Abstract
Objective:
Alzheimer disease (AD) and frontotemporal lobar degeneration (FTLD) are hypothesized to cause clinically distinct forms of primary progressive aphasia (PPA) that predominantly affect expressive speech. AD is thought to cause logopenic progressive aphasia (LPA), and FTLD may cause progressive nonfluent aphasia (PNFA). We sought to determine the value of clinical characterization, neuropsychological analysis, and MRI atrophy in predicting pathology of LPA and PNFA.
Methods:
Patients with LPA (n = 19) and patients with PNFA (n = 19) were evaluated with neuropsychological assessments, structural MRI, CSF analysis, and neuropathologic examination.
Results:
Twelve of 19 patients with LPA (63%) and 6 of 19 patients with PNFA (32%) had neuropathologic findings or CSF biomarkers consistent with AD. Neuropsychological testing showed that naming was more impaired in patients with AD, and letter-guided fluency was more affected in patients with a non-AD disorder. Voxel-based morphometry analysis revealed that in patients with AD, patients with LPA and PNFA had significant posterior-superior temporal atrophy; in patients with non-AD, patients with LPA had peri-Sylvian atrophy and patients with PNFA had dorsolateral prefrontal and insular atrophy. Receiver operator characteristic curve analysis showed that combining neuropsychological testing with MRI atrophy pattern had 90% specificity for pathology or CSF biomarkers consistent with AD, and combining clinical features with neuropsychological analysis had 100% sensitivity for pathology or CSF biomarkers consistent with AD.
Conclusions:
Neither PPA phenotyping nor imaging alone is a reliable predictor of pathology. Multimodal predictors, such as combining neuropsychological testing with MRI analysis, can improve noninvasive prediction of underlying pathology in nonfluent forms of PPA.
GLOSSARY
- AD
= Alzheimer disease;
- AUC
= area under the curve;
- BA
= Brodmann area;
- FTLD
= frontotemporal lobar degeneration;
- LPA
= logopenic progressive aphasia;
- PNFA
= progressive nonfluent aphasia;
- PPA
= primary progressive aphasia;
- ROC
= receiver operating characteristic;
- SemD
= semantic dementia;
- VBM
= voxel-based morphometry.
Primary progressive aphasia (PPA) represents a group of clinical syndromes that involve progressive decline in language functions.1–3 Clinically, 3 forms of progressive aphasic disorders are recognized: progressive nonfluent aphasia (PNFA) with dysfluency and agrammatism; logopenic progressive aphasia (LPA) with nonfluent speech and word-finding pauses; and semantic dementia (SemD) with fluent speech but impaired object knowledge.2,4,5 Patients with PNFA and LPA have reduced fluency (word output per minute), and can be considered to have a nonfluent form of PPA in comparison to SemD. Some clinicopathologic analyses have suggested that each nonfluent PPA syndrome marks a specific pathologic substrate. For example, FTLD-tau pathology underlies PNFA,6–8 while Alzheimer disease (AD) causes LPA.4,9–11 At the same time, PNFA can result from AD in clinicopathologic series,6,12 and FTLD has been implicated in LPA.11 Therefore, significant pathologic heterogeneity exists within each nonfluent PPA syndrome, and improved diagnostic biomarkers remain necessary.
In addition to clinical features, patterns of neuropsychological impairment13 and brain atrophy10 may be useful antemortem assessments for AD or FTLD. Relative differences in impairment across neuropsychological tests persist throughout disease in patients with clinically defined PNFA or LPA and autopsy-defined FTLD or AD,13 and PNFA and LPA are associated with distinct patterns of MRI cortical atrophy.4 However, it is unclear if any of these features is useful in the prediction of underlying pathology at the individual patient level. We examined the value of these modalities—individually and in combination—in predicting AD pathology or CSF biomarkers underlying LPA and PNFA.
METHODS
Protocol approval, registration, and patient consent.
All patients were recruited from the Department of Neurology at University of Pennsylvania School of Medicine. Protocols were approved by the Institutional Review Board, and written informed consent was obtained from all patients and designated guardians.
