Abstract
Background:
Despite recent work, the nosology of nonfluent primary progressive aphasia (PPA) remains unresolved.
Methods:
We describe a clinical and neurolinguistic cross-sectional analysis of a cohort of 24 patients with nonfluent PPA. Patients were initially classified based on analysis of spontaneous speech into 4 groups: apraxia of speech (AOS)/agrammatism (10 patients); AOS/no agrammatism (4 patients); no AOS/agrammatism (3 patients); no AOS/no agrammatism (7 patients). These groups were further characterized using a detailed neurolinguistic and neuropsychological battery. Parkinsonism was present in 3/10 patients in the AOS/agrammatism group. All patients in the no AOS/agrammatism group had mutations in the progranulin (GRN) gene, while 5/7 cases in the no AOS/no agrammatism group had CSF findings compatible with Alzheimer disease.
Results:
The groups without AOS showed more severe neurolinguistic impairments for a given disease stage, and sentence comprehension, speech repetition, and reading were impaired in all groups. Prolonged word-finding pauses and impaired single word comprehension were salient features in the no AOS/agrammatism group. Additional impairments of executive function and praxis were present in both groups with agrammatism, and impaired episodic memory was a feature of the no AOS/no agrammatism group.
Conclusion:
PPA with AOS is aligned with the syndrome previously designated progressive nonfluent aphasia; agrammatism may emerge as the syndrome evolves, or alternatively, the pure AOS group may be pathophysiologically distinct. PPA without AOS resembles the syndrome designated logopenic/phonologic aphasia; however, there is evidence for a distinct subsyndrome of GRN-associated aphasia. The findings provide a rationale for further longitudinal studies with pathologic correlation.
GLOSSARY
- AD
= Alzheimer disease;
- AOS
= apraxia of speech;
- CDR-SB
= Clinical Dementia Rating–sum of boxes;
- LPA
= logopenic progressive aphasia;
- MMSE
= Mini-Mental State Examination score;
- PNFA
= progressive nonfluent aphasia;
- PPA
= primary progressive aphasia;
- SemD
= semantic dementia.

Podcast
Since Mesulam's original case series,1 there has been increasing interest in degenerative disorders that selectively affect the language system: the primary progressive aphasias (PPA).2–5 Two canonical subtypes were originally described: semantic dementia (SemD) and progressive nonfluent aphasia (PNFA).6 PNFA is a heterogeneous syndrome; nonfluent speech may reflect various deficits, including agrammatism (emphasized in the original PNFA criteria6), motor-speech impairment (e.g., apraxia of speech [AOS], i.e., hesitancy and effortfulness attributable to impaired planning of articulation),7 slower speech rate, decreased phrase length, or word-finding difficulty.8 Agrammatism and AOS have been highlighted in the literature on PPA. However, a third, essentially nonfluent, variant of PPA has been more recently described: logopenic or phonologic progressive aphasia (LPA),9–11 with prolonged word-finding pauses but without agrammatism or motor-speech impairment. Various other phenotypes have also been described.4,12–15 Furthermore, nonfluent PPA is pathologically heterogeneous with tau, TDP-43 and Alzheimer pathology all described.16–21 Despite recent progress, a number of key issues remain unresolved: these include the relationship of agrammatism to AOS, and the place of these features in defining nonfluent PPA; relations between PNFA and LPA; and the nosology of nonfluent PPA more broadly. Here we present neurolinguistic and neuropsychological data relevant to these issues.
METHODS
Patient cohort.
Thirty-three consecutive patients presenting with progressive language impairment as the leading feature and not fulfilling criteria for an alternative dementia syndrome (PPA according to current criteria2,3) were recruited. All patients had a structured clinical history and examination by an experienced cognitive neurologist. Based on this initial assessment, 9 patients were diagnosed with SemD.6,22 The remaining 24 patients had nonfluent speech and these patients are the focus of this study. Eighteen cognitively normal age- and gender-matched control subjects also participated. One patient developed a corticobasal syndrome and 2 a progressive supranuclear palsy syndrome. Genetic screening for progranulin (GRN) and tau mutations was performed in all patients; 3 patients had GRN mutations. CSF examination was undertaken in 9 patients; this revealed a profile of total tau/Aβ42 levels consistent with Alzheimer disease (AD) in 5 cases.23 We have previously described neuroimaging and background neuropsychology findings in this cohort.24 Here we describe a detailed neurolinguistic analysis of the cohort.
