Abstract
A pernicious paradox in human motivation is the occasional reduced performance associated with tasks and situations that involve larger-than-average rewards. Three broad explanations that might account for such performance decrements are attentional competition (distraction theories), inhibition by conscious processes (explicit-monitoring theories), and excessive drive and arousal (overmotivation theories). Here, we report incentive-dependent performance decrements in humans in a reward-pursuit task; subjects were less successful in capturing a more valuable reward in a computerized maze. Concurrent functional magnetic resonance imaging revealed that increased activity in ventral midbrain, a brain area associated with incentive motivation and basic reward responding, correlated with both reduced number of captures and increased number of near-misses associated with imminent high rewards. These data cast light on the neurobiological basis of choking under pressure and are consistent with overmotivation accounts.
Contingencies such as competition, presence of an audience, and high reward can sometimes have a detrimental influence on human performance (Baumeister, 1984; Bonner & Spinkle, 2002). Spectacular examples of such impaired performance can be observed in major sporting events, in which highly skilled players sometimes perform catastrophically when on the brink of victory (Jackson & Beilock, 2008). Often called “choking under pressure,” this phenomenon extends beyond sport. For example, similar effects may be seen when highly capable students experience exam anxiety and perform poorly in mathematical problem solving (Beilock & DeCaro, 2007). Moreover, studies from behavioral economics demonstrate that high reward contingencies can result in less-than-optimal performance on a number of tasks, particularly those that involve motor learning and cognitive skill (Ariely, Loewenstein, & Mazar, in press). The neural basis of such underperformance when contingencies have high monetary value has, to date, not been demonstrated.
Researchers have suggested several possible explanations for the paradoxical effects of high rewards, each leading to different predictions about underlying brain activity (Beilock, 2007). Top-down attentional-distraction (Landers, 1980; Nideffer, 1992) and explicit-monitoring (Carver & Scheier, 1978; Jackson & Beilock, 2008) theories predict increases in activity in, for example, working memory systems. According to these theories, top-down interference—for example, from competition (Heaton & Sigall, 1991) or the presence of an audience (Wallace, Baumeister, & Vohs, 2005)—is responsible for the performance decrements associated with high rewards. Such interference consumes working memory load and interrupts proceduralized routines (Beilock, 2007).
Incentive-based, or overmotivation, theories predict that reduced performance is tied to excessive arousal and activity in basic reward pathways, in which “instinctive” mechanisms might interfere with more optimal decision making, which involves working memory or attention (Gladwell, 2000; Short & Sorrentino, 1986). Although the mechanisms accounting for the detrimental effect of high motivation on performance are unclear, it is known that people’s attention becomes more narrow when they are aroused and that such narrowed attention reduces the ability to see the whole picture or to plan ahead (Easterbrook, 1959). This perspective suggests that an increase in reward-circuitry activity associated with high reward incentives may disrupt attention and executive function, resulting in performance decrements.
To investigate the relationship between high and low imminent rewards and performance, we designed a task in which subjects were required to chase an artificial prey around a computerized maze, and were rewarded with either a small (£0.50 ≈ $1) or a large (£5.00 ≈ $10) amount of money for capturing the prey. Our principle aims were to investigate if large reward contingencies impair performance on this task, and, if so, whether they are associated with a shift in activity from prefrontal control areas to more impulsive midbrain systems. Our results showed that increased activity in midbrain is strongly correlated to performance decrements and near-misses induced by high rewards.
METHOD
Subjects
Nineteen healthy subjects underwent functional magnetic resonance imaging (fMRI). All were English speaking, had normal or corrected vision, and had no history of psychiatric or neurological problems. All subjects gave informed consent, and the study was approved by the joint ethics committee of the National Hospital for Neurology and Neurosurgery (University College London Hospital National Health Service Trust) and the Institute of Neurology, University College London. One subject was excluded from the analysis because of poor behavioral performance during scanning, data from 3 subjects were not analyzed because of technical problems with the scanner, and 1 subject was excluded for being left-handed. Thus, the final sample included a total of 14 right-handed subjects (mean age = 25.9 years, SD = 3.9).
