed] [Google Scholar]
- 28.Sahenk Z, Chen L, Mendell JR. Effects of PMP22 duplication and deletions on the axonal cytoskeleton. Ann Neurol 1999;45:16–24. [DOI] [PubMed] [Google Scholar]
- 29.Ryan MM, Jones HR Jr. Delayed neurophysiologic abnormalities in Charcot-Marie-Tooth disease type 1A. Muscle Nerve 2004;30:123–125. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A. Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol 1989;42:403–408. [DOI] [PubMed] [Google Scholar]
- 31.Deyo RA, Patrick DL. Barriers to the use of health status measures in clinical investigation, patient care and policy research. Med Care 1989;27:254–268. [DOI] [PubMed] [Google Scholar]