Standard protocol approvals, registrations, and patient consents.
Ethical approval for the study was obtained from the National Hospital for Neurology and Neurosurgery Local Research Ethics Committee. Written research consent was obtained from all patients participating in the study.
Spontaneous speech analysis.
Initially, a sample of spontaneous speech was obtained by asking subjects to talk about their last holiday and to describe the Cookie Theft Scene from the Boston Diagnostic Aphasia Examination.25 This sample was recorded and subsequently analyzed for the number of agrammatic errors (either morphologic or syntax errors) per minute and for the presence or absence of AOS, defined as a motor-speech disorder with the features of hesitancy, effortfulness with articulatory groping, speech production errors, and dysprosody,26,27 all of which were required to be present. Speech was analyzed using a number of quantitative measures (details in appendix e-1 on the Neurology® Web site at www.neurology.org) including number of words produced per minute, number of speech production errors per minute, length of word-finding pauses, and range of nouns and verbs used (noun and verb frequency28).
From this initial spontaneous speech analysis, 4 groups of patients with nonfluent PPA were identified: AOS/agrammatism, AOS/no agrammatism, no AOS/agrammatism, and no AOS/no agrammatism. Table 1 shows the spontaneous speech data and comparison with the cognitively normal control group and the disease-control SemD group. Analyses were performed using linear regression models within STATA 10.0 (Stata Corporation, College Station, TX). Within-group differences were analyzed using Wilcoxon signed-rank tests.
Table 1 General demographic and spontaneous speech data
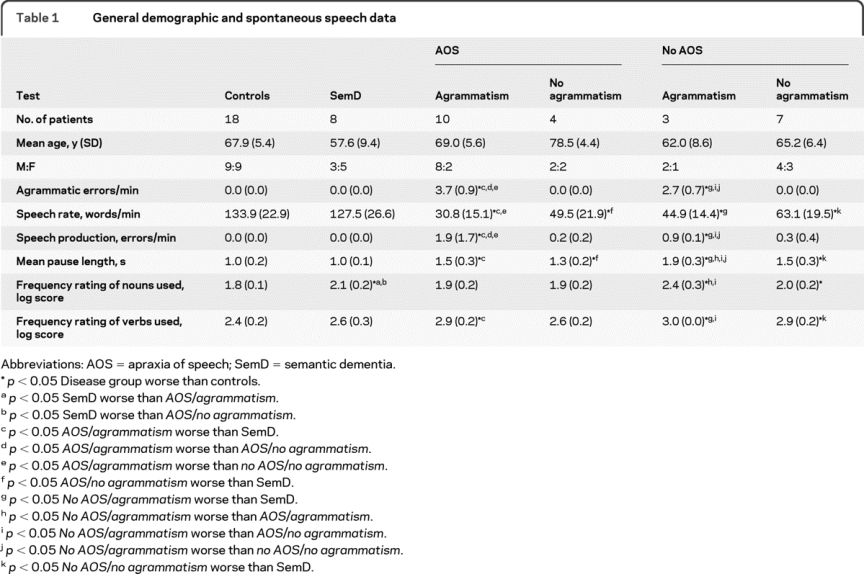
Both groups with AOS had reduced speech rate and increased mean pause length compared with controls and made speech production errors. The range of noun use (noun frequency) was similar to controls. However, the group with agrammatism had significantly more speech production errors and a trend to lower speech rate and longer mean pause duration than the group without agrammatism. Furthermore, there was a higher mean verb but not noun frequency than controls in the AOS/agrammatism group, suggesting a tendency to use more common verbs (the reverse pattern to the SemD group). Patients with no AOS/agrammatism differed from the AOS/agrammatism group in having a significantly longer mean pause length and a higher mean frequency of nouns used (i.e., a tendency to use more common nouns, similar to the SemD group) although they also had a higher mean frequency of verbs used than controls. The no AOS/no agrammatism group had reduced speech rate, occasional speech production errors, and longer mean pause duration compared both with controls and SemD; similar to the no AOS/agrammatism group, there was a higher mean noun and verb frequency.
