The cardiovascular system (CVS) is complex and spatially distributed. The many connections between the system’s components enable efficient regulation of blood flow through a closed system of vessels.
How do we approach the understanding of this complex system? Basically, there are two ways: a microscopic and a macroscopic approach. In the microscopic approach, one tries to analyze the function of each part of the system in great detail. The macroscopic approach, on the other hand, is interested in the collective behavior of all parts. Both approaches are necessary to gain a complete understanding, and the problem is where to start. Microscopic analysis of large complex systems inevitably results in a large system of differential equations. However, there are cases when a system that looks very complicated on the microscopic level exhibits rather simple macroscopic behavior [1], [2].
We show that the CVS is an example of such system. In the next section, we introduce the coupled nonlinear oscillators approach, the framework that we use for our studies of cardiovascular and brain oscillations. As background, we describe the human CVS and present results of time-frequency analysis using wavelet transforms of several noninvasive measurements of cardiovascular signals. Studies of neuronal oscillations have been undertaken since the first human electroencephalographic (EEG) recording, and the recent resurgence of interest in neuronal oscillations has led to an enhanced appreciation of their likely importance and to “the tantalizing conjecture that perception, memory and even consciousness could result from the synchronization of neuronal networks” [3]. The frequency scales for the neuronal oscillations, as presently revised, are presented in the “Neuronal Oscillations” section. Recently, new studies have been initiated to determine the interactions between the cardiovascular oscillations and some of the brain waves [4]. This work is reviewed in the penultimate section, and causal relations between the cardio, respiratory, and brain waves are discussed with a special emphasis on detection of the depth of anesthesia. The final section looks forward to the future and enumerates some of the open questions.
Coupled Oscillators
The coupled nonlinear oscillators approach is marked by two major milestones: the introduction of the entrainment of collective oscillators by Winfree [5] and its analysis using the phase dynamics approach of Kuramoto [6]. Phase dynamics is obtained by reducing the number of degrees of freedom of the original dynamical system. The original dynamics should be perturbed weakly by noise, an external force, or coupling to dynamics with a limit-cycle orbit. The latter applies to a dissipative system, and the form of the phase dynamics is not dependent on the form of the original models. The work of Winfree [5] and Kuramoto [6] further motivated the introduction of the theory of phase synchronization, facilitating studies of the interactions between coupled nonlinear and chaotic oscillators [7]. Coupled oscillators were proposed as a possible description of the dynamics of the CVS [8], [9], and synchronization and modulation between cardiac and respiratory oscillations were examined with particular care [10]-[12]. The emerging picture motivated additional studies, and methods for analysis of the direction of coupling among interacting oscillatory processes have recently been proposed (see [13] and the references therein).
Cardiovascular Oscillations
The System
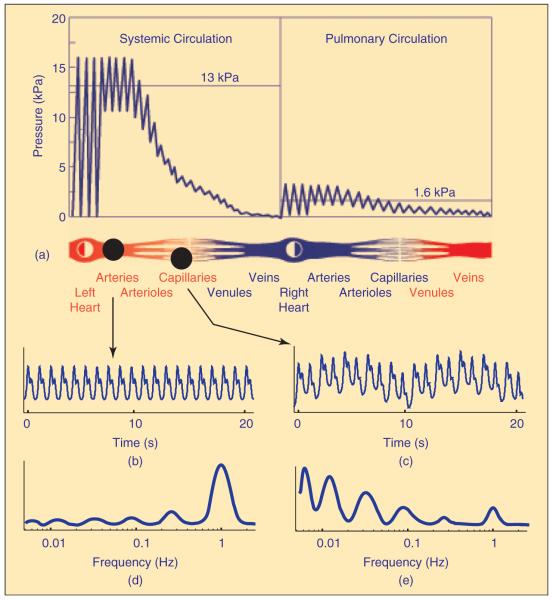
The CVS, which consists of the heart and blood vessels, has one major function—transport. The total volume of blood circulates along the CVS in one minute on average. The circulatory system can be divided into two parts: the pulmonary circulation, which moves blood through the lungs for exchange of oxygen and carbon dioxide, and the systemic circulation, which supplies all other tissues (Figure 1). The systolic and diastolic blood pressures have long been known to differ between the arterial and venous parts and between the systemic and pulmonary circulation [14].
