Abstract
Biological membranes are composed of a large number lipid species differing in hydrophobic length, degree of saturation, and charge and size of the headgroup. We now present data on the effect of hydrocarbon chain length of the lipids and headgroup composition on the lateral mobility of the proteins in model membranes. The trimeric glutamate transporter (GltT) and the monomeric lactose transporter (LacY) were reconstituted in giant unilamellar vesicles composed of unsaturated phosphocholine lipids of varying acyl chain length (14–22 carbon atoms) and various ratios of DOPE/DOPG/DOPC lipids. The lateral mobility of the proteins and of a fluorescent lipid analog was determined as a function of the hydrophobic thickness of the bilayer (h) and lipid composition, using fluorescence correlation spectroscopy. The diffusion coefficient of LacY decreased with increasing thickness of the bilayer, in accordance with the continuum hydrodynamic model of Saffman-Delbrück. For GltT, the mobility had its maximum at diC18:1 PC, which is close to the hydrophobic thickness of the bilayer in vivo. The lateral mobility decreased linearly with the concentration of DOPE but was not affected by the fraction of anionic lipids from DOPG. The addition of DOPG and DOPE did not affect the activity of GltT. We conclude that the hydrophobic thickness of the bilayer is a major determinant of molecule diffusion in membranes, but protein-specific properties may lead to deviations from the Saffman-Delbrück model.
Introduction
The biological membrane is composed of lipids of varying acyl chain length. For instance, in Escherichia coli the cytoplasmic membrane has lipid tails ranging from C12 to C20 with varying degrees of saturation (1). The polar headgroups consist of phosphatidylethanolamine (∼75%), phosphatidylglycerol (∼20%), and cardiolipin (∼5%) as the dominant species (1–3). Membranes from eukaryotic species may additionally contain phosphatidylcholine, sphingolipids, and steroid-type molecules like cholesterol. This diverse composition of the biological membrane is important for the distribution, organization, and function of integral and peripheral membrane proteins (4). Certain combinations of lipids do not mix well and so-called membrane domains can be formed, in which specific proteins may partition and from which others may be excluded (5,6). The lipid composition of the membrane can influence the activity of proteins through specific lipid-protein interactions (lipids acting as cofactors) or through more generic parameters such as fluidity, bilayer thickness/hydrophobic (mis)match, surface charge, membrane tension, and lateral pressure (7). For example, specific lipid-protein interactions play an important role in regulating the sorting of proteins between the Golgi complex and the plasma membrane (5), whereas bulk parameters such as the lateral pressure profile and the hydrophobic thickness affect the gating of mechanosensitive channels (8).
The lateral mobility of proteins in biological membranes can be orders-of-magnitude slower than in synthetic membranes, owing to a high degree of crowding, membrane domains, or interactions with cytoskeletal components (9,10). Although the influence of the bilayer thickness and lipid headgroup composition on lateral diffusion of integral membrane proteins has been only poorly investigated, numerous studies emphasize the importance of these parameters on the activity of transporters and channel proteins. Although the Na+K+-ATPase (11,12) and Ca2+-ATPase (13,14) are relatively insensitive to the bilayer thickness, the branched-chain amino-acid transporter from Lactococcus lactis (15) and the sodium-dependent leucine transporter from Pseudomonas aeruginosa (16) show suboptimal activity when the acyl chain length is less than C16 or greater than C18. Similarly, the function of diglycerolkinase from E. coli (17), the acetylcholine receptor (18), the hexose transporter from human erythrocyte (19), and the melibiose permease from E. coli (20) have been shown to depend on the bilayer thickness. For the mechanosensitive channel of large conductance from E. coli (8) and the Ca2+-voltage-activated K+ channel (21), there is evidence that thin membranes favor channel openings, whereas thick bilayers stabilize the closed configuration.
In the case of hydrophobic mismatch, exposure of hydrophobic surfaces of the proteins to the aqueous environment is thermodynamically unfavorable (22). Mouritsen and Bloom's mattress model (23) provides insight into the hydrophobic (mis)match between integral proteins and the lipid bilayer. The model relates the undulation of the membrane surface to elastic properties of the lipids and the proteins. Under conditions of hydrophobic mismatch between the protein and the lipids, the annular lipids may stretch or compress to the hydrophobic core of the proteins, whereas the protein may change its conformation. Theoretically, the adaptation of a protein to the hydrophobic mismatch results in tilting of its helices, aggregation/self association of the protein, and/or a change in backbone conformation or orientation of the side chains (24–27). These adjustments in the membrane lipids and/or proteins are likely to have consequences for the lateral organization and mobility of the proteins as well as their activity.
Lateral organization of proteins is not only determined by the hydrophobic (mis)match with the surrounding lipids but also by their headgroup composition. Studies in E. coli, altered in the content of anionic lipid (phosphatidylglycerol and cardiolipin), indicate that charge interactions between transmembrane helices and phosphoglycerol headgroups are important for protein topology in the membrane (28). Similarly, the content of phosphatidylethanolamine (PE) has been shown to be important for the activity and topology of the γ-aminobutyric acid permease and phenylalanine permease from E. coli (29–31). Recently, the influence of PE on the lateral mobility of lipids in the membrane was reported. It was shown that the diffusion coefficient of lipids (NBD-lipid probe) decreased nonlinearly with increasing concentration of eggPE in eggPC membranes (32). To the best of our knowledge, a detailed analysis of the effect of phospholipid composition on membrane protein mobility has not been documented yet.