Subjects.
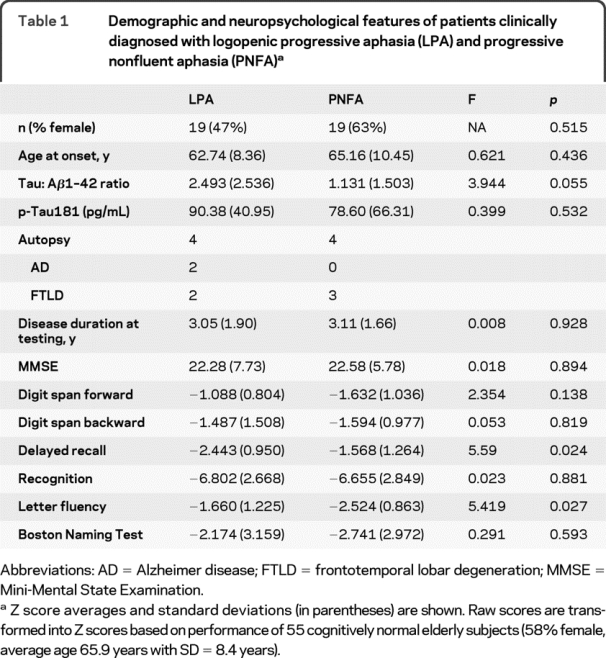
All patients with the diagnosis of LPA or PNFA were included if they underwent antemortem diagnostic lumbar puncture (n = 36) or had neuropathologic analysis at autopsy (n = 8; 4 also underwent lumbar puncture). Four patients with LPA and AD pathology at autopsy were not included for this study as they did not undergo detailed neuropsychological and high-resolution MRI analysis. All patients were evaluated prospectively by a neurologist with expertise in progressive aphasic syndromes (M.G.). Nineteen patients with LPA and 19 with PNFA were identified. Clinical diagnosis was based on modified published criteria.2,3,10 PNFA is diagnosed when there is effortful speech with agrammatism, dysarthria with or without apraxia of speech, and preserved word and object knowledge. LPA is diagnosed when there are significant word-finding pauses, impaired naming, impaired sentence repetition, and preserved word and object knowledge. The diagnosis of each patient was confirmed through a consensus mechanism. The 2 groups were similar in gender, age at onset, and disease duration at time of evaluation (table 1). Neuropsychological testing assessed language, memory, and executive and visual domains during the initial clinical visit as described previously (table 1).13 CSF was obtained at the time of clinical evaluation,14 and analyzed in duplicate using a sandwich ELISA for total tau (Innogenetics, Belgium) and Aβ1–42 levels. A CSF tau:Aβ42 ratio cutoff of 1.05 was predictive of AD pathology in our previous clinicopathologic cohort, achieving a sensitivity of 78.9% and specificity of 96.6% in distinguishing AD from FTLD.14 Thus, we included the CSF tau:Aβ42 ratio as an AD biomarker for patients without autopsy confirmation (n = 30).
Table 1 Demographic and neuropsychological features of patients clinically diagnosed with logopenic progressive aphasia (LPA) and progressive nonfluent aphasia (PNFA)
Voxel-based morphometry analysis.
High-resolution volumetric MRI was obtained in a subset of patients with LPA (n = 12) and patients with PNFA (n = 11) at the time of CSF and neuropsychological evaluation, and in age- and gender-matched cognitively normal controls. Images were acquired by a Siemens Trio 3-T MRI scanner. High-resolution axial T1-weighted 3-dimensional spoiled gradient echo images were acquired with repetition time = 1,620 msec, echo time = 3 msec, slice thickness = 1.0 mm, flip angle = 15°, matrix = 192 × 256, and in-plane resolution = 0.9 × 0.9 mm. Brain volumes were normalized to the MNI template and segmented using the segmentation algorithm implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm5/). Statistical comparisons of modulated gray matter volumes were conducted using a 2-sample t test in SPM5. After anatomic brain regions distinguishing between the 2 pathologic causes of nonfluent PPA were identified, the volume for each region was calculated by multiplying average signal intensity in the segmented brain region and template volume for that region. A ratio of adjusted volumes in regions reflecting AD or non-AD grouping membership (region atrophic in non-AD:region atrophic in AD) was calculated in each individual to account for relative atrophy pattern and interindividual intensity differences. Other patients underwent routine clinical diagnostic MRI, which were visually inspected to ensure that there was no alternate cause of PPA, although these images did not have sufficient spatial resolution to support volumetric analysis.