Experimental Task
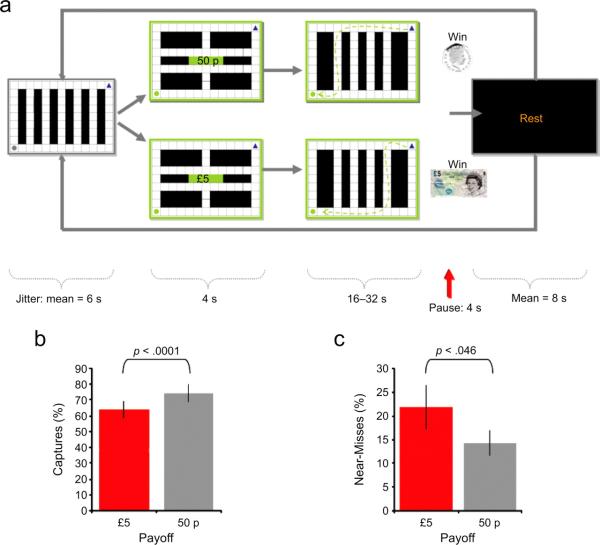
Each trial commenced with a neutral period, during which a preprogrammed artificially intelligent agent (a gray circle) appeared at the bottom left of the maze (Fig. 1a). A blue triangle marking the subject’s position appeared at the upper right, but could not yet be moved. The artificial agent was programmed to wander the maze indiscriminately and was presented on average for 6 s (jitter = ±2 s). The circle then started to flash (alternating between green and gray), signaling that the artificial agent was about to become the artificial prey. The flashing lasted for 2 s, during which time the artificial agent continued to wander the maze indiscriminately. Next, a 2-s display indicated the amount of money the subject would receive (i.e., £0.50 or £5.00) if he or she captured the artificial prey. Once the message indicating the reward level disappeared from the screen, the subject could move the blue triangle and start chasing the artificial prey. If the subject caught the prey, the screen showed a “win” message for 4 s, and then the screen turned black for an average duration of 8 s. Then, the next neutral period began. The maximum duration of the chase was 16 to 32 s (determined randomly), and the trial ended if there was no capture during this time. Twenty low-payoff trials and 18 high-payoff trials (2 missing because of program error) were presented in random order.
Fig. 1.
Schematic representation of the experimental paradigm and behavioral results. Each trial of the task (a), began with a neutral period, during which subjects viewed an artificial agent of no intrinsic value (represented by a gray circle) wandering the maze. Next, they were informed that they would begin chasing an artificial prey (represented by a green circle) and were told whether the payoff for catching the prey would be low (50 pence) or high (£5). Subjects then began to pursue the prey; their position in the maze was indicated by a blue triangle. At the end of this phase, a blank rest screen appeared for an average of 8 s before the next neutral phase began. The task was interleaved with a separate task in which subjects were pursued by an artificial predator (Mobbs et al., 2007). The graphs show the (c) percentage of successful captures and (d) percentage of near-misses for low- and high-payoff preys.
Movement of the Artificially Intelligent Prey
A recursive breadth-first flood-fill search algorithm was implemented to control the behavior of the artificial prey (Russell & Norvig, 2003). All valid positions (i.e., not wall blocks) that were adjacent to the current position in the maze (maximum = 4) were considered for the next movement, and the distance from the subject’s position was computed for each. Then, the position the furthest from the subject’s blue triangle was chosen as the next position for the artificial prey. For mazes with no dead ends, as were used in this study, this is the optimal strategy for the escaping artificial prey. To dissociate spatial and temporal elements of imminence, and also allow for more variation in distance for the parametric analyses, we introduced a small jitter to the speed of the artificial prey, which randomly changed from the starting speed every 4 s.
Speed Calibration
During practice trials (see the next paragraph), we determined the speed at which each subject could catch the artificial prey at least 50% of the time. For each subject, the artificial prey was programmed to be about 10% slower than this calibrated speed.
Procedure
Subjects used a keypad to navigate the blue triangle and were given time to practice the experimental task in and out of the scanner. After each 10-min practice, the number of times the subject caught the artificial prey was calculated, and the speed of the prey was reduced if the capture rate was low. Subjects practiced the task outside the scanner until they could fully control the navigation of the blue triangle and could catch the artificial prey more than 50% of the time, so that learning effects during scanning would be diminished. Subjects then practiced inside the scanner. The experimental task was interleaved with a separate task in which the subject was pursued by an artificial predator (described in Mobbs et al., 2007). Following the experiment, we used a questionnaire to explore how motivated the subjects were to acquire the money.
