Abstract
A single enhancing lesion in the brain parenchyma, also called an inflammatory granuloma, is a frequent neurologic diagnosis. One of the commonest causes of this lesion is human neurocysticercosis, the infection by the larvae of the pork tapeworm, Taenia solium. Following the demonstration that viable cysticercosis cysts survive in good conditions for several years in the human brain, single cysticercal granulomas have been consistently interpreted as representing late degeneration of a long-established parasite. On the basis of epidemiologic, clinical, and laboratory evidence detailed in this article, we hypothesize that in most cases these inflammatory lesions correspond to parasites that die in the early steps of infection, likely as the natural result of the host immunity overcoming mild infections.
GLOSSARY
- NCC
= neurocysticercosis;
- SCG
= single cysticercal granuloma.

CME
A single inflammatory lesion in the brain parenchyma is a common diagnostic problem in neurology. These lesions are mostly called single enhancing lesions due to their enhancement in CT or MRI after the injection of contrast media. Numerous etiologic agents may cause this lesion (cysticercosis, tuberculosis, toxoplasmosis, mycoses, small abscesses, brain tumors, and even vascular malformations),1 but by far its most frequent cause is neurocysticercosis (NCC, the infection by the larvae of the pork tapeworm Taenia solium) and thus many authors name them single cysticercal granuloma (SCG).2,3 We present information supporting the hypothesis that most SCGs in NCC are the result of early parasite death (likely soon after encystment) by the host's immune response instead of the currently accepted interpretation that SCGs represent long-established cysts that cannot maintain their active immune evasion mechanisms and thus are discovered and killed.
CYSTICERCOSIS
T solium infection and NCC are present in most of the world. The infection is endemic in most developing countries, and increasingly diagnosed in industrialized countries due to tourism and immigration of NCC cases and tapeworm carriers from endemic zones.4 In the life cycle of T solium, humans are the only definitive host and harbor the adult tapeworm (taeniasis), whereas both humans and pigs are intermediate hosts and harbor the larvae or cysticerci. Cysticercosis is caused by ingestion of the eggs of the adult tapeworm by fecal contamination. Embryos are liberated from the eggs by the action of gastric acid and intestinal fluids; they cross the bowel wall and enter the bloodstream to be carried to the muscles and other tissues where they establish and encyst, reaching their definitive size of about 1 cm in 2–3 months.5
Outside the nervous system, human cysticercosis causes no major symptomatology. Subcutaneous cysticercosis presents as small, movable, painless nodules that are usually noticed in the arms or chest. After a period of months or a few years, the nodules get swollen, tender, and inflamed, and then gradually disappear. Muscular cysticercosis is a casual finding, appearing as dot-shaped or ellipsoidal calcifications following the muscle bundles in the thighs or arms, when X-rays are performed for an unrelated reason. The heart is another occasional location of cysticerci, infected in approximately 5% of patients. As much as it is known, cardiac cysticercosis is usually asymptomatic. Neurocysticercosis, conversely, is a pleomorphic clinical disorder associated with seizures and other neurologic symptoms in endemic areas. NCC presents with epileptic seizures in 50% to 90% of symptomatic patients with parenchymal brain cysts or calcifications, and intracranial hypertension or hydrocephalus in 20%–30% of cases. The proportion of patients with intracranial hypertension varies according to the origin of the cases, being higher in neurosurgical series.4
Evolution of intraparenchymal neurocysticercosis.
Vesicles vary in contents according to their evolutionary stage. Viable cysts have an opaline membrane through which the scolex is visible as a small 2- to 3-mm nodule. When cyst degeneration begins, the vesicular fluid becomes opaque and dense, and the cyst's edges become irregular and shrink. Later, calcification starts in the cephalic portion and progresses to the vesicular wall, to finally leave a round, whitish, residual calcified nodule.6
Disease pathogenesis.
Scarce data exist on the pathogenesis of NCC. After entering the CNS, cysticerci establish as viable cysts and elicit few inflammatory changes in the surrounding tissues. Cysticerci may remain for a long time in this stage. After a variable and undetermined time, neurologic symptoms appear, frequently associated with degeneration of the parasite due to immune mechanisms. The main evidence showing that symptoms occur long after infection originated from a classic series of articles describing seizure cases in English soldiers returning from India. These patients had been exposed to infection during a well-defined time period but developed seizures and other neurologic symptoms after having returned to England (where no transmission occurs). Most cases developed neurologic symptoms beginning 3 to 5 years after return to England.7–9 This was inconsistent with previous data showing that cysts in infected pigs reach their definitive size in 2 to 3 months and thus the time to symptom development in the series by Dixon and colleagues refuted the intuitive interpretation that the entry of the parasites to the brain was responsible for the symptoms.