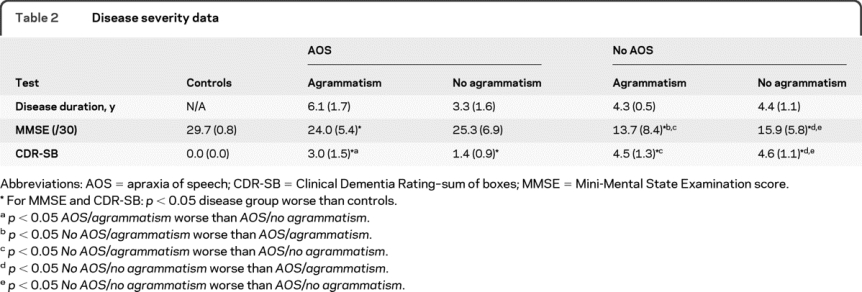
Disease duration and disease severity.
One problem with comparing patients cross-sectionally (as here) is that within a single study they will be at various disease stages. This is compounded by variability in the rate of progression. We therefore compared disease duration from symptom onset with disease severity measured using both a cognitive index (the Mini-Mental State Examination score [MMSE]29) and a functional index (the Clinical Dementia Rating–sum of boxes [CDR-SB]30) (table 2). Each of the patient groups had decreasing MMSE and increasing CDR-SB with increasing disease duration, but for a given disease duration patients without AOS had lower MMSE and higher CDR-SB scores.
Table 2 Disease severity data
Neurolinguistic and neuropsychological analyses.
Having defined the 4 nonfluent PPA patient groups, we examined linguistic and other neuropsychological features in each group (see appendix e-1). We adjusted for disease severity (MMSE) in subsequent statistical analyses comparing disease groups.
RESULTS
Results are detailed in table 3.
Table 3 Neurolinguistic and neuropsychological data
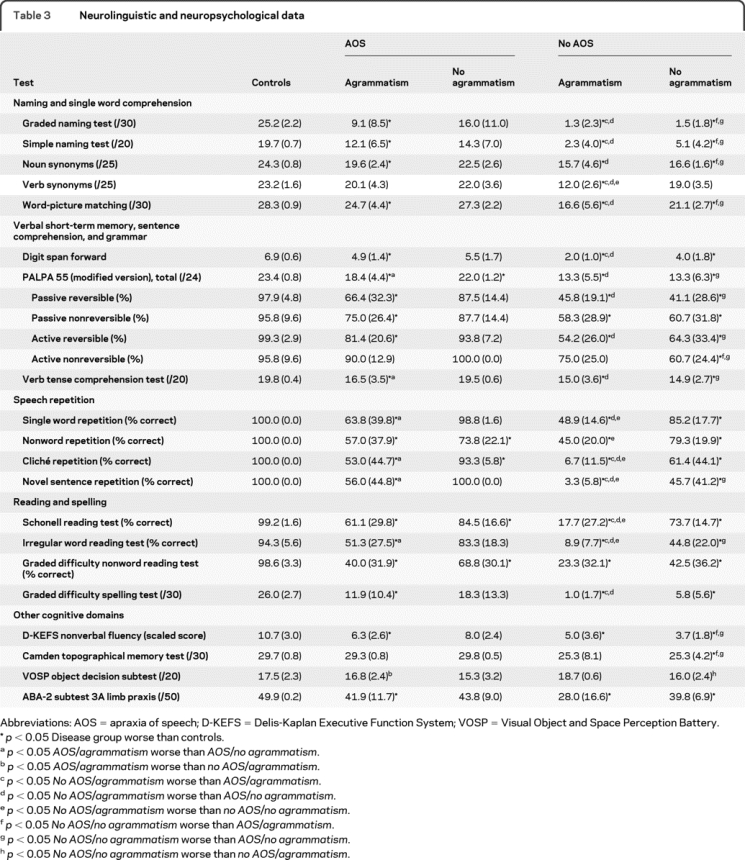
Naming and single word comprehension.