Fig. 1.
The pulmonary and systemic circulation and the pressure distribution (modified from Folkow and Neil [14]). The maximum is known as the systolic and the minimum as the diastolic pressure. In addition, on the basis of recent measurements and analysis, the blood flow through the aorta and the capillaries and the amplitudes of their time-averaged wavelet transforms are indicated. While the amplitude of the oscillations generated by the heart as the main pump into the system dominates the aortic flow (d), the amplitude and the corresponding power of oscillations in the capillary flow result from the central and peripheral regulatory mechanisms acting on a wide frequency scale (e). The six spectral peaks are usually estimated from 30-min recordings, while, for the sake of clarity, only 20-sec segments are presented here (for details see, e.g., [9], [15], [16]).
Observed on the macroscopic level, the heart acts as a pump that drives the blood through a closed circuit of elastic vessels. The respiratory activity is a generator of pressure that assists in the return of blood to the heart. The flow of blood also depends on the resistance of the vessels that is controlled by adjustment of their diameters. Consequently, the power of cardiac oscillations dominates the aortic flow and is significantly decreased in blood flow through the capillaries.
Oscillations
Time-frequency analysis of signals derived from respiration, cardiac function, and blood flow revealed the existence of five almost periodic frequency components in the frequency interval 0.0095–2.0 Hz [9], [15]. Recently, a sixth oscillatory component was identified in the interval 0.005–0.0095 Hz [16]. The frequency intervals are summarized in Figure 2.
Fig. 2.

The characteristic frequencies of cardiovascular oscillations in humans in the frequency interval from 0.005 to 2 Hz as defined or used in [9] and [15]-[21].
The cardiac and respiratory oscillations have frequencies of around 1.0 and 0.3 Hz, respectively. They originate centrally and are propagated through the system. In contrast, the low-frequency oscillations involved in the regulation of the vessels’ resistance are generated locally. However, it is the continuous circulation of blood through the system of closed tubes that coordinates the local oscillatory activity of each individual mechanism and evidently synchronizes it for much of the time. Hence, each physiological mechanism manifests as a single almost periodic process that we can observe at the macroscopic level. Consequently, the peaks in the low-frequency interval are broadened and can be best distinguished using logarithmic frequency resolution.
The physiological origin of the low-frequency oscillations has been investigated using laser Doppler flowmetry (LDF). The oscillations at around 0.1, 0.04, 0.01, and 0.007 Hz, respectively, have thus been associated with the intrinsic myogenic activity of vascular smooth muscle, the neurogenic activity of the vessel wall, and two different mechanisms of vascular endothelial function [17], [18]. Nitric oxide and endothelium-derived hyperpolarizing factors are hypothesized to be involved in the oscillations near 0.01 and 0.007 Hz [16]; however, the precise mechanisms giving rise to them need to be further elucidated.
These results suggest that the CVS as a whole, including the microcirculation, can usefully be treated as a single entity. In terms of frequencies, it is irrelevant at which point we observe the system or which function we choose to measure: each regulatory mechanism is reflected on every site, and can be detected in each cardiovascular function; however, its amplitude may differ with respect to the function and the site of observation (see Figure 1). Moreover, the characteristic frequencies of the oscillatory components are shown to be confined to the same intervals in resting healthy subjects as in resting subjects with cardiovascular diseases [15]. The power within each interval can be used as a quantitative measure for characterizing the state of the system [9], [15].
Clinical and experimental studies have included patients with diabetes mellitus, myocardial infarction, and cardiac failure. The perturbations of the cardiovascular oscillations brought about by exercise, anesthesia, and aging have also been examined. For example, based on the logarithmic frequency resolution of the wavelet transform of the heart rate variability (HRV) signal, cardiac autonomic dysregulation was detected [19] in diabetic patients prior to clinical signs of cardiac autonomic neuropathy. Anesthesia is another state where clinical application of the cardiovascular oscillations approach may be significant. In a recent study [20], a substantial decrease of the low-frequency components was demonstrated in the blood flow recorded by LDF during local anesthesia.