The aim of this study is to investigate the influence of the hydrocarbon chain length and composition of the lipids on the lateral mobility of integral membrane proteins. The model proteins studied are the structurally stable glutamate transporter (GltT) from Bacillus stearothermophilus (33) and the conformationally flexible lactose permease (LacY) from E. coli (34). The proteins were purified and fluorescently labeled, and reconstituted in large unilamellar vesicles (LUVs) composed of synthetic lipids. Subsequently, the LUVs were converted into giant unilamellar vesicles and the protein diffusion coefficients were measured using fluorescence correlation spectroscopy.
Materials and Methods
Protein purification and labeling
The single cysteine mutant (Q412C) of the glutamate transporter GltT from B. stearothermophilus was prepared by standard molecular biology methods (35). The lactose permease LacY (C154G/S401C) mutant from E. coli (36) was a gift of Prof. H. R. Kaback (University of California, Los Angeles). For protein expression, E. coli strain MC1061 (for GltT) or XL1-blue line (for LacY) was grown in Luria broth, and, in the midexponential growth phase (OD600 ∼0.8), the cells were induced for 2 h with 100 μg/L L-arabinose (for GltT) or 0.5 mM isopropyl-β-D-thiogalactopyranoside (for LacY). The cells were harvested by centrifugation, resuspended in 50 mM potassium phosphate buffer, pH 7.0 to a final OD600 ∼100 and lysed by a single passage through a French press at 25,000 psi. The membranes were collected by centrifugation at 180,000 × g for 1 h at 4°C, resuspended in 50 mM potassium phosphate buffer, pH 8.0 to a protein concentration of 5 mg/mL and solubilized for 30 min at 4°C by using either 1% (w/v) n-dodecyl β-D-maltoside (DDM) (for GltT) or 2% DDM (for LacY).
The solubilisate was cleared by centrifugation for 15 min at 280,000 × g, after which the solubilized proteins were purified by nickel-affinity chromatography, essentially as described previously (37,38). Solubilization buffers were 50 mM potassium phosphate (KPi), pH 8.0, 300 mM NaCl, 10% (w/v) glycerol, supplemented with 15 mM imidazole (for GltT); 50 mM KPi, pH 8.0, 200 mM NaCl, supplemented with 5 mM imidazole (for LacY). The solubilized material was incubated with Ni2+-Sepharose resin for 1 h at 4°C while rotating (25 mg of resin per 1 mg of total membrane protein). Subsequently, the resin was drained and washed with 20 column volumes of solubilization buffer containing 0.05% (w/v) DDM (for GltT) or 0.01% (w/v) DDM (for LacY), supplemented with either 60 mM (GltT) or 25 mM imidazole (LacY). The proteins were labeled with Alexa Fluor 488 (AF488; Invitrogen, Carlsbad, CA), bound to the Ni2+-sepharose resin, at a 1:30 molar ratio of protein over AF488 (37). After incubation for 2 h, the column was washed with 20 column volumes of solubilization buffer without imidazole supplemented with 0.05% (w/v) DDM (GltT) or 0.01% DDM (LacY) to remove free AF488 dye. Subsequently, the proteins were eluted with solubilization buffer containing 400 mM of imidazole without glycerol. The concentration of purified proteins was determined by the Bradford assay (39), using bovine serum albumin as a protein standard, and by measuring the absorbance at 280 nm, using extinction coefficients 0.625 and 1.169 (mg/mL)−1 cm−1 for GltT and LacY, respectively. The Alexa Fluor 488-labeling of the proteins was verified by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry, using α-cyano-4-hydroxycinnaminic acid as matrix.
Vesicle formation and membrane reconstitution
Large unilamellar vesicles (LUVs) were formed from 1,2-dimyristoyl-sn-glycero-3 phosphocholine (diC14:1PC), 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine (diC16:1PC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (diC18:1PC;DOPC), 1,2-dieicosenoyl-sn-glycero-3-phosphocholine (diC20:1PC), 1,2-dierucoyl-sn-glycero-3-phosphocholine (diC22:1PC), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DOPG), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Avanti Polar Lipids, Alabaster, AL), and the lipid probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-dicarbocyanine perchlorate (DiD) (excitation at 644 nm, emission at 665 nm; Invitrogen) was incorporated at 1:150,000 mol/mol ratio. Briefly, 40 mg of lipid mixture was dried in a rotary evaporator for 1 h to remove the chloroform, as described previously (37,38).
The thin film of dried lipids was rehydrated to a final concentration of 20 mg of lipid/mL in 50 mM KPi, pH 7.0 for 30 min, and, subsequently, the lipids were flash-frozen in liquid nitrogen and thawed at room temperature; the freezing-thawing cycles were repeated three times. Before membrane reconstitution, the multilamellar vesicles, obtained after freezing-thawing, were extruded through 400-nm polycarbonate filters to obtain LUVs. Next, the LUVs were diluted to 4 mg/mL (volume of 2.5 mL) and titrated stepwise with 10 μL aliquots of 10% Triton X-100 (Sigma-Aldrich, St. Louis, MO); typically 80 μL of 10% Triton X-100 was used per 2.5 mL of LUVs (10 mg of lipids in total) (40). The purified proteins were added to the detergent-destabilized LUVs at 1:150 protein/lipid ratio (wt/wt), unless indicated otherwise. The detergent-lipid-protein complex was incubated while gently shaking for 45 min at room temperature. The mixture was then incubated with 40 mg/mL of polystyrene beads (Bio-Beads SM2 from Bio-Rad Laboratories, Hercules, CA) to remove the detergent (41). Subsequently, the proteo-LUVs were dried under vacuum in the presence of 3 mM sucrose for at least 12 h, and giant unilamellar vesicles (GUVs) were formed as reported previously (37,38). The dried lipid film was rehydrated in 10 mM KPi, pH 7.0, and GUV formation was monitored on a confocal microscope.