Neuropathologic examination.
Eight patients (4 LPA, 4 PNFA) had detailed neuropathologic examination at autopsy. As described previously,13 each case was examined by 2 board-certified neuropathologists (M.S.F., J.Q.T.) with extensive experience in neurodegenerative disorders. Neuropathologic diagnoses were established according to consensus criteria,15–18 with immunohistochemistry for phosphorylated tau (PHF1; Dr. P. Davies)19; β-amyloid (4G8; Senetek, Maryland Heights, MO); α-synuclein (Syn303)20; and TDP-43 (PolyTech, Chicago, IL). Semiquantitative ratings (0 = absent, 1 = mild, 2 = moderate, and 3 = severe) were used to assess the density of senile plaques, as well as tau-positive, α-synuclein-positive, and TDP-43-positive lesions.
Statistical analysis.
Statistical analysis was performed using SPSS 12.0 (Chicago, IL) unless otherwise specified. χ2 test and one-way analysis of variance were used to evaluate demographic features and neuropsychological performance. Neuropsychological measures were converted to Z scores in each individual relative to 55 age-matched and education-matched healthy control subjects. With such a conversion, relative performance could be compared across measures of different length and difficulty to derive significant deficits.
For clinicopathologic prediction, results from univariate analyses were integrated to create a multimodal scale with higher values suggestive of AD. For qualitative clinical characterization, features associated with AD (word-finding pauses, circumlocution, memory complaints) were each assigned a value of 1, and features associated with non-AD (effortful speech and agrammatism) were each assigned a value of −1; the sum of these scores formulated the composite clinical score. Neuropsychological raw scores were converted to Z scores. A composite neuropsychological score was created by subtracting BNT Z score (worse performance in AD) from the FAS Z score (worse performance in non-AD). For brain atrophy, a composite imaging score was derived by the ratio of volume in regions uniquely associated with atrophy in FTLD—inferior prefrontal cortex (Brodmann area [BA] 47) and insula—to volume in regions uniquely associated with atrophy in AD—angular gyrus (BA 39)—as defined by an automated atlas.21 Each score was individually analyzed in a receiver operating characteristic (ROC) curve analysis to derive the optimal cutoff for predicting AD pathology or CSF biomarker pattern. After ROC analysis of each predictor, combinations of 2 factors and all 3 factors were further tested in ROC analyses by summing predictor values. Areas under the curve (AUC) were compared across predictor combinations to derive the optimal sensitivity and specificity for predicting AD pathology of CSF biomarker.
RESULTS
Clinical, neuropsychological, and imaging analyses in syndromic LPA and PNFA.
The clinical characteristics of patients with LPA and patients with PNFA reflected their syndromic diagnosis, although there were overlapping features (figure 1). Syndromic diagnosis alone (LPA) has limited sensitivity (66.7%) and specificity (65%) for AD pathology or CSF biomarkers. Word-finding pauses (χ2 = 7.134, p = 0.019), impaired naming (χ2 = 5.700, p = 0.042), and circumlocution (χ2 = 3.800, p = 0.103) were more common in LPA. Dysarthria (χ2 = 10.378, p = 0.02), effortful speech (χ2 = 19.760, p < 0.001), and agrammatism (χ2 = 13.328, p = 0.001) were more common in PNFA.

Figure 1 Clinical characteristics of patients with clinically diagnosed logopenic progressive aphasia (LPA) and progressive nonfluent aphasia (PNFA)
*Significantly different characteristic between patients with LPA and patients with PNFA.