The existence of characteristic peaks leads to the inference that each subsystem can be described mathematically as an oscillator [21], [22]. The systems are mutually dependent via couplings that lead to amplitude/frequency fluctuations and hence further broadening of the characteristic peaks.
Interactions
Simultaneous measurements of the cardiac and respiratory functions enable analysis of the cardiorespiratory interactions. The resultant modulation of the cardiac frequency, known as respiratory arrhythmia, has long been known to play an essential role in the overall performance of the system. Synchronization analysis [7], [10], [11] has confirmed that, in a conscious healthy subject at rest, the two systems can synchronize. We have shown that synchronization and modulation can coexist [11] and that the respiratory system is the driving system at all respiration frequencies, whether paced or spontaneous [13]. Two different methods, time-phase bispectral analysis [23] and a new inference technique [24], were used to demonstrate the existence of a nonlinear cardiorespiratory interaction.
Phase synchronization between the cardiac and respiratory oscillations has been investigated during anesthesia in rats [9]. Synchrograms and the time-evolution of synchronization indices were used to show that the system passes reversibly through a sequence of different phase-synchronized states as the anesthesia level changes, indicating that it can undergo phase transition-like phenomena. It was found that the synchronization state may be used to characterize the depth of anesthesia.
Neuronal Oscillations
Studies of the interactions between the cardiovascular oscillations and brain waves were then initiated [4]. The frequency scales for the neuronal oscillations in the rat cortex, as recently revised by Buzsáki and Draguhn [3], are summarized in Figure 3. Neuronal oscillations span an even wider frequency interval than cardiovascular oscillations, covering frequencies from 0.025 to 600 Hz.
Fig. 3.
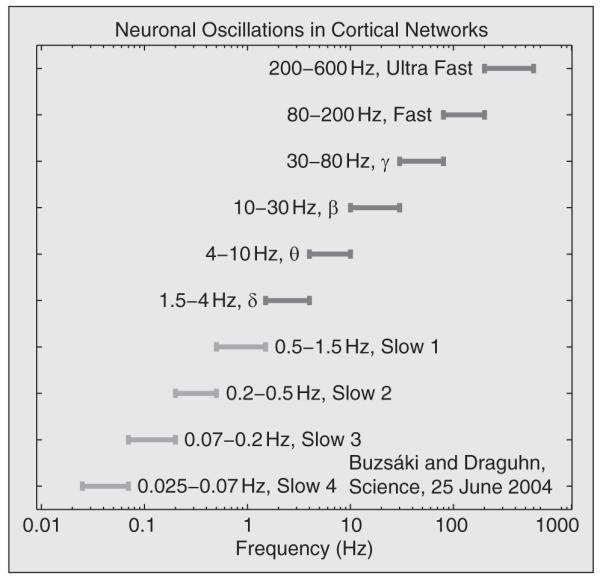
The frequency scales of neuronal oscillations in rats as recently revised by Buzsaki and Draguhn [3]. The conventional frequency intervals consisting of δ, θ, β, and γ bands are extended to incorporate fast and ultrafast γ waves and several slow δ waves.
Interactions Between Neuronal and Cardiovascular Oscillations
Experiments were performed on ten adult, male Wistar rats, each weighing approximately 250 g. The animals were anesthetized with a single intraperitoneal injection of ketamine hydrochloride [45 mg/kg body weight (b.w.)] and xylazine hydrochloride (7 mg/kg b.w.) and placed in a darkened Faraday cage. The depth of anesthesia was assessed at 5-min intervals by a nociceptive stimulus, the skin pinch-test, applied to the sole of the animal’s front paw. Simultaneous recordings were made of EEG over the left and right parietal cortex, of the electrical activity of the heart (electrocardiogram), and of respiration measured with a piezo probe attached over the animal’s chest. The three signals were sampled at 1 KHz with 16 b resolution. The study will be presented in detail elsewhere [4], while here we summarize briefly the main results.