With the advent of modern biology and neuroimaging techniques, the prevalent view is now that the cysts can survive in the human brain protected by the blood–brain barrier, and by using a series of active immune evasion mechanisms.4,5 In this view, acute inflammation and symptoms are usually the result of the death of an established parasite either by natural immunity (if the cyst cannot keep its active immune evasion mechanisms and is detected by the host's immune system) or by antiparasitic treatment. Treatment-associated parasite death is not immediate but takes a few weeks. The effect of the antiparasitic drug seems to occur by exposing the parasites to the host's immune system and thus accelerating the process of destruction, rather than by a direct, immediate drug effect on the parasite as a whole.10
THE SINGLE ENHANCING LESION
A particular presentation of NCC is the so-called single enhancing lesion, corresponding to an intraparenchymal small inflammatory lesion seen on CT or MRI as a hyperintense nodule or ring after the injection of contrast dye (figure). A SCG is the most frequent presentation of NCC in the Indian subcontinent, as well as in travelers or individuals from nonendemic countries exposed to the parasite.11 In other endemic areas (Latin America, Southeast Asia), SCGs contribute only ∼20% of all cases with active NCC.12–15 Patients with SCGs in India are usually young teenagers or young adults presenting with newly developed seizures.
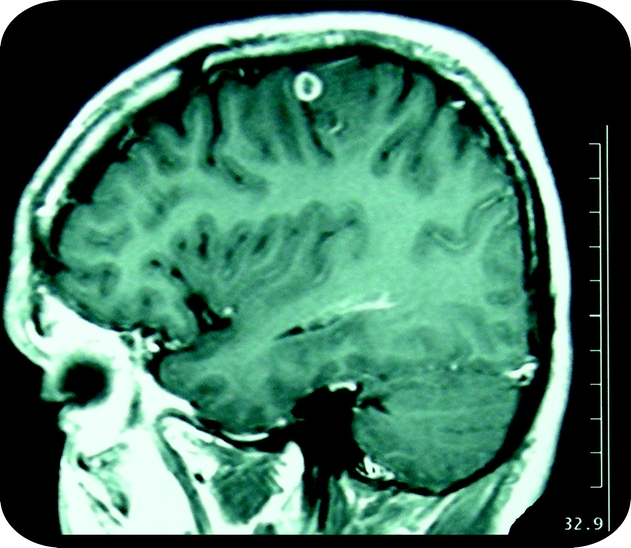
Figure Typical MRI (postcontrast T1) of a single enhancing lesion (single cysticercal granuloma)
SCGs were recognized as early as 1980 in CT images of Indian patients with seizures but were thought to be “microtuberculomas.”16 They were initially labeled disappearing or vanishing lesions as these lesions resolved spontaneously without specific therapy.17 A histologic study of these lesions subsequently showed that the vast majority of these lesions were degenerating T solium cysts and they were thus called cysticercal granulomas.18 Most of these will resolve spontaneously without cysticidal drug therapy by 1 year after presentation,19 leaving a calcified scar in approximately 20% of cases. Most patients with a SCG will have no further seizures, although 20% to 30% of cases will have seizure relapses. Further studies documented their occurrence in other NCC-endemic regions. SCGs are thus one of the most common forms of presentation of NCC and a major cause of acquired epilepsy all over the world. It cannot be ruled out, of course, that some patients presenting with a SCG had harbored one or more other brain parasitic larvae which died without leaving discernible scars.
The underlying theory supporting the benign evolution of SCGs is that they are a late stage of destruction of a previously established cyst. A major obstacle in understanding the pathophysiology of SCGs is that the time of ova ingestion is not known in most patients since longitudinal neuroimaging data on untreated patients from the time of ingestion are not available. However, there are major pieces of evidence that do not fit with this late-stage interpretation and suggest that in most cases, SCGs are parasites that degenerate and die in the early metacestode phase, likely as a result of the host immunity overcoming a mild infection. The following arguments support this hypothesis.