Transport assays
The activity of GltT, reconstituted in LUVs (lipid composition specified in the Fig. 1 legend), was determined as described previously (42). LUVs were thawed, extruded as described above, and centrifuged for 20 min (280,000 × g, 4°C, TLA 100.4 rotor; Beckman Coulter, Brea, CA). Subsequently, the LUVs were resuspended in 50 mM potassium phosphate, pH 7.5 (internal buffer), to a protein concentration of 4 μg/μL. In the transport assays, proteoliposomes were diluted 100-fold into 200 μL of 64 mM sodium phosphate, pH 6.0 (external buffer), supplemented with 0.2 μM valinomycin plus 1 μM [14C]glutamate (Amersham Bio-Sciences, Buckinghamshire, UK)). All assays were performed at 30°C, except when otherwise indicated, and internal and external buffers were iso-osmotic. Transport reactions were stopped by addition of 2 mL of ice-cold 100 mM LiCl, followed by rapid filtration over BA-85 nitrocellulose filters and an additional wash step with lithium chloride. Levels of radioactivity were determined by addition of 2 mL of emulsifier scintillator plus liquid (Perkin Elmer, Waltham, MA), and analyzed in a Tricarb 2800 TR isotope counter (Perkin Elmer).
Figure 1.
Confocal images of GUVs prepared from phosphatidylcholine lipids with varying acyl chain length: (A) diC14:1PC, (B) diC16:1PC, (C) diC18:1PC, (D) diC20:1PC, and (E) diC22:1PC. GltT was reconstituted into the GUV at a molar protein/lipid ratio 1:30,000.
Fluorescence correlation spectroscopy
Measurements were carried out on a dual-color laser scanning confocal microscope (37,38) based on an inverted microscope Axiovert S 100 TV (Zeiss, Jena, Germany) in combination with a galvanometer optical scanner (model 6860; Cambridge Technology, Cambridge, MA) and a microscope objective nanofocusing device (P-721; Physik Instrumente, Karlsruhe, Germany). Two laser beams, a 488-nm argon ion laser (Spectra Physics, Mountain View, CA) and a 633-nm He-Ne laser (JDS Uniphase, Milpitas, CA), were focused by a C-Apochromat infinity-corrected 1.2 NA 40× water immersion objective (Zeiss) for excitation of the Alexa Fluor 488 and DiD fluorophores. The fluorescence was collected through the same objective, separated from the excitation beams by a beam pick-off plate (BSP20-A1; Thorlabs, North Newton, NJ), and finally directed through emission filters (HQ 550/100 and HQ675/50; Chroma Technology, Rockingham, VT) and pinholes (diameter 20 μm) onto two avalanche photodiodes (SPCM-AQR-14; EG&G, Quebec, Canada). The fluorescence signals were digitized and auto- and cross-correlation curves were calculated using a multiple τ-algorithm.
The diffusion of fluorescent particles within lipid membrane occurs in two dimensions and thus the autocorrelation curves were fitted using a two-dimensional diffusion model (43)
| (1) |
where N is the average number of fluorescent particles in the detection area. The diffusion time τD is related to the diffusion coefficient D through the expression
| (2) |
where ω is lateral radii, defined as the point where the fluorescence count rate drops e2 times. The setup was calibrated based on the known diffusion coefficients of Alexa Fluor 488 and 633 in water (D = 380 μm2 s−1) at 20°C (44). The lateral radii, ω, were ∼200 nm for Alexa Fluor 488 and 270 nm for Alexa Fluor 633. Error bars in figures and text (± values) refer to standard deviations obtained from diffusion measurements of at least three independent data sets, each consisting of minimally seven measurements.
To accurately position the focal volume on the pole of the GUV, a z-scan, parallel to the optical axis, was performed. Next, the fluorescence signals in the red (DiD fluorescence, 633-nm excitation) and green (AF488-labeled protein fluorescence, 488-nm excitation) channels, F1(t) and F2(t), respectively, were recorded. The time-dependent fluctuations of F1(t) and F2(t) were evaluated by calculating the autocorrelation functions G(τ). The obtained autocorrelation functions G(τ) could be fitted reasonably well to a one-component two-dimensional diffusion model, yielding values of the protein and DiD diffusion coefficients as described previously (37). To avoid undulations in the case of large GUVs, and possible curvature effects in the case of small vesicles, only GUVs with diameters of 15–40 μm were used for the measurements. The details of the data evaluation have been published elsewhere (37).
Results
Protein reconstitution and giant unilamellar vesicle formation
The large unilamellar vesicles (LUVs) were prepared from phosphocholine lipids varying in diacyl chain length from 14 to 22 C-atoms, with DiD incorporated as the fluorescent lipid probe. To study the effect of bilayer thickness on the diffusion of integral membrane proteins, we incorporated into the vesicles the lactose permease LacY (monomer; lateral radius (R) ∼2 nm, hydrophobic length (h) ∼2.7 nm) from E. coli (36,45) and the glutamate transporter GltT (trimer; R ∼4 nm, h ∼3.1 nm) from B. stearothermophilus (35). To obtain R and h for GltT, we used the crystal structure of the glutamate transporter from Pyrococcus horikoshii (46). Selective fluorescent labeling of a single cysteine residue in each of the membrane proteins was done using Alexa Fluor 488 C5 maleimide (AF488). The purity and labeling of the proteins was checked by sodium dodecyl sulfate polyacrylamide gel electrophoresis and imaging of the gels as described previously (37). AF488-labeled proteins were reconstituted into Triton X-100-destabilized LUVs composed of the phosphocholine lipids at molar ratios of 1:30,000 (for trimeric GltT) and 1:8,000 (LacY), unless stated otherwise. The GUVs were prepared by drying the LUVs in the presence of low amounts of sucrose to stabilize the proteins during dehydration, as described previously (38). GUV formation was monitored by means of confocal microscopy and measurements were carried out at room temperature (20°C). Fig. 1 shows the confocal images of GUVs prepared from phosphocholine lipids of different chain lengths.