Neuropsychological evaluation revealed that, as a group, patients with LPA performed worse on delayed recall than patients with PNFA (F = 5.59, p = 0.024, table 1), and patients with PNFA performed worse on letter-guided category naming fluency than patients with LPA (F = 5.419, p = 0.027, table 1).
Analyses of MRI largely replicated reported differences between patients with LPA and patients with PNFA when compared with age- and gender-matched cognitively normal subjects. As a group, patients with LPA had cortical atrophy throughout the left temporal and parietal regions extending into the insula (table e-1 on the Neurology® Web site at www.neurology.org). Patients with PNFA had cortical atrophy most prominently along the left Sylvian fissure, and particularly involved anterior regions (table e-1).
Pathologic and biomarker analysis.
Analysis of these 38 patients revealed that AD and non-AD pathology each contributed to the clinical syndromes of LPA and PNFA. Among 19 patients with LPA, 12 (63.2%) had CSF biomarker (n = 11) or autopsy findings (n = 2, 1 with both autopsy and CSF) consistent with AD (LPA-AD, table 2). Among the rest of the patients with LPA, 2 had autopsy confirmation of FTLD-TDP (1 with a mutation in the progranulin gene). Patients with LPA with FTLD-TDP (LPA-FTLD) had higher lesion density in the temporal neocortex and angular gyrus compared to the frontal neocortex, but this pattern was not seen in AD (table e-2). The remaining patients had CSF biomarker patterns inconsistent with AD.
Table 2 Demographic and neuropsychological features of logopenic progressive aphasia (LPA) and progressive nonfluent aphasia (PNFA) patients according to their pathologic grouping
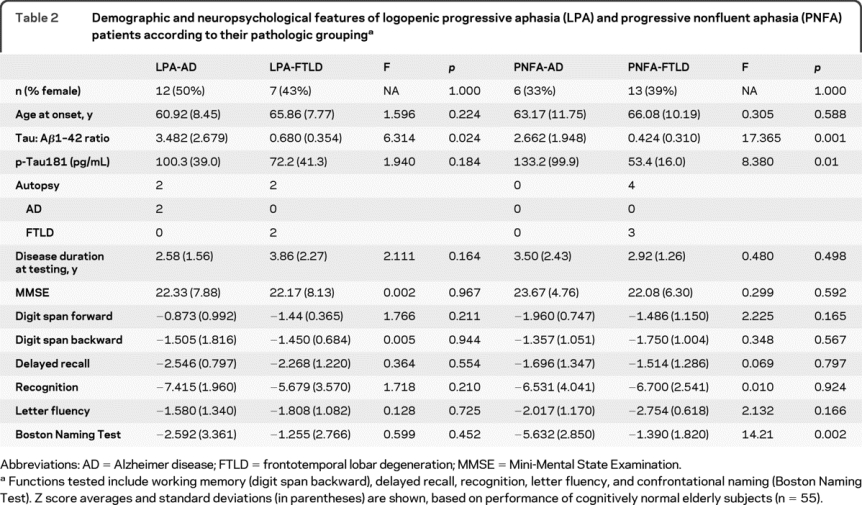
All 19 patients with PNFA had CSF analysis (table 2), with 6 patients (31.6%) having CSF tau:Aβ42 ratio >1.05 (PNFA-AD, none with autopsy). Of the remaining 13 patients with PNFA, 3 had autopsy-confirmed FTLD (2 with CBD and 1 with PSP), and 1 had Lewy body disease, which is a rare cause of PNFA in our center. Overall, 13 patients with PNFA (68.4%) had autopsy or CSF biomarker findings that are not consistent with AD pathology (PNFA-FTLD, table 2).
Clinical, neuropsychological, and imaging prediction of pathology or CSF biomarker consistent with AD.
We identified clinical, neuropsychological, and imaging features to combine into multimodal measures for distinguishing AD and FTLD. Qualitative clinical features of nonfluent PPA showed a trend associating AD with word-finding pauses (χ2 = 2.991, p = 0.130), circumlocution (χ2 = 2.702, p = 0.119), and memory complaints (χ2 = 2.788, p = 0.144), and associating FTLD with effortful speech (χ2 = 3.788, p = 0.087) and agrammatism (χ2 = 3.702, p = 0.096). These 5 features were used to formulate a composite clinical score for ROC analysis.