Epidemiologic
1. SCGs are the most frequent clinical presentation of NCC in India, where viable cysts are rarely seen except in a very small minority of patients with very heavy parasite loads. The epidemiologic scenario in India is clearly different from that in other parts of the world, with only a few individuals raising pigs and a vast subgroup being vegetarian or not eating pork. The particular epidemiologic characteristics of the Indian subcontinent are likely associated with few tapeworm carriers and less-direct transmission, thus favoring the occurrence of mild infections likely through some (yet unknown) mechanism of dispersion of transmission.
2. SCGs are the most frequent presentation of NCC seen in US or European travelers returning from endemic regions. It is likely that this population was exposed to mild egg challenges for a short period, compared to local people continuously at risk in endemic regions.
3. In the few imaging studies performed in the general population of endemic villages or other asymptomatic populations, brain calcifications are overwhelmingly more common than viable cysts or SCGs, demonstrating that many infections are mild and heal by natural evolution.20,21
4. The fact that SCGs occur in younger individuals than do viable cyst infections strongly argues against considering all SCGs as the result of the degeneration of an established infection. To eliminate the effect of the number of lesions, we compared the ages of a consecutive group of patients presenting to our unit with a SCG (n = 59) and those presenting with a single viable cyst (n = 49). Again, patients with a SCG were slightly younger than patients with a single viable cyst with or without inflammation (median age 22 years, interquartile range 19–29.5 vs median 28 years, interquartile range 21–39.5, p = 0.0960, one-way analysis of variance) (unpublished data, CWGP 2009).
Biological
5. Most helminth infections are overaggregated, meaning that only a few individuals harbor many parasites and most individuals have 1 or a few parasites. It follows that mild infections will be the most frequent biologic event. This has been clearly shown in the few series of necropsies of pigs from endemic villages and population-based CT studies in endemic villages.21–24
6. Almost by definition, SCGs are associated with low parasite burdens, and are not associated with extraneural cysticercosis. In pig necropsies, the number of cysts in the brain is proportional to the number of cysts found in the entire carcass.
7. In the pig model, degenerating cysts can be found both in artificially and naturally infected pigs, mostly animals with a few parasites. Pigs with heavier parasite burdens usually show homogeneously viable cysts.25 This is consistent with the host's immunity overcoming light infections but not heavy infections.
8. In heavily endemic regions, unlike the Indian subcontinent, most cases of symptomatic NCC present with multiple parasites and established cystic infections are frequent. Unlike SCGs, these cysts do not disappear in the short term (even 50% or more of those showing marked signs of inflammation persist as cysts after a period of 6 months),26 and the vast majority (∼80%) will leave a residual calcification.
Immunologic
9. Immunity to cysticercosis is not restricted to a reaction to the established cyst. First there is innate immunity. Second, stage-specific immunity anti-oncosphere and anti-immature metacestode has already been demonstrated in T solium and other cestodes.27,28 On the contrary, established cysts mount a complex immune evasion system which actively blocks the host's cellular response.29
10. Despite the fact that cysticercosis-specific antibodies are long-lasting,30 the antibody response in individuals with a single viable cyst is stronger than that in individuals with a SCG. If SCGs were the degeneration of a long-established cyst, the antibody response should be similar or even stronger (as happens in viable cysts even after treatment) given that parasite antigens are exposed at the time of the death of the parasite. In the above mentioned series from our group, SCGs were more frequently seronegative than were viable cysts (18/59 vs 7/49, p = 0.047, Pearson χ2 test), and were less likely to have stronger antibody reactions (4 or more bands on Western blot, 10/59 vs 17/49, p = 0.339, Pearson χ2 test).
DISCUSSION
Cysticercosis presents diverse clinical and imaging presentations. SCGs are the commonest presentation of NCC in India and in travelers, and are also found in ∼20% of NCC cases elsewhere. The therapeutic approach to a SCG has been the subject of intense controversy, varying from conservative observation, to routine use of antiparasitic therapy, and even a role for diagnostic brain biopsies has been considered in certain cases.12,31 Understanding SCG's physiopathology is crucial for sound diagnostic and therapeutic approaches.