Measures
The percentage of trials on which subjects caught the artificial prey, at both levels of monetary reward, was recorded. A near-miss was arbitrarily defined as the subject’s blue triangle coming within two squares of the artificial prey and then falling back more than seven squares away.
fMRI Acquisition and Analysis
A 3-T Allegra head scanner with standard transmit-receive head coil was used to acquire functional data with a single-shot gradient echo isotropic high-resolution echo-planar imaging (EPI) sequence. The matrix size was 128 × 128, with a field of view of 192 × 192 mm2 and an in-plane resolution of 1.5 × 1.5 mm2. Fifty slices with interleaved acquisition and a slice thickness of 1.5 mm, with no gap between slices, were used. Echo time was 30 ms, and asymmetric echo shifted forward by 26 phase-encoding lines. Acquisition time per slice was 102 ms, with a repetition time of 5,100 ms and echo spacing of 560 μs. The receiver bandwidth was 250 kHz, with a 30% ramp sampling and twofold read oversampling to allow for k-space regridding. A z-shim gradient-compensation prepulse of −1.4 mT/m*ms was used, with a read gradient amplitude of 34.47mT/m and a read gradient slew rate of 344.7 mT/m/ms. In order to maximize statistical power, we used only 50 slices that were optimized to cover the brainstem and angled at −30° to cover the anterior cingulate cortex (ACC) and medial orbitofrontal cortex (mOBFC). The slice tilt, z-shim, and high spatial resolution further reduced susceptibility-related signal loss in the mOBFC (Deichmann, Gottfried, Hutton, & Turner, 2005). In addition, field maps using a double-echo fast low-angled shot (FLASH) sequence were recorded for correction of susceptibility-related geometric distortions in the EPI images. A high-resolution T1-weighted structural scan was obtained for each subject (1-mm isotropic resolution) and coregistered to the subject’s mean EPI image. The mean of all individual structural images permitted the anatomical localization of the functional activations at the group level.
Statistical parametric mapping (SPM2; Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) was used to preprocess all fMRI data and included correction of motion and EPI distortion, spatial normalization, and smoothing (see Mobbs et al., 2007, for additional details). Statistical analysis was performed to determine each subject’s voxel-wise activation while chasing the artificial prey. Parametric analysis was modeled with delta functions representing onsets convolved with the canonical hemodynamic response function and time derivative to provide for a varying lag in the event-related blood-oxygenation-level-dependent (BOLD) signal. Contrasts included the effect of distance from the artificial prey (the distance from the subject to the artificial prey, in squares, modeled every second) as a parametric regressor. We tested the interaction between prey proximity and reward magnitude by calculating the difference between the distance regressors in the two payoff conditions (£5.00 condition minus £0.50 condition). Therefore, the kind of interaction tested for is slightly different from the interactions in factorial design structures. Random-effects analysis (Penny & Friston, 2003) was used for group statistics. For a priori hypothesized regions, we used a statistical threshold of prep = .99 uncorrected, and when significance was not reached at the uncorrected level, we used a threshold of prep = .88 (small-volume-corrected, or SVC). In addition, only clusters involving 30 or more contiguous voxels are reported. The a priori regions of interest were ventromedial striatum (VMS), dorsolateral striatum (DLS), midbrain, amygdala, mOBFC, ventromedial prefrontal cortex, and ACC (Bunzeck & Düzel, 2006; O’Doherty et al., 2003; O’Doherty, Dayan, Friston, Critchley, & Dolan, 2004).
RESULTS
Behavioral Results
Consistent with choking under pressure, the data showed that subjects were less successful in catching the high-payoff prey (M = 63.9%, SD = 19.9%) than in catching the low-payoff prey (M = 74.3%, SD = 19.4%), t(13) = 5.19, prep = .99, d = 0.53 (Fig. 1b). To explore this finding further, we quantified near-misses, defined as instances in which the subject, having been within two squares of the prey, dropped back to a distance of more than seven squares, an indication of an erroneous action. We found significantly more missed turns in the high-payoff condition (M = 21.8%, SD = 17.4%) than in the low-payoff condition (M = 14.3%, SD = 9.6%), t(13) = 2.210, prep = .88, d = 0.56 (Fig. 1c).