Lateral mobility of lipid analog
The diffusion coefficient of DiD (lipid probe) as a function of hydrophobic bilayer thickness h is presented in Fig. 2 A. The bilayer thickness h corresponds to the hydrophobic thickness of the membrane and was taken from Lewis and Engelman (47). The diffusion coefficient of DiD decreased with increasing h, as was reported previously (48,49). The diffusion coefficient of DiD was not affected by the presence of LacY or GltT, co-reconstituted with DiD at lipid/protein molar ratios of 1:8,000 and 1:30,000, respectively.
Figure 2.
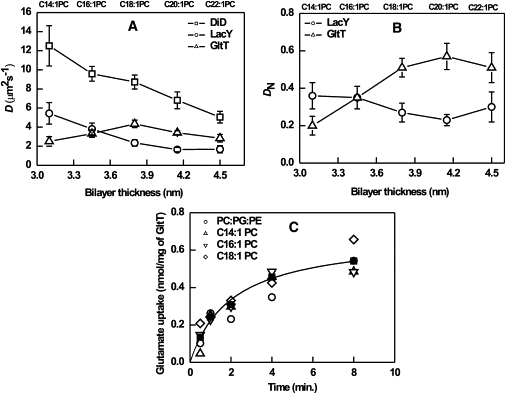
(A) Diffusion coefficient of DiD, GltT(Q412C), and LacY (C154G/S401C) in GUVs as a function of bilayer hydrophobic thickness. D of the lipid analog DiD was not affected by the incorporation of the proteins. The protein/lipid molar ratio was 1:30,000 and 1:8000 for GltT and LacY, respectively. (B) Normalized diffusion coefficient DN (DP/DL) for GltT and LacY. (C) Electrochemical sodium gradient-driven uptake of glutamate in GltT containing LUVs (see Materials and Methods for further details). The transport activity of GltT reconstituted in diCn/PC (n = 14, 16, 18) and DOPC/DOPG/DOPE (1:1:1) is presented; the symbols representing the different lipid compositions are indicated in the figure; the hyperbolic fit is based on all the data points.
Lateral mobility of membrane proteins
Next, we measured the lateral mobility of two integral membrane proteins, i.e., GltT and LacY, as a function of bilayer thickness (Fig. 2 A). The diffusion coefficients of LacY decreased on increasing the thickness of the bilayer, whereas GltT displayed a clear maximum at diC18:1PC lipids. In the thickest membrane (diC22:1PC), D of GltT and LacY were not significantly decreased when compared diC20:1PC membranes. The diffusion coefficients of GltT and LacY normalized to that of DiD are plotted versus bilayer thickness in Fig. 2 B. In the case of LacY, the normalized diffusion coefficient was nearly constant, while that of GltT increased up to diC18:1PC and remained nearly constant upon a further increase in membrane thickness.
Because of the unexpected trend in the (normalized) diffusion of GltT, we determined the transport activity of GltT in diCn:1PC (for n = 14, 16, and 18) and compared the activity to that of proteoliposomes composed of equal fractions of DOPC, DOPG and DOPE. Fig. 2 C shows that GltT was relatively insensitive to the hydrophobic thickness and headgroup composition of the membrane lipids.
It has been shown that PG lipids can have pronounced effects on the stability, orientation, and topology of the membrane proteins (28,50). Because short-tailed, unsaturated PG is not available, we incorporated DOPG in diCn:1PC (n = 14, 18, and 22) at 1:3 molar ratio and investigated the lateral mobility of DiD, GltT, and LacY as a function of DOPG concentration (Table 1). The diffusion coefficient of DiD decreased linearly with increasing bilayer thickness; the values were ∼20% higher than in pure PC lipids. The diffusion coefficient of GltT remained constant, whereas LacY showed a linear decrease on increasing bilayer thickness, comparable to what was observed in pure PC lipids.
Table 1.
Diffusion coefficient of DiD, LacY, and GltT in membranes composed of pure PC lipids (differing in acyl chain length) and binary mixtures PC and DOPG (3:1 molar ratio)
| Chain length (n) | DiD |
LacY |
GltT |
|||
|---|---|---|---|---|---|---|
| diCn:1PC | PC/DOPG | diCn:1PC | PC/DOPG | diCn:1PC | PC/DOPG | |
| 14 | 13 ± 2 | 14.9 ± 0.9 | 6.4 ± 1.5 | 5.7 ± 0.5 | 3.0 ± 0.6 | 3.7 ± 0.4 |
| 18 | 8.7 ± 0.7 | 11.1 ± 0.8 | 2.9 ± 0.4 | 3.9 ± 0.4 | 4.9 ± 0.5 | 3.7 ± 0.5 |
| 22 | 5.0 ± 0.6 | 7.7 ± 0.6 | 2.1 ± 0.4 | 2.8 ± 0.3 | 3.4 ± 0.3 | 3.6 ± 0.5 |
Mean and standard deviation obtained from at least 15 GUVs.