The instantaneous phases of the cardiac (i.e., HRV) and respiratory oscillations were obtained by the marked events method, f (t) = 1/(tk+1 – tk), where tk and tk+1 are the times of two consecutive peaks. The instantaneous phases were then obtained as φ(t) = 2π((t – tk)/(tk+1 – tk)) + 2πk, tk≤t<tk+1, linearly interpolated, and resampled with Δt = 0.05 s. The analytic signal concept was used to obtain the instantaneous frequency corresponding to the slow-1 δ-oscillations in the EEG. An analytic signal ζ(t) was constructed from the original time series. This is a complex function of time defined as ζ(t) = s(t) + lsH = A(t)elφ(t), where A(t) is the amplitude, φ(t) is the phase of the signal s(t), and sH(t) is its Hilbert transform. The instantaneous phase is given by φ(t) = arctan (sH(t)/s(t)).
For inferring information about the coupling, we use a method based on information theory [10] that enabled us to evaluate both the strength and asymmetry (directionality) of the coupling. The reliability of these measures was checked through the use of surrogate data.
In all ten rats, injection of the single bolus of anesthetic resulted in two distinctly different stages of anesthesia. The first period is characterized by the presence of strong slow-1 δ-oscillations, but no significant presence of any other oscillations in the EEG. During the second period, slow-1 δ-oscillations are markedly decreased.
The causal relationships between slow-1 δ, cardiac, and respiratory oscillations during deep anesthesia are presented in Figure 4. Their statistical significance is checked using surrogate data (for details, see [4]). It is evident that respiration drives the cardiac oscillations strongly, whereas there is no significant influence in the opposite direction. On the other hand, the respiratory and slow-1 δ-oscillations are bidirectionally coupled; however, statistically significant evidence only for respiration driving the cortical δ-oscillations is established. Finally, the slow-1 δ-oscillations drive the cardiac activity, but without any evidence of significant influence in the opposite direction. No signature of cardiac-δ interactions is obtained for any of the rats, and there is a high probability of there being no driving in either direction. Note that the analysis is based on the maximum possible window length. This means that possible short episodes of interaction will not be observed, but makes for a more reliable estimate of the average interaction.
Fig. 4.

Interactions between cardiac (C), respiratory (r), and slow-1 δ EEG (δ) oscillations in rats anesthetised by ketamine-xylazine during deep anesthesia. The strengths of coupling are indicated by the number of stars.
We conclude that interactions occur between the oscillatory processes, both within and between the cardiovascular and the neuronal systems. The strengths and directions of these interactions may be used, in principle, for characterization of the state of the organism as demonstrated here for the case of deep anesthesia.
Perspective and Open Questions
We have briefly discussed the oscillatory nature of the CVS and the neuronal activity in the brain. We have also mentioned that there exist deterministic interactions among these two vital systems, the CVS providing for energy transfer through the organism and the brain serving its information-processing needs. Although a clear picture is starting to emerge, the list of open questions is still very long and we will now list some of them.
-
•
The origin of time-variability: There is a clear signature of oscillations in both systems. However, their amplitudes and frequencies vary in time. At present, the origin of these variations is unclear. One plausible explanation of their origin is that each of the oscillatory components observed at macroscopic level results from an ensemble of oscillators at microscopic level that are spatially distributed and not fully synchronized. To illustrate this possibility, let us take two extreme examples: the cardiac and the endothelial oscillations. The cardiac oscillatory component is maintained through the activation of thousands of cardiac cells. They are spatially localized and their activity is paced by stimuli from the sinoatrial node. Thus, the activity of the cardiac cells is largely synchronized and their synchrony is manifested as a single, distinct, oscillatory component at the system level. Endothelial cells, on the other hand, are distributed throughout the whole CVS, forming an average human network >40,000 km in length. Although the blood circulation through the system synchronizes their activity most of the time, the effect of this spatial distribution results in strong amplitude and frequency variability of their oscillatory components. The other important source of variability most probably comes from an interaction between the ensembles of oscillators themselves.