The AOS/agrammatism group and both groups without AOS were significantly anomic compared with healthy controls and anomia was significantly more severe in the groups without AOS compared with those with AOS. A similar pattern was seen on tests of noun comprehension although verb comprehension was only significantly impaired relative to controls in the no AOS/agrammatism group (with a trend to better performance on nouns compared to verbs in this group). Word-picture matching performance was significantly worse than controls in all disease groups apart from the AOS/no agrammatism group and significantly worse in the 2 groups without AOS compared with those with AOS.
Verbal short-term memory, sentence comprehension, and grammar.
Compared with controls, all groups apart from the AOS/no agrammatism group had decreased digit span and digit span was significantly lower in the no AOS/agrammatism group compared with the 2 groups with AOS. Performance on the modified PALPA55 subtest was impaired in all groups compared with controls. The AOS/agrammatism group performed significantly worse on comprehension of passive reversible than active nonreversible sentences (p = 0.01, suggesting a true grammatic comprehension deficit). The no AOS/agrammatism group performed poorly on all sentences but there was a trend to worse performance on the passive reversible sentences compared to active nonreversible sentences (p = 0.10). The no AOS/no agrammatism group performed similarly on all sentences and did not benefit from the effect of nonreversibility in simpler active sentences. Verb tense comprehension was affected similarly in all groups apart from the AOS/no agrammatism group, who performed normally.
Speech repetition.
The AOS/agrammatism group and the 2 groups without AOS performed worse than controls on all tests while the AOS/no agrammatism group performed worse than controls only on the nonword and cliché repetition tasks. The groups with AOS did not show significant differences between words and sentences. The no AOS/no agrammatism group performed significantly worse on novel sentence repetition compared to cliché, nonword, or word repetition with a similar trend in the no AOS/agrammatism group.
Reading and spelling.
Word and nonword reading was impaired in all groups although most significantly in the no AOS/agrammatism group. Nonword reading was more impaired than irregular word reading for both the AOS groups; performance reading nonwords and irregular words was comparable in the no AOS/no agrammatism group, while irregular word reading was most severely affected in the no AOS/agrammatism group. Spelling performance was significantly worse than controls for all groups apart from the AOS/no agrammatism group.
Other cognitive domains.
Executive function was impaired in all but the AOS/no agrammatism group. Episodic memory was impaired relative to controls only in the no AOS/no agrammatism group, with a trend to worse performance in the no AOS/agrammatism group. Limb praxis was impaired in all groups apart from the AOS/no agrammatism group. Visual object perception was comparable to controls in all groups. The AOS/no agrammatism group performed normally on all nonlinguistic tests.
Summary of findings in each group.
AOS/agrammatism.
This group had reduced speech rate with speech production errors and increased pause length with nonfluency due to the dual deficits of AOS and agrammatism. These features distinguished the speech of these patients from the SemD group, with in addition reduced verb but normal noun frequency (completing a double dissociation with SemD; table 1). Other key features were anomia, impaired sentence comprehension (particularly for more complex sentences), impaired speech repetition that was similarly severe for both words and sentences, impaired reading (particularly nonwords), and in addition executive dysfunction and limb apraxia. There was also evidence of a mild single word comprehension deficit, particularly in more severely affected patients. This profile is consistent with previous descriptions of PNFA. Of note, the 3 patients with parkinsonism all fell within this group.
AOS/no agrammatism.
This group had shorter mean disease duration than the AOS/agrammatism group and showed a trend toward a qualitatively similar though less severe profile of deficits. Mean speech rate was reduced and pause length prolonged in relation to both healthy controls and the SemD group. Despite the absence of expressive agrammatism, this group performed significantly worse than controls on the PALPA55 sentence comprehension test (suggesting a deficit of receptive grammar). These patients also had mild dyslexia (affecting nonwords). These features suggest that this pure AOS group may represent an earlier stage of PNFA prior to development of expressive agrammatism, though this remains unresolved in the absence of longitudinal data.
No AOS/agrammatism.