Lateral mobility of lipids and membrane proteins in ternary mixtures of DOPC/DOPG/DOPE
To determine the effect of lipid compositions on the lateral mobility of DiD, GltT, and LacY, GUVs were prepared at different ratios of DOPC and DOPG, keeping the DOPE concentration constant at 10 mol %; DOPG was varied from 5 to 60 mol %. The diffusion coefficients of DiD, GltT, and LacY versus DOPG concentration are depicted in Fig. 3 A. The diffusivity of the DiD, GltT, and LacY was not influenced by DOPG lipids in the membrane. Next, we varied the ratio of DOPC/DOPE (up to 50 mol % DOPE), while keeping the DOPG concentration constant at 25 mol %. The presence of 25 mol % DOPG lipids enhanced rapid formation of GUV but did not influence the lateral mobility of the proteins and lipids (Fig. 3 A). On the contrary, we observed a significant decrease in lateral mobility of DiD, GltT, and LacY as a function of DOPE concentration in the membrane.
Figure 3.
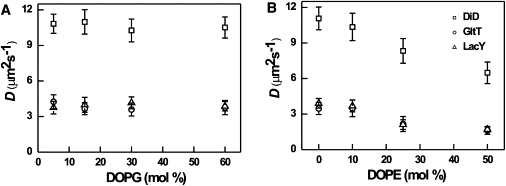
(A) Diffusion coefficient of DiD, GltT (Q412C), and LacY (C154/S401C) in GUV as a function of DOPG concentration; DOPC was varied reciprocally and DOPE was kept constant of 10%. (B) Diffusion coefficient of DiD, GltT, and LacY in GUV as a function of DOPE; DOPC was varied reciprocally and DOPG was kept constant at 25%. The protein/lipid molar ratio was 1:30,000 and 1:8000 for GltT and LacY, respectively.
Discussion
Saffman-Delbrück's continuum hydrodynamic model describes lateral and rotational diffusion of objects moving in a two-dimensional fluid, e.g., a lipid membrane (51,52). The protein is considered as a cylindrical entity moving in a continuous viscous fluid of defined height (h), which is separated by fluids of lower viscosity (aqueous environment). The lateral diffusion coefficient (D) can be expressed as
| (3) |
where kB is the Boltzmann constant, T is absolute temperature, h is the thickness of the bilayer, μ is viscosity of the membrane, μ′ is viscosity of the outer liquid, R is the radius of the diffusing object, and γ is Euler's constant. In this model, D is logarithmically dependent on the radius of the diffusing object and inversely proportional to the thickness of the bilayer and viscosity of the lipid membrane.
In this study, the bilayer thickness was increased by increasing the hydrocarbon chain length of the lipids (47). Fig. 2 A shows that the diffusion coefficient of DiD decreased on increasing the bilayer thickness h of the membrane. This dependence can be rationalized by the fact that a thin membrane allows fewer van der Waals interactions between lipids and lipid probe than a thick membrane (53–55). The trends in lipid diffusion were similar when 25 mol % DOPG was incorporated in the bilayers of different thickness (Table 1).
The effect of hydrophobic (mis)match on diffusion of membrane proteins
For the evaluation of the effect hydrophobic mismatch on protein diffusion, the normalized thickness dependence DN (DP/DL) (Fig. 2 B) was used because it eliminates possible bilayer viscosity variations; DP and DL are the diffusion coefficient for membrane protein and lipid analog DiD, respectively. For instance, in the case of a constant hydrodynamic radius, DN should be independent of the thickness, at least in a limited thickness range. The lateral mobility of LacY continuously slows down on increasing the bilayer thickness (and DN was nearly constant) as the Saffman-Delbrück model predicts for a protein diffusing in a two-dimensional fluid. GltT, on the other hand, displayed a maximum in diffusion around a bilayer thickness of 3.8 nm (Fig. 2 A).
One way of looking at the data is that the diffusion coefficient of GltT in thin membranes (C14 lipids) is approximately twofold lower than could be expected. In terms of the Saffman-Delbrück model, this would imply an approximately fourfold increase in hydrodynamic radius of the protein, which cannot be explained by increased tilting of the helices. Alternatively, a protein highly mismatched in terms of hydrophobic surface may aggregate. However, our functional data show that GltT is equally active in C14 as in C18 lipids (Fig. 2 C), making it unlikely that (part of) the protein has (irreversibly) aggregated in the thin membranes. According to the mattress model (23), the inclusion of a protein into a (mismatch) membrane perturbs the surrounding lipids significantly to avoid exposure of transmembrane helices to the aqueous phase. For the glycerol channel (GlpF, homo-tetramer, aquaporin) in a POPC/POPE bilayer, it was calculated that within a radial distance of 10 Å, lipids were disturbed due to the insertion of the protein (56). Recent neutron scattering studies have shown that the S1–S4 helices of the voltage sensory domains of the KVAP channel, reconstituted in membranes composed of POPC/POPG (3:1), decrease the local bilayer thickness (h) from ∼52.3 to ∼49.3 Å (57). It is evident that inclusions of proteins into membranes distort the bilayer locally, an effect that will increase with increasing mismatch. From theoretical calculations and molecular dynamics simulations, it has been proposed that the membrane deformation in the lateral dimension may be in the order of the protein radius or bilayer thickness (58,59). The fact that the diffusion coefficient of LacY changes almost inversely proportional with the hydrophobic thickness may imply that this protein influences the membrane structure less than GltT. In fact, LacY is a conformationally flexible protein (34,60,61) that may adjust the tilting of its helices more than GltT does. The diffusional behavior as a function of bilayer thickness of LacY is very similar to that of transmembrane peptides (49).
What is known of LacY and GltT, and what sets the two proteins apart?