-
•
The nature of interactions: The evidence that the CVS oscillations interact with each other is growing. It is also becoming obvious that the interactions change with disease. At present, however, the phases of low-frequency oscillatory components cannot be reliably traced in time and hence the nature of their interactions is completely unknown. We can possibly study some of the characteristics of the interactions using models. Indeed, several types of oscillator have been proposed as the basic oscillator, ranging from Poncaré, Lienard, Van der Pol, to FitzHugh-Nagumo. Again, it is not obvious what is the benefit of each of this oscillators and whether, in fact, the simplest, Poincaré, oscillator possesses all the important properties needed for a basic oscillator, or the features of the other are essential in the modeling of the cardiovascular dynamics.
-
•
The cardiorespiratory interactions: The fact that the two systems interact has been known for centuries [25], [26], but the exact pathways of interaction are still not understood, although much progress has been made. With the theory of generalized synchronization, and the recently introduced algorithms for analysis of the direction of coupling, we may expect further rapid progress. However, the problem is difficult, in part because of the huge amount of work already done and the diversity of opinions already established. Conventional wisdom is often difficult to overcome; but in this case, the need is clear [27], [28].
-
•The origin of low-frequency oscillations and their potential connection to the immune system: To facilitate the resolution of this question several other problems have to be addressed as well.
-
•Most of what is known about the low-frequency oscillatory components is based on measurements by LDF [17], [18], [29]. Although this technique is noninvasive and simple to use, as applied to the microvascular flow presently, it has a clear drawback, that is, the vascular area from which the reading is taken cannot be straight-forwardly defined. With a laser emitting with a wavelength of 780 nm it is generally assumed that a 1 mm3 hemisphere is being observed. However, to determine the number and the type of vessels within this area, one needs an imaging system that will enable visualization of the vascular content within the hemisphere. Such an imaging system is presently not available.
-
•The role of the venous flow in the CVS oscillations is practically unknown. While the heart is the main pump in the arterial system, preliminary results based on invasive monitoring of venous pressure have demonstrated that the respiratory pump dominates the venous flow [30]. Further work is needed to elucidate the involvement of the venous flow in CVS oscillations.
-
•Lymphatic oscillations have recently been discussed [31]. Mechanisms similar to the ones responsible for the vascular oscillations seems to be involved—originating from the myogenic and endothelial regulation. Whether or not the lymphatic oscillations have something in common with the cardiovascular oscillations is an intriguing yet fully open question.
-
•
-
•
The functional unit between the cardiovascular and neuronal oscillations: The results briefly discussed earlier (but in more detail in [4]) have indicated the existence of a functional linkage between certain oscillations of the two systems. Little is known about this interaction. At the same time, an avalanche of studies points to an existence of structural linkage between the two systems. The astrocytes, pericytes, and other forms of glial cells [32], [33] are being found to play a structural link between the vasculature and the neurons. Basically, they bring substances, mainly electrolytes, from the blood vessels to the neurones, which they then use to maintain membrane potential and to enable action potentials. If a similar avalanche could be initiated to reveal the functional link, one might expect that the picture of the cardiovascular and brain interactions will soon become clear. Simple and useful applications of this knowledge in diagnosis and prediction may be expected to follow. For an understanding of this functional link, nonlinear and stochastic dynamics techniques will be crucial. Here, as well as with the other open questions discussed earlier, the role of physics and engineering will be essential.
Acknowledgments
This work has been supported by the Wellcome Trust (U.K.), EPSRC (U.K.), ARRS (Slovenia), and the EC NEST-Pathfinder project BRACCIA (Brain, Respiratory and Cardiac Causalities in Anesthesia). This article is based in part on a presentation made at the Special Symposium Coordination of Physiological Rhythms at the 28th IEEE International Conference of the Engineering in Medicine and Biology Society, New York, August 2006. The author is grateful to the Royal Society of London for a travel grant. She warmly acknowledges fruitful collaboration and numerous useful discussions over the past years with Peter McClintock and with all coauthors listed later in the reference list. She greatly enjoys working with numerous young collaborators and is looking forward to the work to come.