These patients were more severely affected than the 2 groups with AOS (based on MMSE and CDR scores) with impairments on most linguistic tests. However, speech rate and speech production errors were similar to the groups with AOS. In addition, visual object perception and episodic memory were preserved, indicating a predominantly aphasic syndrome. The most notable linguistic problems were profound anomia, impaired single word comprehension (particularly verbs), severely reduced digit span (phonologic short-term memory deficit), impaired sentence comprehension and repetition, and severe dyslexia. Expressive agrammatism was found on formal speech analysis but difficult to assess at the bedside because of the slow speech rate and word-finding pauses. This group comprised the patients with GRN mutations.
No AOS/no agrammatism.
The most prominent features in this group were anomia, decreased forward digit span, impaired sentence comprehension (both simple and complex), impaired sentence repetition with relatively spared single word repetition, dyslexia (particularly for nonwords), and relatively intact single word comprehension. These features are consistent with current descriptive criteria for LPA. In addition these patients had an extralinguistic deficit of episodic memory impairment. Of note, most patients (5 of 7) in this group had CSF biomarkers consistent with AD pathology.
DISCUSSION
We describe 4 distinct syndromic groups within a cohort of patients with nonfluent PPA. We delineated the groups based initially on the presence or absence of AOS and expressive agrammatism in spontaneous speech followed by detailed linguistic analysis. These groups comprised an AOS-only group, an AOS-plus-agrammatism group, an agrammatism-only group, and a group without AOS or agrammatism. The AOS groups together constitute the majority of patients and might be described as PNFA or PNFA/AOS. It remains unclear whether the AOS group without agrammatism represents a less severe form of PNFA, consistent with the observation that agrammatism may supervene later in the course of progressive AOS, or whether pure AOS constitutes a pathophysiologically distinct group within the PPA spectrum. Indeed, the relation between these AOS syndromes is a key issue for future work. The group without AOS or agrammatism has a syndrome equivalent to LPA as previously described.10,11 This syndrome is likely to be underpinned by AD pathology in a high proportion of cases. The agrammatism-only group is more problematic; while the presence of agrammatism would tend to align such cases with PNFA, this syndrome has some linguistic and neuropsychological similarity to LPA (including long word-finding pauses, a severe phonologic verbal short memory deficit, impaired sentence processing, and nonlinguistic dominant parietal lobe features). All patients in this group here had GRN mutations, suggesting that GRN mutations may lead to a distinct aphasia syndrome albeit overlapping PNFA/AOS and LPA.31 If indeed agrammatism is a defining feature of GRN-associated PPA, this supports recent work suggesting that TDP-43 pathology may be a substrate for agrammatic PPA,32 though this group may include both cases with GRN mutations and other patients lacking such mutations. More fine-grained analyses of PPA with expressive agrammatism may identify further subdivisions with clinically meaningful associations (e.g., an association with tau pathology19,33,34).
This study underlines the importance of an initial clinical assessment of the patient's spontaneous speech, and in particular the presence or absence of AOS and agrammatism, in classifying nonfluent PPA syndromes at presentation. However, we do not wish to imply that clinical characterization of PPA syndromes is straightforward; analysis of spontaneous speech may be difficult where this is severely impoverished. It is likely that a particular syndrome will change in character as disease evolves: disease duration and severity therefore need to be taken into account. Moreover, there is a need for new operational and clinical measures of PPA that can characterize positively the no AOS/no agrammatism group defined negatively here, and potentially, other less-common PPA syndromes not captured by the simple classification scheme we present (for example, the controversial entity of cortical anarthria).9
This study further illustrates that a number of standard neuropsychological measures are of limited use in differentiating PPA syndromes. However, within particular cognitive domains, certain features may allow more detailed neuropsychological stratification of these syndromes. Consistent with previous work,10,11,35–37 this study indicates that sentence comprehension deficits occur in the nonfluent PPA spectrum. The LPA (no AOS/no agrammatism) group here exhibited more severe deficits of sentence syntax and verb tense processing than the groups with AOS, and in contrast to the patients with AOS, showed impaired processing of both simple (active) and complex (passive) sentences with limited sensitivity to nonreversibility (a semantic cue based on agency). Considering the PPA spectrum as a whole, various deficits may potentially contribute to impaired sentence processing, including impaired verbal working memory as well as primary grammatic or semantic deficits.38,39 These potential mechanisms of impaired sentence comprehension remain to be elucidated fully.