E. coli lactose permease is a monomeric protein of 12 transmembrane helices, heart-shaped with a surface radius of 2 nm and hydrophobic length of ∼2.7 nm (45). The optimal match between lipids and hydrophobic core of the protein is expected for diC16:1PC, which has a hydrophobic thickness of ∼2.6 nm (47). The adaptation of LacY to hydrophobic mismatch may be reconstructed from NMR and other spectroscopic data and molecular dynamics simulation studies. Overall, these data indicate a highly flexible structure, with the majority of amino acids readily accessible to water (62), and helices that readily adjust their tilting to mismatching environments and during translocation (61). The B. stearothermophilus glutamate transporter is a trimeric protein, with each subunit composed of eight transmembrane helices and two reentrant loops forming the catalytic heart of the protein. Based on crystal structure of GltT from P. horikoshii, Groeneveld and Slotboom (33) designed cross-links at the subunit interface. These and other studies (63) indicate that the trimerization domain of GltT results in an overall rigid protein structure.
Proteins are less compressible than lipids in membranes. The volumetric compressibility moduli of globular proteins in water are 1010–1011 Nm−2 (64), which is 1–2 orders-of-magnitude larger than those of liquid-crystalline phospholipid bilayers (∼109 Nm−2 (65); and 2–3 orders-of-magnitude larger than the moduli for bilayer thickness compressibility (∼108 Nm−2 (66)). While this is true for the effects of hydrostatic pressure on proteins in solution, it is not necessarily the case for membrane proteins. For instance, upon changing the lateral pressure (e.g., thinning or thickening of the bilayer) a protein may change its conformation, as is well known for mechanosensitive channels (8). In such cases, the structure of a protein can change without major changes in its volume. By analogy, LacY may undergo large structural changes upon hydrophobic mismatch, whereas GltT may be more robust and deform the bilayer. Although there is no experimental evidence for bilayer deformation due to hydrophobic mismatch with the protein (that is, under the conditions of low protein/lipid ratio as used here), theoretical calculations suggest that the magnitude of bilayer deformation can be equivalent to the radius of the proteins or the membrane thickness. Under such conditions, the effective mobility may scale to 1/R rather than ln(1/R), as proposed previously (67). We speculate that the anomalous diffusion behavior of GltT may be due to such (relatively) large membrane deformations.
The role of lipid composition on the diffusion coefficient of proteins and lipids
We also investigated the role of anionic and nonbilayer lipids on the lateral diffusion of GltT, LacY, and DiD, in particular because these lipids are often needed for optimal activity of membrane transport proteins (68). We did not observe any significant effect of the surface charge (fraction of DOPG) on the diffusion of lipids and proteins in the membrane. Importantly, we observed a linear decrease in the lateral mobility of lipids and proteins on increasing the concentration of DOPE in the membrane. An increase of PE in the membrane results in a high molecular packing due to a decrease in lipid-lipid spacing, thereby increasing the van der Waals interactions. Moreover, compared to PC, PE can make additional hydrogen bonds with neighboring molecules through its amine headgroup (32,55,69–71). These interactions increase the membrane viscosity. Moreover, the bilayer thickness increases slightly when the fraction of PE is increased relative to PC (71). Although the increase in membrane viscosity is difficult to quantify, our data strongly suggest that the lateral mobility of proteins in PE-containing membranes is mainly determined by the bulk properties such as viscosity (e.g., DN is nearly constant as a function of DOPE) rather than specific interaction between lipids and proteins.
In conclusion, the lateral mobility of GltT and LacY is affected differently in membranes with hydrophobic mismatch. Whereas the diffusion of LacY is in line with the Saffmann-Delbrück model, GltT mobility exhibits abnormal thickness-dependence. This anomaly is not likely due to changes in conformation (or aggregation state), as this would require unrealistic alterations in the hydrodynamic radius of the protein. We propose that the structure of the bilayer is altered by the inclusion of GltT and that the diffusion no longer follows a logarithmic dependence of the hydrodynamic radius of protein. By varying the DOPG and/or DOPE concentration in the membrane (at the expense of DOPC), it becomes evident that specific lipid-protein interactions do not play a determining role in the mobility of the proteins. Instead, the viscosity of the membrane seems to be the major determinant of lateral diffusion.
Acknowledgments
We acknowledge financial support from The Netherlands Organisation for Scientific Research (Top-Subsidy grant 700.56.302), SysMo via the Biotechnology and Biological Sciences Research Council-funded KosmoBac program coordinated by Ian R. Booth (Aberdeen, UK), and the Zernike Institute for Advanced Materials. We thank Prof. H. R. Kaback from the University of California, Los Angeles, for a gift of LacY (plasmid pT7-5).