Biography

Aneta Stefanovska completed her Ph.D. in 1992 combining biocybernetics and synergetics, working partly in Ljubljana and partly in Stuttgart. She then introduced the coupled oscillators approach to cardiovascular dynamics and invested much energy in the improvement of data acquisition and analysis. She headed the Nonlinear Dynamics and Synergetics Group in Ljubljana from 1993. In 2006, she came to Lancaster University as reader in medical physics where she is developing major new initiatives involving the application of nonlinear dynamics to biology and medicine.
References
- [1].Haken H. Cooperative phenomena in systems far from thermal equilibrium and in nonphysical systems. Rev. Mod. Phys. 1975;47(1):67–121. [Google Scholar]
- [2].Haken H. Synergetics, An Introduction. Springer-Verlag; Berlin: 1983. [Google Scholar]
- [3].Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- [4].Musizza B, Stefanovska A, McClintock PVE, Paluš M, Petrovčič J, Ribarič S, Bajrović FF. Interactions between cardiac, respiratory and EEG-delta oscillations in rats during anaesthesia. J. Physiol. London. 2007;580(1):315–326. doi: 10.1113/jphysiol.2006.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Winfree AT. Biological rhythms and the behavior of populations of coupled oscillators. J. Theor. Biol. 1967;16(1):15. doi: 10.1016/0022-5193(67)90051-3. [DOI] [PubMed] [Google Scholar]
- [6].Kuramoto Y. Chemical Oscillations, Waves, and Turbulence. Springer-Verlag; Berlin: 1984. [Google Scholar]
- [7].Pikovsky A, Rosenblum M, Kurths J. Synchronization—A Universal Concept in Nonlinear Sciences. Cambridge Univ. Press; Cambridge, U.K.: 2001. [Google Scholar]
- [8].Stefanovska A, Krošelj P. Correlation integral and frequency analysis of cardiovascular functions. Open Syst. Inform. Dynam. 1997;4(4):457–478. [Google Scholar]
- [9].Stefanovska A, Bračič M. Physics of the human cardiovascular system. Contemp. Phys. 1999;40(1):31–55. [Google Scholar]
- [10].Schäfer C, Rosenblum MG, Kurths J, Abel HH. Heartbeat synchronised with ventilation. Nature. 1998;392(6673):239–240. doi: 10.1038/32567. [DOI] [PubMed] [Google Scholar]
- [11].Lotrič MB, Stefanovska Synchronization and modulation in the human cardiorespiratory system. Physica A. 2000;283(3–4):451–461. [Google Scholar]
- [12].Stefanovska A, Haken H, McClintock PVE, Hožič M, Bajrović F, Ribarič S. Reversible transitions between synchronization states of the cardiorespiratory system. Phys. Rev. Lett. 2000;85(22):4831–4834. doi: 10.1103/PhysRevLett.85.4831. [DOI] [PubMed] [Google Scholar]
- [13].Paluš M, Stefanovska A. Direction of coupling from phases of interacting oscillators: An information-theoretic approach. Phys. Rev. E, Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2003;67(5):055201(R). doi: 10.1103/PhysRevE.67.055201. [DOI] [PubMed] [Google Scholar]
- [14].Folkow B, Neil E. Circulation. Oxford Univ. Press; New York: 1971. [Google Scholar]
- [15].Bračič M, McClintock PVE, Stefanovska A. Characteristic frequencies of the human blood distribution system. In: Broomhead DS, Luchinskaya EA, McClintock PVE, Mullin T, editors. Stochastic and Chaotic Dynamics in the Lakes. American Institute of Physics; Melville, NY: 2000. pp. 146–153. [Google Scholar]