The different syndromes have distinct patterns of speech repetition that may help to distinguish them. Previous studies have suggested that patients with LPA have significantly worse performance on sentences compared to single words11 and this pattern was also seen in the LPA (no AOS/no agrammatism) group here. A similar but more severe dichotomy between single word and sentence repetition was seen in the GRN (no AOS/agrammatism) group. In comparison to these groups without AOS, the AOS/agrammatism group performed similarly on words, nonwords, clichés, and sentences, while the AOS/no agrammatism group showed deficits of nonword and cliché repetition.
Our findings further highlight dyslexia and dysgraphia as key components of the nonfluent PPA variants; performance on nonwords was worse for both the AOS groups and the LPA (no AOS/no agrammatism) group, in keeping with a phonologic dyslexia.40 In the GRN (no AOS/agrammatism) group, reading of all word types was affected, suggesting a more severe dyslexia. Cognitive domains beyond language may provide further information; episodic memory impairment (on the relatively easy test used here) was a consistent feature only of the LPA (no AOS/no agrammatism) group.
This study has the limitations of small case numbers, absence of a longitudinal arm to track the evolution of deficits, and lack of pathologic correlation. We focused on a relatively small number of neurolinguistic measures with clear clinical relevance. More fine-grained psycholinguistic analyses (for example, to characterize motor-speech deficits, and intrasentential vs intersentential pauses) may further refine the distinction between PPA subgroups. These caveats notwithstanding, the findings provide a rationale for future studies of the nosology of PPA syndromes. It is likely that there are at least 3 nonfluent PPA syndromes and that these are distinct rather than variations on a single continuum.24,31,32,34 The PNFA/AOS syndrome can be associated with a corticobasal or progressive supranuclear palsy syndrome during life17 and based on previous evidence is most commonly underpinned by tau pathology, while the LPA syndrome without AOS or agrammatism is closely associated with AD pathology, and GRN-associated aphasia has TDP-43 pathology. The phenotype of GRN-associated aphasia is of neurobiological interest since it is associated with a specific molecular dysfunction.24,31 Though detailed neuropsychological studies are few, previous reports include descriptions of a syndrome of progressive “nonfluent anomic aphasia.”12 Clearly, additional unidentified factors are likely to influence the particular phenotype of GRN aphasia, and we do not suggest a precise correspondence between GRN mutations and the no AOS/agrammatism aphasia syndrome delineated here. The GRN-aphasia syndrome may bear some neuropsychological and neuroanatomic24 similarity to LPA; however, there are certain key points of distinction. While detection of expressive agrammatism may be difficult at the bedside, for the reasons outlined above, our findings suggest that additional neurolinguistic features may help discriminate this GRN-associated aphasia syndrome from cases of LPA (e.g., impaired single word comprehension). It is unlikely that the GRN-associated no AOS/agrammatism group simply represents a more severe syndrome than LPA; both groups without AOS here had very similar disease durations and disease severity as indexed by MMSE and CDR. Detailed neuropsychological evaluation may be required to differentiate the GRN-associated and AD-associated syndromes and this could in turn potentially help guide investigation of patients with PPA. Systematic, hypothesis-led longitudinal neurolinguistic analyses with neuroanatomic, genetic, and pathologic correlation in larger patient cohorts will be important directions for future work.
DISCLOSURE
Dr. Rohrer has received research support from the Wellcome Trust (Clinical Research Fellowship) and Brain (Exit Scholarship). Dr. Rossor serves on a scientific advisory board for Elan Corporation and Wyeth; serves as Editor-in-Chief of the Journal of Neurology, Neurosurgery and Psychiatry, and on the editorial boards of Practical Neurology, Dementia and Geriatric Cognitive Disorders, Neurodegenerative Diseases, and the British Medical Journal; receives royalties from the publication of Brain's Diseases of the Nervous System, 11th ed. (Oxford University Press, 2001) and Brain's Diseases of the Nervous System, 12th ed. (Oxford University Press, 2009); and receives research support from the Department of Health and the Alzheimer's Research Trust. Dr. Warren has received research support from the Wellcome Trust (Intermediate Clinical Fellowship).