References
- 1.Oursel D., Loutelier-Bourhis C., Lange C.M. Lipid composition of membranes of Escherichia coli by liquid chromatography/tandem mass spectrometry using negative electrospray ionization. Rapid Commun. Mass Spectrom. 2007;21:1721–1728. doi: 10.1002/rcm.3013. [DOI] [PubMed] [Google Scholar]
- 2.Oursel D., Loutelier-Bourhis C., Lange C.M. Identification and relative quantification of fatty acids in Escherichia coli membranes by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:3229–3233. doi: 10.1002/rcm.3177. [DOI] [PubMed] [Google Scholar]
- 3.Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh T.J., Simon S.A. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 2006;35:177–198. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher M.S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- 6.Sprong H., van der Sluijs P., van Meer G. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2001;2:504–513. doi: 10.1038/35080071. [DOI] [PubMed] [Google Scholar]
- 7.Andersen O.S., Koeppe R.E., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 8.Perozo E., Kloda A., Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 9.Owen D.M., Williamson D., Gaus K. Quantitative microscopy: protein dynamics and membrane organization. Traffic. 2009;10:962–971. doi: 10.1111/j.1600-0854.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 10.Frick M., Schmidt K., Nichols B.J. Modulation of lateral diffusion in the plasma membrane by protein density. Curr. Biol. 2007;17:462–467. doi: 10.1016/j.cub.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 11.Caffrey M., Feigenson G.W. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981;20:1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- 12.East J.M., Lee A.G. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 1982;21:4144–4151. doi: 10.1021/bi00260a035. [DOI] [PubMed] [Google Scholar]
- 13.Johannsson A., Smith G.A., Metcalfe J.C. The effect of bilayer thickness on the activity of (Na+ + K+)-ATPase. Biochim. Biophys. Acta. 1981;641:416–421. doi: 10.1016/0005-2736(81)90498-3. [DOI] [PubMed] [Google Scholar]
- 14.Cornelius F., Turner N., Christensen H.R. Modulation of Na,K-ATPase by phospholipids and cholesterol. II. Steady-state and presteady-state kinetics. Biochemistry. 2003;42:8541–8549. doi: 10.1021/bi034532e. [DOI] [PubMed] [Google Scholar]
- 15.In 't Veld G., Driessen A.J., Konings W.N. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim. Biophys. Acta. 1991;1065:203–212. doi: 10.1016/0005-2736(91)90231-v. [DOI] [PubMed] [Google Scholar]
- 16.Uratani Y., Wakayama N., Hoshino T. Effect of lipid acyl chain length on activity of sodium-dependent leucine transport system in Pseudomonas aeruginosa. J. Biol. Chem. 1987;262:16914–16919. [PubMed] [Google Scholar]
- 17.Pilot J.D., East J.M., Lee A.G. Effects of bilayer thickness on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry. 2001;40:8188–8195. doi: 10.1021/bi0103258. [DOI] [PubMed] [Google Scholar]
- 18.Criado M., Eibl H., Barrantes F.J. Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor-mediated ion translocation. J. Biol. Chem. 1984;259:9188–9198. [PubMed] [Google Scholar]
- 19.Carruthers A., Melchior D.L. Human erythrocyte hexose transporter activity is governed by bilayer lipid composition in reconstituted vesicles. Biochemistry. 1984;23:6901–6911. doi: 10.1021/bi00321a096. [DOI] [PubMed] [Google Scholar]
- 20.Dumas F., Tocanne J.F., Lebrun M.C. Consequences of hydrophobic mismatch between lipids and melibiose permease on melibiose transport. Biochemistry. 2000;39:4846–4854. doi: 10.1021/bi992634s. [DOI] [PubMed] [Google Scholar]
- 21.Yuan C., O'Connell R.J., Treistman S.N. Regulation of the gating of BKCa channel by lipid bilayer thickness. J. Biol. Chem. 2007;282:7276–7286. doi: 10.1074/jbc.M607593200. [DOI] [PubMed] [Google Scholar]
- 22.White S.H., Wimley W.C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 23.Mouritsen O.G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys. J. 1984;46:141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb R.J., East J.M., Lee A.G. Hydrophobic mismatch and the incorporation of peptides into lipid bilayers: a possible mechanism for retention in the Golgi. Biochemistry. 1998;37:673–679. doi: 10.1021/bi972441+. [DOI] [PubMed] [Google Scholar]
- 25.Freites J.A., Tobias D.J., White S.H. Interface connections of a transmembrane voltage sensor. Proc. Natl. Acad. Sci. USA. 2005;102:15059–15064. doi: 10.1073/pnas.0507618102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White S.H., von Heijne G. Transmembrane helices before, during, and after insertion. Curr. Opin. Struct. Biol. 2005;15:378–386. doi: 10.1016/j.sbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Killian J.A., Nyholm T.K. Peptides in lipid bilayers: the power of simple models. Curr. Opin. Struct. Biol. 2006;16:473–479. doi: 10.1016/j.sbi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.van Klompenburg W., Nilsson I., de Kruijff B. Anionic phospholipids are determinants of membrane protein topology. EMBO J. 1997;16:4261–4266. doi: 10.1093/emboj/16.14.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Campbell H.A., Dowhan W. Phospholipids as determinants of membrane protein topology. Phosphatidylethanolamine is required for the proper topological organization of the γ-aminobutyric acid permease (GabP) of Escherichia coli. J. Biol. Chem. 2005;280:26032–26038. doi: 10.1074/jbc.M504929200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W., Bogdanov M., Dowhan W. Reversible topological organization within a polytopic membrane protein is governed by a change in membrane phospholipid composition. J. Biol. Chem. 2003;278:50128–50135. doi: 10.1074/jbc.M309840200. [DOI] [PubMed] [Google Scholar]
- 31.Dowhan W., Bogdanov M. Lipid-dependent membrane protein topogenesis. Annu. Rev. Biochem. 2009;78:515–540. doi: 10.1146/annurev.biochem.77.060806.091251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seu K.J., Cambrea L.R., Hovis J.S. Influence of lipid chemistry on membrane fluidity: tail and headgroup interactions. Biophys. J. 2006;91:3727–3735. doi: 10.1529/biophysj.106.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groeneveld M., Slotboom D.J. Rigidity of the subunit interfaces of the trimeric glutamate transporter GltT during translocation. J. Mol. Biol. 2007;372:565–570. doi: 10.1016/j.jmb.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 34.Ermolova N.V., Smirnova I.N., Kaback H.R. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 35.Slotboom D.J., Sobczak I., Lolkema J.S. A conserved Serine-rich stretch in the glutamate transporter family forms a substrate-sensitive reentrant loop. Proc. Natl. Acad. Sci. USA. 1999;96:14282–14287. doi: 10.1073/pnas.96.25.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majumdar D.S., Smirnova I., Kaback H.R. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc. Natl. Acad. Sci. USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramadurai S., Holt A., Poolman B. Lateral diffusion of membrane proteins. J. Am. Chem. Soc. 2009;131:12650–12656. doi: 10.1021/ja902853g. [DOI] [PubMed] [Google Scholar]