- [16].Kvandal P, Landsverk SA, Bernjak A, Stefanovska A, Kvernmo HD, Kirkebøen K-A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc. Res. 2006;72(3):120–127. doi: 10.1016/j.mvr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [17].Kvernmo HD, Stefanovska A, Kirkebøen K-A, Kvernebo K. Oscillations in the human cutaneous blood perfusion signal modified by endothelium-dependent and endothelium-independent vasodilators. Microvasc. Res. 1999;57(3):298–309. doi: 10.1006/mvre.1998.2139. [DOI] [PubMed] [Google Scholar]
- [18].Söderström T, Stefanovska A, Veber M, Svenson H. Involvement of sympathetic nerve activity in skin blood flow oscillations in humans. Am. J. Physiol. Heart Circ. Physiol. 2003;284(5):H1638–H1646. doi: 10.1152/ajpheart.00826.2000. [DOI] [PubMed] [Google Scholar]
- [19].Urbančič-Rovan V, Meglič B, Stefanovska A, Bernjak A, Ažman-Juvan K, Kocijančič A. Incipient diabetic cardiovascular autonomic imbalance revealed by wavelet analysis of heart rate variability. Diabet. Med. 2007;24(1):18–26. doi: 10.1111/j.1464-5491.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- [20].Landsverk SA, Kvandal P, Kjelstrup T, Bernjak A, Benko U, Stefanovska A, Kvernmo H, Kirkebøen K-A. Human skin microcirculation after brachial plexus block evaluated by wavelet transform of laser Doppler flowmetry signals. Anesthesiology. 2006;105(3):478–484. doi: 10.1097/00000542-200609000-00010. [DOI] [PubMed] [Google Scholar]
- [21].Stefanovska A, Bračič Lotrič M, Strle S, Haken H. The cardiovascular system as coupled oscillators? Physiol. Meas. 2001;22(3):535–550. doi: 10.1088/0967-3334/22/3/311. [DOI] [PubMed] [Google Scholar]
- [22].Stefanovska A, Luchinsky DG, McClintock PVE. Modelling couplings among the oscillators of the cardiovascular system. Physiol. Meas. 2001;22(3):551–564. doi: 10.1088/0967-3334/22/3/312. [DOI] [PubMed] [Google Scholar]
- [23].Jamšek J, Stefanovska A, McClintock PVE. Nonlinear cardiorespiratory interactions resolved by time-phase bispectral analysis. Phys. Med. Biol. 2004;18(8):4407–4425. doi: 10.1088/0031-9155/49/18/015. [DOI] [PubMed] [Google Scholar]
- [24].Smelyanskiy VN, Luchinsky DG, Stefanovska A, McClintock PVE. Inference of a nonlinear stochastic model of the cardiorespiratory interaction. Phys. Rev. Lett. 2005;94(9):098101. doi: 10.1103/PhysRevLett.94.098101. [DOI] [PubMed] [Google Scholar]
- [25].Hales S. Statistical Essays II, Hæmastatisticks. Innings Manby; London, U.K.: 1773. [Google Scholar]
- [26].Ludwig C. Beiträge zur Kenntniss des Einflusses der Respirationsbewegungen auf den Blatlauf im Aortensysteme. Arch. Anat. Physiol. Wiss. Med. 1847;13:242–302. [Google Scholar]
- [27].Eckberg DL. Sympathovagal balance—A critical appraisal. Circulation. 1997;96(9):3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- [28].Eckberg DL. Point: Counterpoint: Cardiovascular variability is/is not an index of autonomic control of circulation. J. Appl. Physiol. 2006;101(2):688. doi: 10.1152/japplphysiol.00584.2006. [DOI] [PubMed] [Google Scholar]
- [29].Stern MD. In vivo evaluation of microcirculation by coherent light-scattering. Nature. 1975;254(5495):56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- [30].Stefanovska A, Bračič M. Reconstructing cardiovascular dynamics. Control Eng. Pract. 1999;7(2):161–172. [Google Scholar]
- [31].van Helden DF, Hosaka K, Imtiaz MS. Rhythmicity in miccrocirculation. Clin. Hemorheol. Microcirc. 2006;34(1–2):59–66. [PubMed] [Google Scholar]
- [32].Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- [33].Peppiatt CM, Howarth C, Mobbs CP, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]