Neuroimaging Results
We first assessed the parametric effect of distance (measured as squares in the maze) on reward systems. As subjects approached both high- and low-payoff prey, activity increased in the DLS (£5.00: x = 27, y = −1, z = 18; Z = 3.67, prep = .99 uncorrected; £0.50: x = 26, y = 4, z = 16; Z = 3.22, prep = .986 uncorrected) and right mOBFC (£5.00: x = 12, y = 45, z = −10; Z = 3.24, prep = .99 uncorrected; £0.50: x = 15, y = 54, z = −16; Z = 3.92, prep = .99 uncorrected). Moreover, both high- and low-payoff prey elicited activity in the VMS that increased with increasing proximity of the prey (£5.00: x = 4, y = 6, z = −10; Z = 3.05, prep = .99 uncorrected; £0.50: x = 14, y = 17, z = −4; Z = 4.49, prep = .986 uncorrected) and rostral ACC (£5.00: x = −4, y = 40, z = 2; Z = 4.08, prep = .99 uncorrected; £0.50: x = −3, y = 35, z = 11; Z = 3.32, prep = .99 uncorrected). Although these results confirm that basic reward systems are engaged by the task, they do not specifically isolate those regions that integrate the prey’s proximity and value.
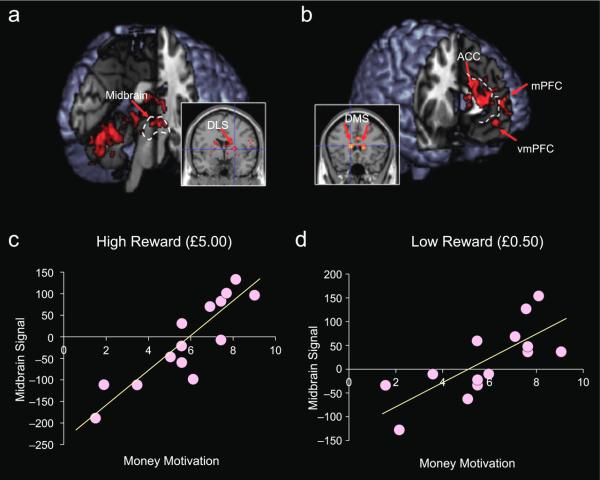
We next identified activity corresponding to the interaction between prey proximity (parametric effect of reducing prey distance) and reward magnitude. That is, we tested for brain areas in which the increase in activity with decreasing distance was significantly greater when subjects were chasing the high-payoff prey than when they were chasing the low-payoff prey. This analysis revealed activity in the left ventral midbrain, encompassing the ventral tegmental area/substantia nigra (Fig. 2a), right DLS (x = 23, y = 0, z = 5; Z = 4.86, prep = .99 whole-brain-corrected; Fig. 2a), and bilateral ventral premotor area (left: x = −50, y = 5, z = 10; Z = 3.86, prep = .99 whole-brain-corrected; right: x = 5, y = 16, z = 5; Z = 3.76, prep = .99 whole-brain-corrected). These results suggest that these regions are specifically involved in computing reward incentive as a function of goal distance. Regions in which the increase in activity with decreasing distance was greater for low than for high reward) were the right ACC, medial prefrontal cortex (mPFC; x = 1, y = 63, z = 15; Z = 3.90, prep = .99 uncorrected; Fib. 2b), and dorsomedial striatum (left: x = −13, y = 22, z = 12; Z = 3.71, prep = .99 uncorrected; right: x = 20, y = 30, z = 1; Z = 3.68, prep = .99 uncorrected; see Fig. 2b).
Fig. 2.