- 38.Doeven M.K., Folgering J.H., Poolman B. Distribution, lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys. J. 2005;88:1134–1142. doi: 10.1529/biophysj.104.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Knol J., Veenhoff L., Poolman B. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem. 1996;271:15358–15366. doi: 10.1074/jbc.271.26.15358. [DOI] [PubMed] [Google Scholar]
- 41.Girard P., Pécréaux J., Bassereau P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 2004;87:419–429. doi: 10.1529/biophysj.104.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaillard I., Slotboom D.J., Konings W.N. Purification and reconstitution of the glutamate carrier GltT of the thermophilic bacterium Bacillus stearothermophilus. Biochemistry. 1996;35:6150–6156. doi: 10.1021/bi953005v. [DOI] [PubMed] [Google Scholar]
- 43.Magde D., Elson E.L., Webb W.W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974;13:29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- 44.Petrásek Z., Schwille P. Precise measurement of diffusion coefficients using scanning fluorescence correlation spectroscopy. Biophys. J. 2008;94:1437–1448. doi: 10.1529/biophysj.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abramson J., Smirnova I., Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 46.Yernool D., Boudker O., Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 47.Lewis B.A., Engelman D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 1983;166:211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- 48.Kahya N., Schwille P. How phospholipid-cholesterol interactions modulate lipid lateral diffusion, as revealed by fluorescence correlation spectroscopy. J. Fluoresc. 2006;16:671–678. doi: 10.1007/s10895-006-0108-6. [DOI] [PubMed] [Google Scholar]
- 49.Ramadurai S., Holt A., Poolman B. Influence of hydrophobic mismatch and amino acid composition on the lateral diffusion of transmembrane peptides. Biophys. J. 2010 doi: 10.1016/j.bpj.2010.05.042. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahidullah K., London E. Effect of lipid composition on the topography of membrane-associated hydrophobic helices: stabilization of transmembrane topography by anionic lipids. J. Mol. Biol. 2008;379:704–718. doi: 10.1016/j.jmb.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saffman P.G., Delbrück M. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saffman P.G. Brownian motion in thin sheets of viscous fluid. J. Fluid Mech. 1976;73:593–602. [Google Scholar]
- 53.Kucerka N., Tristram-Nagle S., Nagle J.F. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 2005;208:193–202. doi: 10.1007/s00232-005-7006-8. [DOI] [PubMed] [Google Scholar]
- 54.Kucerka N., Liu Y., Nagle J.F. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using x-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 2005;88:2626–2637. doi: 10.1529/biophysj.104.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damodaran K.V., Merz K.M. Head group water interactions in lipid bilayers—a comparison between DMPC-based and DLPE-based lipid bilayers. Langmuir. 1993;9:1179–1183. [Google Scholar]
- 56.Jensen M.O., Mouritsen O.G. Lipids do influence protein function—the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta. 2004;1666:205–226. doi: 10.1016/j.bbamem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Krepkiy D., Mihailescu M., Swartz K.J. Structure and hydration of membranes embedded with voltage-sensing domains. Nature. 2009;462:473–479. doi: 10.1038/nature08542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aranda-Espinoza H., Berman A., Safran S. Interaction between inclusions embedded in membranes. Biophys. J. 1996;71:648–656. doi: 10.1016/S0006-3495(96)79265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brannigan G., Brown F.L. A consistent model for thermal fluctuations and protein-induced deformations in lipid bilayers. Biophys. J. 2006;90:1501–1520. doi: 10.1529/biophysj.105.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett M., D'Rozario R., Yeagle P.L. Asymmetric stability among the transmembrane helices of lactose permease. Biochemistry. 2006;45:8088–8095. doi: 10.1021/bi060355g. [DOI] [PubMed] [Google Scholar]
- 61.Yeagle P.L., Bennett M., Watts A. Transmembrane helices of membrane proteins may flex to satisfy hydrophobic mismatch. Biochim. Biophys. Acta. 2007;1768:530–537. doi: 10.1016/j.bbamem.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 62.le Coutre J., Narasimhan L.R., Kaback H.R. The lipid bilayer determines helical tilt angle and function in lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1997;94:10167–10171. doi: 10.1073/pnas.94.19.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes N., Ginter C., Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462:880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gekko K., Noguchi H. Compressibility of globular-proteins in water at 25°C. J. Phys. Chem. 1979;83:2706–2714. [Google Scholar]
- 65.Liu N.I., Kay R.L. Redetermination of the pressure dependence of the lipid bilayer phase transition. Biochemistry. 1977;16:3484–3486. doi: 10.1021/bi00634a030. [DOI] [PubMed] [Google Scholar]
- 66.Hochmuth R., Worthy P., Evans E. Measurement of membrane viscosity. Biophys. J. 1978;21:A27. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naji A., Levine A.J., Pincus P.A. Corrections to the Saffman-Delbruck mobility for membrane bound proteins. Biophys. J. 2007;93:L49–L51. doi: 10.1529/biophysj.107.119222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Damodaran K.V., Merz K.M., Jr. A comparison of DMPC- and DLPE-based lipid bilayers. Biophys. J. 1994;66:1076–1087. doi: 10.1016/S0006-3495(94)80889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vries A.H., Mark A.E., Marrink S.J. The binary mixing behavior of phospholipids in a bilayer: a molecular dynamics study. J. Phys. Chem. B. 2004;108:2454–2463. [Google Scholar]
- 71.Leekumjorn S., Sum A.K. Molecular simulation study of structural and dynamic properties of mixed DPPC/DPPE bilayers. Biophys. J. 2006;90:3951–3965. doi: 10.1529/biophysj.105.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]