Supplementary Material
Address correspondence and reprint requests to Dr. Jason Warren, Dementia Research Centre, Institute of Neurology, Queen Square, London WC1N 3BG, UK warren@dementia.ion.ucl.ac.uk
Editorial, page 582
See pages 588 and 595
Supplemental data at www.neurology.org
Study funding: This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer's Research Trust Coordinating Centre. This work was also funded by the Medical Research Council UK and the Wellcome Trust.
Disclosure: Author disclosures are provided at the end of the article.
Received October 21, 2009. Accepted in final form March 29, 2010.
REFERENCES
- 1.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–432. [PubMed] [Google Scholar]
- 3.Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med 2003;349:1535–1542. [DOI] [PubMed] [Google Scholar]
- 4.Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase 2004;10:3–18. [DOI] [PubMed] [Google Scholar]
- 5.Rohrer JD, Knight WD, Warren JE, et al. Word-finding difficulty: a clinical analysis of the progressive aphasias. Brain 2008;131:8–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 7.Ogar JM, Dronkers NF, Brambati SM, et al. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007;21:S23–S30. [DOI] [PubMed] [Google Scholar]
- 8.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology 2007;69:200–213. [DOI] [PubMed] [Google Scholar]
- 9.Kertesz A, Davidson W, McCabe P, et al. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc 2003;9:710–719. [DOI] [PubMed] [Google Scholar]
- 10.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snowden JS, Pickering-Brown SM, Du Plessis D, et al. Progressive anomia revisited: focal degeneration associated with progranulin gene mutation. Neurocase 2007;13:366–377. [DOI] [PubMed] [Google Scholar]
- 13.Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain 2008;131:721–731. [DOI] [PubMed] [Google Scholar]
- 14.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain 2007;130:2636–2645. [DOI] [PubMed] [Google Scholar]
- 15.Kartsounis LD, Crewes H. Phonological buffer and selective deficits of grammar, with distinct time onsets, in a patient with a focal degenerative disorder. Neurocase 2007;13:65–80. [DOI] [PubMed] [Google Scholar]
- 16.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004;56:399–406. [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 2007;114:31–38. [DOI] [PubMed] [Google Scholar]
- 19.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008;64:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 2000;123:484–498. [DOI] [PubMed] [Google Scholar]
- 22.Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 2006;129:3066–3080. [DOI] [PubMed] [Google Scholar]
- 23.Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology 1999;52:1555–1562. [DOI] [PubMed] [Google Scholar]
- 24.Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage 2010;49:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 26.Croot K. Diagnosis of AOS: definition and criteria. Semin Speech Lang 2002;23:267–280. [DOI] [PubMed] [Google Scholar]
- 27.Ogar J, Slama H, Dronkers N, et al. Apraxia of speech: an overview. Neurocase 2005;11:427–432. [DOI] [PubMed] [Google Scholar]
- 28.Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical data base on CD-ROM. Philadelphia: Linguistic Data Consortium; 1993. [Google Scholar]
- 29.Folstein M, Folstein S, McHugh P. The “Mini mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 31.Rohrer JD, Crutch SJ, Warrington EK, Warren JD. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia 2010;48:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 2010;74:42–49. [DOI] [PubMed] [Google Scholar]
- 33.Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol 2009;66:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knibb JA, Woollams AM, Hodges JR, Patterson K. Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain 2009;132:2734–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee J, Antiquena P, Grossman M. Verb comprehension in frontotemporal degeneration: the role of grammatical, semantic and executive components. Neurocase 2001;7:173–184. [DOI] [PubMed] [Google Scholar]
- 36.Grossman M, Moore P. A longitudinal study of sentence comprehension difficulty in primary progressive aphasia. J Neurol Neurosurg Psychiatry 2005;76:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peelle JE, Cooke A, Moore P, et al. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. J Neurolinguistics 2007;20:482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiebach CJ, Schlesewsky M, Friederici AD. Syntactic working memory and the establishment of filler-gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res 2001;30:321–338. [DOI] [PubMed] [Google Scholar]
- 39.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci 2002;6:78–84. [DOI] [PubMed] [Google Scholar]
- 40.Brambati SM, Ogar J, Neuhaus J, et al. Reading disorders in primary progressive aphasia: a behavioral and neuroimaging study. Neuropsychologia 2009;47:1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