Neuropsychological analysis according to AD pathology or CSF biomarker pattern showed that patients with AD performed worse in confrontation naming (F = 6.060, p = 0.019), and patients with FTLD performed worse in letter-guided fluency (F = 3.402, p = 0.075). This is in keeping with our previous observation that pathologic AD cases performed worse in confrontation naming than in letter-guided fluency over time, regardless of the clinical syndromic diagnosis, with patients with FTLD having the opposite pattern.13 Given the group-level differences, we formulated a composite neuropsychological score by subtracting the Z score of confrontation naming from the Z score of letter-guided fluency for ROC analysis.
A subset of patients (6 LPA-AD, 6 LPA-FTLD, 5 PNFA-AD, 6 PNFA-FTLD) had high-resolution MRI studies for voxel-based morphometry (VBM) analysis (figure 2). VBM analysis showed that AD pathology or CSF biomarkers were associated with atrophy in the left temporal-parietal regions (green regions in figure 2, peak voxels in BA 7, 21, 37, 39, 40, p < 0.05, familywise error–corrected). FTLD pathology or biomarkers were associated with significant atrophy in the bilateral frontotemporal regions including left insula and BA47 (red regions in figure 2, table e-1). Analysis based on autopsy findings or CSF biomarkers associated atrophy in BA 37 (angular gyrus) with AD, and atrophy in left insula and BA 47 (inferior prefrontal cortex) with FTLD. Ratio of atrophy in these regions (insula + BA47:BA37) was used to create the composite atrophy score for each individual.
Figure 2 Regions of cortical atrophy according to clinical syndromic diagnosis or pathologic grouping compared with age- and gender-matched cognitively normal subjects (n = 24; 58.3% female, mean age at scan = 65.2 years, SD = 8.6 years)
Patients with logopenic progressive aphasia (A) and progressive nonfluent aphasia (B) demonstrated frontotemporal atrophy (red) when their CSF biomarkers or autopsy results are consistent with frontotemporal lobar degeneration, and temporal-parietal atrophy (green) when their CSF biomarkers or autopsy results are more consistent with Alzheimer disease. A statistical height threshold for these analyses was set at p < 0.005 and only clusters comprised of 100 or more adjacent voxels that survived a peak voxel significance of p < 0.05 (corrected for familywise error) were accepted.
Multimodal clinicopathologic prediction in LPA and PNFA.
Finally, we assessed the value of combining the 3 scales to predict AD pathology or CSF biomarkers in nonfluent PPA. We used the major differences from univariate analyses between pathologic AD and FTLD to derive a predictor scale for each measure (see Methods). Neuropsychological analysis had the largest AUC of 0.817 (86.7% sensitivity and 68.7% specificity for AD pathology or CSF biomarkers), followed by clinical features alone (AUC of 0.729, 72.2% sensitivity and 65.0% specificity for AD) and MRI patterns of atrophy alone (AUC of 0.636, 72.7% sensitivity and 66.7% specificity for AD). Predictor combinations were then tested in patients with all 3 measures available (n = 19). Combining neuropsychological analysis with clinical features (AUC = 0.878) or MRI patterns of atrophy (AUC = 0.878) improved the distinction between FTLD and AD (figure 3), but adding volumetric MRI analysis to clinical features resulted in a higher false-positive rate (AUC = 0.544). Combining neuropsychological testing and volumetric MRI analysis achieved 77.8% sensitivity and 90.0% specificity in identifying AD pathology or CSF biomarkers in nonfluent PPA (positive predictive value = 87.5%, negative predictive value = 81.8%), and combining neuropsychological testing and clinical features improved sensitivity at the price of diminished specificity, achieving 100% sensitivity and 80% specificity (positive predictive value = 82.4%, negative predictive value = 80.9%). Use of all 3 factors improved the prediction model marginally (AUC = 0.900, 100% sensitivity and 80% specificity). This range of accuracy was additionally confirmed with random forest analysis using clinical features and neuropsychological scores, with the model achieving 81.2% accuracy (81.3% sensitivity and 82.4% specificity).