Brain regions showing differential effects of the artificial prey’s proximity in the high- and low-payoff conditions and correlations of the effect of proximity with money motivation. In (a), the highlighting indicates regions in which the effect of proximity on activation was greater for the high-payoff prey than for the low-payoff prey; in (b), the highlighting indicates regions in which the effect of proximity on activation was greater for the low-payoff prey than for the high-payoff prey. The scatter plots illustrate the correlations between money motivation and the increase in midbrain activation with increasing proximity of the (c) high-payoff prey and (d) low-payoff prey. ACC = anterior cingulate cortex; DLS = dorsolateral striatum; DMS = dorsomedial striatum; mPFC = medial prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
Individual Differences
Financial Motivation
A regression analysis revealed a positive association between financial motivation (i.e., subjects’ ratings of how much they wanted the money per se; M = 57.8%, SD = 22.2%) and the main effect of proximity to the high-payoff prey on midbrain activation (x = 7, y = −18, z = −10; Z = 4.16, prep = .99 SVC; Fig. 2c); a similar midbrain pattern was found for the low-payoff prey (x = 1, y = −14, z = −14; Z = 3.26, prep = .99 uncorrected; Fig. 2d). Directly subtracting the effect of proximity in the low-money condition from the effect of proximity in the high-money condition (i.e., high-reward prey minus low reward-prey) revealed a positive correlation between financial motivation and midbrain activity (x = 6, y = −25, z = −12; Z = 3.14, prep = .99 uncorrected). The opposite contrast revealed a correlation between financial motivation and activity in the rostral ACC (x = −7, y = 51, z = 8; Z = 4.09, prep = .99 uncorrected).
Performance
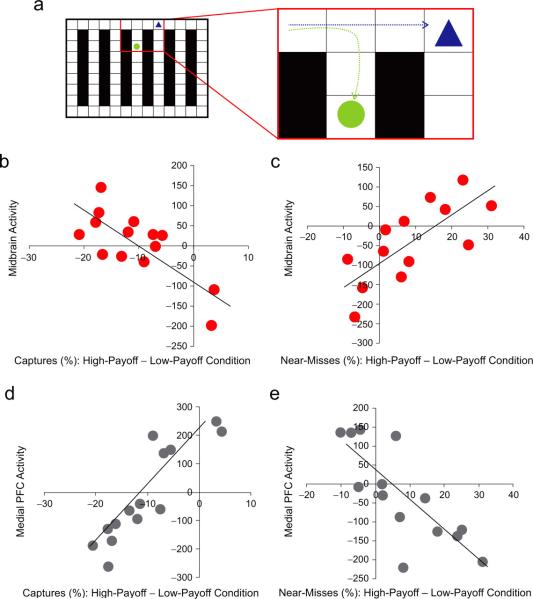
To directly examine the key relationship between brain activity and task performance (i.e., captures), we calculated correlations between the brain activity encoding the Proximity × Reward interaction and subject-specific behavioral measures of performance. Taking overall performance (i.e., number of captures) as a subject-specific covariate, we found that increasing ventral midbrain activity was positively correlated with reduced performance (x = 6, y = −25, z = −12; Z = 3.14, prep = .99 SVC) for high compared with low reward (Fig. 3b). In contrast, increased activity in the mPFC (x = −8, y = 30, z = 17; Z = 3.48, prep = .99 uncorrected) predicted better performance for high than for low reward (Fig. 3d).
Fig. 3.
Example of an error in performing the task (a) and subject-specific correlations between performance and activation in the midbrain and medial prefrontal cortex (medial PFC; b–e). In the illustrated chase (a), the subject (triangle) was following the path of the prey (circle), but missed a turn. An error was classified as a near-miss if the subject was within two squares of the prey and made a mistake resulting in falling back seven squares from the prey. The upper graphs illustrate the correlation between midbrain activity associated with proximity and (b) the difference between the percentage of captures in the high- and low-payoff conditions and (c) the difference in the percentage of near-misses in the high- and low-payoff conditions. The lower graphs illustrate the correlation between medial PFC activity associated with proximity and (d) the difference between the percentage of captures in the high- and low-payoff conditions and (e) the difference between the percentage of near-misses in the high- and low-payoff conditions.
Performance was also measured in terms of the frequency of near-misses (Fig. 3a), and this index, too, was correlated with brain activity. Paralleling the results for the number of captures, analysis of near-misses revealed that an increase in near-misses in the high-payoff condition relative to the low-payoff condition was associated with increased activity in ventral midbrain (see Fig. 3c), as well as in DLS (x = 25, y = 13, z = 11; Z = 4.31, prep = .99 uncorrected). Conversely, a decrease in near-misses in the high-payoff condition was associated with increased activity in the mPFC (x = −2, y = 49, z = 18; Z = 3.34, prep = .99 uncorrected; Fig. 3e), ventromedial prefrontal cortex (x = 8, y = 53, z = −5; Z = 3.88, prep = .99 SVC), and right ventrolateral prefrontal cortex (x = 48, y = 38, z = −12; Z = 3.62, prep = .99 uncorrected).