Figure 3 Predictive value of combining clinical features, neuropsychological analysis, and MRI patterns of atrophy for Alzheimer disease pathology in receiver operating characteristic analysis
AUC = area under the curve; Clin = clinical characterization; MRI = volumetric MRI analysis; NPsy = neuropsychological analysis.
DISCUSSION
AD and FTLD are the 2 main pathologic substrates that can manifest clinically as nonfluent PPA. Qualitative clinical features, neuropsychological performance, and MRI atrophy each represents a potential predictor for AD or FTLD. Our findings suggest that relative performance in neuropsychological tests helps identify patients with AD pathology or CSF biomarkers, and can achieve highly specific and sensitive prediction of AD when combined with clinical characterization or high-resolution neuroimaging analysis. We discuss these findings below.
Previous observations hypothesized a direct syndrome-pathology relationship within subgroups of PPA. According to this hypothesis, LPA is a marker of AD and PNFA is a marker of FTLD-tau. Exactly what proportion of patients with LPA have AD pathology at autopsy remains to be confirmed in large clinicopathologic series. In 1 study, 7/11 clinically diagnosed patients with LPA had AD pathology, with the remaining showing FTLD-TDP pathology.11 We found a similar proportion of patients with LPA with AD pathology or CSF biomarker, plus 4 patients with LPA and AD at autopsy who were excluded from this study due to absence of detailed neuropsychological and MRI data. Thus, among those with autopsy findings, we confirmed that LPA itself is pathologically heterogeneous, with a high proportion of cases showing pathologic findings of AD (75%).
It is equally problematic to use PNFA as a reliable marker of FTLD pathology. While PNFA is often associated with FTLD spectrum pathology,22 PNFA also can be a clinical manifestation of AD.6 PNFA is characterized by effortful, agrammatic, and dysarthric speech, but nonfluent speech could be related to limited category naming fluency23 in AD. Indeed, we found some patients with LPA and patients with PNFA to share clinical features presumed specific for each syndrome. Furthermore, while a syndromic distinction between LPA and PNFA has limited sensitivity (66.7%) and specificity (65%) for pathology, detailed clinical feature tabulation only modestly improved sensitivity without improving specificity. Therefore, our findings do not support the use of syndromic diagnosis or qualitative characterization alone as a predictor for pathology.
Differences in the anatomic distribution of cortical atrophy across clinical phenotypes or pathologic substrates suggested the potential of volumetric MRI analysis as a predictor. We found that patients with AD generally had a temporal-parietal pattern of atrophy, and patients with FTLD had more frontal-temporal atrophy. This pattern is consistent with findings from previous series of aphasic or behavioral/ dysexecutive patients with autopsy findings of AD and FTLD-TDP.24,25 Patients with FTLD also had parietal atrophy in this study that could give rise to word-finding deficits in LPA, suggesting again that involvement of common brain regions by different pathologic substrates can result in overlapping clinical phenotype. At the individual patient level, the relative distribution of atrophy performed only as well as qualitative clinical characterization in predicting AD pathology or CSF biomarker.
In contrast, patterns of quantitative neuropsychological performance significantly differed across biomarker/pathology groupings regardless of the syndrome. We previously observed the unique association between relative performance on neuropsychological measures and underlying pathology across syndromes in patients followed to autopsy.13 This relative difference in performance may reflect differential vulnerability of brain regions to AD or FTLD pathology independent of the dominant syndrome or disease duration, and thus provided superior sensitivity and specificity in predicting the underlying pathology. The syndrome-independent nature of this relative neuropsychological performance may also explain why the composite neuropsychological scores and clinical features may improve prediction of AD pathology or CSF biomarkers synergistically rather than redundantly.