DISCUSSION
Choking under pressure occurs in situations in which the desire for optimal performance is maximum (Beilock, 2007; Jackson & Beilock, 2008). Our behavioral results show that high reward contingences result in less-than-optimal performance. The effect of proximity on midbrain activity was significantly greater in the high-payoff condition than in the low-payoff condition, and there was a strong correlation between how much subjects wanted the money and ventral midbrain response to proximity for both high- and low-payoff prey. Both of these results support the midbrain’s role in incentive motivation (Tobler, Fletcher, Bullmore, & Schultz, 2007). Critically, activity in ventral midbrain was strongly correlated with performance decrements induced by high, relative to low, reward. Midbrain activity was also correlated with an increase in near-misses. Given the absence of top-down distractors, such as an audience or competition, our findings support an overmotivation, or incentive-based, account of choking.
Studies show that execution of well-learned sensorimotor skills is highly susceptible to performance decrements, which might be accounted for by poor execution focus (Beilock, 2007; Beilock & DeCaro, 2007). Although working memory is prone to interference from task-irrelevant cues, such as distraction, it plays an important role in attentional focus and task execution (Ashcraft & Kirk, 2001). Why incentive-based motivation causes performance deficits is open to argument; however, the anatomical location of the activity we observed (i.e., ventral midbrain and striatum) is consistent with a dopaminergic basis. Dopamine is implicated in increased motivational vigor (Dalley et al., 2007; Schultz, 2007; Wise, 2004) and increased sensitivity to positive outcomes, yet can impair performance (Frank, Seeberger, & O’Reilly, 2004; Murphy, Arnsten, Goldman-Rakic, & Roth, 1996). One possibility that could be explored further is whether incentive-based motivation results in increased attentional narrowing (Easterbrook, 1959) or simply actions without foresight (Robbins, 2002).
Alternatively, it is conceivable that high rewards are framed in terms of losses in some situations, such that performance decreases in conditions of high reward might arise predominantly from aversive states, such as anxiety. Anxiety can occupy working memory devoted to skill execution and in turn reduce performance (Ashcraft & Kirk, 2001). It seems quite plausible that the performance of an individual who strongly expects to attain a goal is driven by fear of loss, rather than by the excitement of possible success. Serotonin has been implicated in the mediation of such anticipatory anxiety, and there is good evidence for an opposition between serotonin and dopamine in reward-motivated behavior (Daw, Kakade, & Dayan, 2002).
An incentive-based account of performance in this task might predict that increased cortical control is associated with increased performance. Indeed, in this study, increased activation in regions of prefrontal cortex, notably medial and lateral prefrontal cortex, predicted better performance (i.e., more captures; Fig. 3d) and reduced susceptibility to incentive-induced errors (Fig. 3e) in the high-payoff condition relative to the low-payoff condition. This suggests that the mPFC may exert an opposing influence over ventral midbrain in controlling performance (Ridderinkhof, Ullperger, Crone, & Nieuwenhuis, 2004). It is worth noting that the mPFC is directly connected to the midbrain (i.e., ventral tegmental area; Au-Young, Shen, & Yang, 1999), and that these regions have been frequently implicated in cognitive control, in which more explicit representations of goals guide performance in complex tasks (Pessoa, 2008). Monitoring of performance errors is critical for the ability to shift performance strategies, and thus the mPFC, through possible interactions with the lateral prefrontal cortex, may be involved in on-line behavioral adjustments (Ridderinkhof et al., 2004).
CONCLUSION
Clearly, many variables contribute to choking under pressure, but the fact that our task yielded performance decrements in a controlled experimental setting suggests that a simple incentive-based account may be one of the core explanations in more complex situations. Indeed, similar emotional explanations are implicated in other deleterious influences of reward on economic behavior across a variety of rewarding tasks (Beilock, 2007; Beilock & DeCaro, 2007; Camerer, Loewenstein, & Prelec, 2005). One striking finding of this study is that a high reward that is a remarkably modest amount of money can impair performance in a relatively simple motor task. Our findings have implications for making sense of the conditions that elicit suboptimal performance in sport and vocational pursuits.
Acknowledgments
We thank Predrag Petrovic, Marcus Gray, Cindy Hagan, and Christian Buchel for helpful comments and help with the experimental setup. This research was supported by the Wellcome Trust. D.M. is supported by a Brain Research Trust Prize Studentship and by the MRC. R.J.D. is a Wellcome Trust Programme Grant holder. C.D.F. is supported by the Wellcome Trust and the Danish National Research Foundation.