While each predictor alone was useful in pathologic prediction, we reasoned that a combination of these could improve the ability to predict pathology. This combinatorial process has been demonstrated in recent work,26 although not in a comparative manner. The reduction in false-positive rate that we observed likely results from complementary interaction between neuropsychological testing and other factors, in contrast to the increased false-positive rate when clinical characterization and MRI analysis were combined. Likewise, the combination of all 3 factors improved the overall diagnostic accuracy only modestly. One possible explanation is that multiple factors contributing to a multimodal analysis must be relatively independent of each other to improve the overall predictive power. Regional MRI atrophy did not improve the syndrome-based prediction of underlying pathology nor the most inclusive multimodal predictor model, possibly due to the association between brain atrophy and clinical symptoms. Nevertheless, the modification of a single predictor's performance by another is promising evidence that independent biomarkers can be analyzed in predictive combinations to improve overall diagnostic accuracy. Additional biomarkers under investigation should also be assessed in a combinatorial fashion to maximize their utility, including cerebral amyloid imaging,27 diffusion tensor imaging,28 and plasma or CSF proteomics.29
This study has a number of limitations, including the use of CSF biomarkers as surrogate for pathology. While CSF tau:Aβ42 ratio is associated with high specificity over 90%, the modest sensitivity under 85% may have underestimated the number of AD cases within each syndrome. Not all patients had high-resolution MRI, also limiting the sensitivity and specificity contributed by this modality, and automated high-throughput volumetric measurements of specific brain regions—once identified—is currently available only at selected centers.30 The multimodal combination identified in the current study should be tested in an independent group of autopsy-confirmed patients, and future studies should incorporate other biomarkers for AD, FTLD-TDP, and tauopathies. With these caveats in mind, we propose that the pathology causing nonfluent PPA can be identified by combining qualitative clinical features, quantitative neuropsychological analysis, and MRI pattern of atrophy.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. W.T. Hu, Dr. C. McMillan, and Dr. M. Grossman.
DISCLOSURE
Dr. Hu has a patent pending re: Cerebrospinal fluid and plasma biomarkers for AD and receives research support from the American Academy of Neurology Foundation (Clinical Research Training Fellowship). Dr. McMillan receives research support from the NIH (NICHHD HD060406 [PI]). Dr. Libon reports no disclosures. S. Leight receives research support from the NIH (AG017586-09 [research lab manager]) and from the Cure Alzheimer's Fund. Dr. Forman is an employee of and holds stock options in Merck Serono and serves on the editorial advisory board of the Journal of Neuropathology and Experimental Neurology. Dr. Lee has received funding for travel or speaker honoraria from Takeda Pharmaceutical Company Limited, Genentech, Inc., and Pfizer Inc.; serves on the editorial boards of Laboratory Investigation, Neurorehabilitation and Neural Repair, NeuroSignals, Neuron, and Experimental Neurology, and on the Board of Reviewing Editors for Science Magazine; holds and has patents pending re: Modified avidin-biotin technique, Method of stabilizing microtubules to treat Alzheimer's disease, Method of screening for Alzheimer's disease or disease associated with the accumulation of paired helical filaments, Compositions and methods for producing and using homogeneous neuronal cell transplants, Animal model for Alzheimer's disease, Method of identifying, diagnosing and treating α-synuclein positive neurodegenerative disorders, Identification and characterization of AB-negative plaques and methods of diagnosing Alzheimer's disease, and Mutation-specific functional impairments in distinct tau isoforms of hereditary frontotemporal dementia and parkinsonism linked to chromosome-17; and receives research support from the NIH (NIA P30-AG 009215-19 [project leader]) and Ware Benaroya. Dr. Trojanowski has received funding for travel and honoraria from Takeda Pharmaceutical Company Ltd. and to attend numerous conferences not funded by industry; serves as an Associate Editor of Alzheimer's & Dementia; may accrue revenue on patents re: Modified avidin-biotin technique, Method of stabilizing microtubules to treat Alzheimer's disease, Method of detecting abnormally phosphorylated tau, Method of screening for Alzheimer's disease or disease associated with the accumulation of paired helical filaments, Compositions and methods for producing and using homogeneous neuronal cell transplants, Rat comprising straight filaments in its brain, Compositions and methods for producing and using homogeneous neuronal cell transplants to treat neurodegenerative disorders and brain and spinal cord injuries, Diagnostic methods for Alzheimer's disease by detection of multiple mRNAs, Methods and compositions for determining lipid peroxidation levels in oxidant stress syndromes and diseases, Compositions and methods for producing and using homogenous neuronal cell transplants, Method of identifying, diagnosing and treating alpha-synuclein positive neurodegenerative disorders, Mutation-specific functional impairments in distinct tau isoforms of hereditary frontotemporal dementia and parkinsonism linked to chromosome-17: Genotype predicts phenotype, Microtubule stabilizing therapies for neurodegenerative disorders, and Treatment of Alzheimer's and related diseases with an antibody; and receives research support from the NIH (NIA P01 AG 09215-20 [PI], NIA P30 AG 10124-18 [PI], NIA PO1 AG 17586-10 [Project 4 Leader], NIA 1PO1 AG-19724-07 [Core C Leader], NIA 1 U01 AG 024904-05 [Co-PI Biomarker Core Laboratory], NINDS P50 NS053488-02 [PI], NIA UO1 AG029213-01 [Co-I], RC2NS069368 [PI], RC1AG035427 [PI], and NIA P30AG036468 [PI]) and the Marian S. Ware Alzheimer Program. Dr. Grossman serves on a scientific advisory board for Allon Therapeutics Inc.; serves as Editor of Cognitive and Behavioral Neurology; serves as a consultant for Pfizer Inc., Forest Laboratories, Inc., and Allon Therapeutics Inc.; and receives research support from the NIH (AG17586 [project and core leader], AG15116 [PI], NS44266 [PI], and NS53488 [project leader]).
Supplementary Material
Address correspondence and reprint requests to Dr. Murray Grossman, Department of Neurology, University of Pennsylvania School of Medicine, 3400 Spruce Street, Philadelphia, PA 19106 mgrossma@mail.med.upenn.edu
Editorial, page 582
See pages 588 and 603
Supplemental data at www.neurology.org
Study funding: Supported by American Academy of Neurology Clinical Research Training Fellowship and the NIH (AG17586, AG15116, NS53488, NS44266).
Disclosure: Author disclosures are provided at the end of the article.
Received September 8, 2009. Accepted in final form March 11, 2010.
REFERENCES
- 1.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598. [DOI] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001;58:1803–1809. [DOI] [PubMed] [Google Scholar]
- 4.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman M, McMillan C, Moore P, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain 2004;127:628–649. [DOI] [PubMed] [Google Scholar]
- 6.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006;59:156–165. [DOI] [PubMed] [Google Scholar]
- 7.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 2000;123:484–498. [DOI] [PubMed] [Google Scholar]
- 10.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene JD, Patterson K, Xuereb J, Hodges JR. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol 1996;53:1072–1078. [DOI] [PubMed] [Google Scholar]
- 13.Grossman M, Xie SX, Libon DJ, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology 2008;70:2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 2008;70:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:1095–1097. [DOI] [PubMed] [Google Scholar]
- 16.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007;114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002;61:935–946. [DOI] [PubMed] [Google Scholar]
- 18.Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–2019. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 1990;87:5827–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000;290:985–989. [DOI] [PubMed] [Google Scholar]
- 21.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia: longitudinal course, neuropsychological profile, and language features. Arch Neurol 1990;47:1329–1335. [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA, Whitwell JL, Duffy JR, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology 2008;70:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 2007;22:474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarkers signature in Alzheimer's Disease Neuroimaging Initiative subjects. Ann Neurol Epub 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leinonen V, Alafuzoff I, Aalto S, et al. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol 2008;65:1304–1309. [DOI] [PubMed] [Google Scholar]
- 28.Borroni B, Brambati SM, Agosti C, et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch Neurol 2007;64:246–251. [DOI] [PubMed] [Google Scholar]
- 29.Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med 2007;13:1359–1362. [DOI] [PubMed] [Google Scholar]
- 30.Kovacevic S, Rafii MS, Brewer JB. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis Assoc Disord 2009;23:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