REFERENCES
- Ariely DG, Loewenstein G, Mazar N. Large stakes and big mistakes. Quarterly Journal of Economics. (in press) [Google Scholar]
- Ashcraft MH, Kirk EP. The relationships among working memory, math anxiety, and performance. Journal of Experimental Psychology: General. 2001;130:224–237. doi: 10.1037//0096-3445.130.2.224. [DOI] [PubMed] [Google Scholar]
- Au-Young SMW, Shen H, Yang CR. Medial prefrontal cortical output neurons to the ventral tegmental area (VTA) and their responses to burst-patterned stimulation of the VTA: Neuroanatomical and in vivo electrophysiological analyses. Synapse. 1999;34:245–255. doi: 10.1002/(SICI)1098-2396(19991215)34:4<245::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Choking under pressure: Self-consciousness and paradoxical effects of incentives on skillful performance. Journal of Personality and Social Psychology. 1984;46:610–620. doi: 10.1037//0022-3514.46.3.610. [DOI] [PubMed] [Google Scholar]
- Beilock SL. Choking under pressure. In: Baumeister RF, Vohs KD, editors. Encyclopedia of social psychology. Sage; Thousand Oaks, CA: 2007. pp. 140–141. [Google Scholar]
- Beilock SL, DeCaro MS. From poor performance to success under stress: Working memory, strategy selection, and mathematical problem solving under pressure. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:983–998. doi: 10.1037/0278-7393.33.6.983. [DOI] [PubMed] [Google Scholar]
- Bonner SE, Sprinkle GB. The effects of monetary incentives on effort and task performance: Theories, evidence, and a framework for research. Accounting Organizations and Society. 2002;27:303–345. [Google Scholar]
- Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Camerer C, Loewenstein G, Prelec D. Neuroeconomics: How neuroscience can inform economics. Journal of Economic Literature. 2005;43:9–64. [Google Scholar]
- Carver CS, Scheier MF. Self-focusing effects of dispositional self-consciousness, mirror presence, and audience presence. Journal of Personality and Social Psychology. 1978;36:324–332. [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Networks. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2005;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effects of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–202. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gladwell M. The art of failure: Why some people choke and others panic. The New Yorker. 2000 Aug 21;:84–92. [Google Scholar]
- Heaton AW, Sigall H. Self-consciousness, self-presentation, and performance under pressure: Who chokes, and when. Journal of Applied Social Psychology. 1991;21:175–188. [Google Scholar]
- Jackson R, Beilock SL. Attention and performance. In: Farrow D, Baker J, MacMahon C, editors. Developing elite sports performers: Lessons from theory and practice. Routledge; New York: 2008. pp. 104–118. [Google Scholar]
- Landers DM. The arousal-performance relationship revisited. Research Quarterly for Exercise and Sport. 1980;51:77–90. doi: 10.1080/02701367.1980.10609276. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant J, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: Threat imminence elicits prefrontal-periaqueductal grey shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proceedings of the National Academy of Sciences, USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nideffer RM. Psyched to win. Leisure Press; Champaign, IL: 1992. [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Penny WH, Friston K. Random effects analysis. In: Frackowiak RSJ, Friston K, Frith C, Dolan R, Price CJ, Zeki S, et al., editors. Human brain function. Academic Press; London: 2003. pp. 843–861. [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robbins TW. ADHD and addiction. Nature Medicine. 2002;8:24–25. doi: 10.1038/nm0102-24. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Norvig P. Artificial intelligence: A modern approach. 2nd ed Prentice Hall; Upper Saddle River, NJ: 2003. [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in Neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Short JAC, Sorrentino RM. Achievement, affiliation, and group incentives: A test of the overmotivation hypothesis. Motivation and Emotion. 1986;10:115–131. [Google Scholar]
- Tobler PN, Fletcher PC, Bullmore ET, Schultz W. Learning-related human brain activations reflecting individual finances. Neuron. 2007;54:167–175. doi: 10.1016/j.neuron.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wallace HM, Baumeister RF, Vohs KD. Audience support and choking under pressure: A home disadvantage? Journal of Sports Sciences. 2005;23:429–438. doi: 10.1080/02640410400021666. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]