Transient seropositive reactions32 and frequent cases of asymptomatic calcified NCC21–24 are consistent with a threshold under which parasite destruction normally occurs, potentially determined by the number of parasites, the immune status of the host, the age at infection, or any combination of these factors. If some kinds of parasite challenge (i.e., infections with low numbers of parasite eggs or those in immune individuals) are rapidly overcome by the host's immune response, it would explain the frequency of degenerating cysts clustered in younger ages, its relation to infections with a single parasite (90% of cases with only degenerating cysts), and its frequency in India, where because of vegetarian habits, direct exposure to tapeworm carriers its less frequent.33 Patients with only viable cysts as those seen frequently in South America, China, or other endemic regions would represent cases in which the parasites survive and establish for a long time. This would explain why the serologic diagnosis of SCGs is much less productive, the prognosis is better, and the effects of antiparasitic treatment are less apparent when compared to cystic, multilesional NCC. Alternatively or concomitantly, genetic differences in the human host population or the parasite could contribute to explain these differences.
COINVESTIGATORS
Cysticercosis Working Group in Peru: Manuel Martinez, MD; Manuel Alvarado, MD; Miguel Porras, MD; Herbert Saavedra, MD (Instituto Nacional de Ciencias Neurológicas, Lima, Peru, clinical neurology advisers); Manuela Verastegui, PhD; Holger Mayta, PhD (Universidad Peruana Cayetano Heredia, Lima, Peru, immunology and molecular biology advisers); Genaro Herrera, MD (Universidad Peruana Cayetano Heredia, Lima, Peru, neuroradiologist); Andres G. Lescano, PhD; Mirko Zimic, PhD (Universidad Peruana Cayetano Heredia, Lima, Peru, consultants in epidemiology and biostatistics); Maria T. Lopez, DVM, PhD; Cesar M. Gavidia, DVM, PhD; Maria Silva, DVM, PhD (School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru, veterinary parasitologists); Guillermo Gonzalvez, MD, MPH; Luz M. Moyano, MD; Viterbo Ayvar, DVM; and Andre Diaz, DVM (Cysticercosis Elimination Program, Tumbes, Peru, field epidemiologists).
DISCLOSURE
Dr. García serves as an Associate Editor of PLoS Neglected Tropical Diseases, as an editorial consultant for The Lancet, and on the editorial boards of the American Journal of Tropical Medicine and Hygiene, Experimental Parasitology, the World Journal of Gastroenterology, Annals of Neurosciences (India), and the Journal of Neuroparasitology; and receives research support from the NIH (NINDS R01 NS054805 [PI] and TW001140 [PI]), the Gates Foundation, and The Wellcome Trust. Dr. Gonzalez receives research support from the NIH (TW008273 [PI]). Dr. Rodriguez reports no disclosures. Dr. Tsang holds or has pending patents re: Diagnostic assay (EITB) for human cysticercosis; Compositions and methods for detecting adult Taenia solium; Method for detecting Cryptosporidium parvum oocysts; Method for detecting Cryptosporidium parvum oocysts, viability assay; Synthetic antigens for diagnosis of cysticercosis; and Synthetic antigens for diagnosis of taeniasis. Dr. Pretell and Dr. Gonzales report no disclosures. Dr. Gilman serves on a scientific advisory board for the Wellcome Trust and receives research support from the NIH (R21 AI072093 [PI], D43 TW006581 [PI], T35 AI065385 [PI], R01 HD059005 [PI], and R01 AI087776 [PI]) and the OPTIMUS Foundation.
Address correspondence and reprint requests to Dr. Hector H. Garcia, Cysticercosis Unit, Instituto Nacional de Ciencias Neurologicas, Jr. Ancash 1271, Barrios Altos, Lima 1, Peru hgarcia@jhsph.edu
Disclosure: Author disclosures are provided at the end of the article.
Received February 5, 2010. Accepted in final form May 5, 2010.
REFERENCES
- 1.Garcia HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Trop 2003;87:71–78. [DOI] [PubMed] [Google Scholar]
- 2.Rajshekhar V, Haran RP, Prakash GS, Chandy MJ. Differentiating solitary small cysticercus granulomas and tuberculomas in patients with epilepsy: clinical and computerized tomographic criteria. J Neurosurg 1993;78:402–407. [DOI] [PubMed] [Google Scholar]
- 3.Garg RK. Solitary cysticercus granulomas. J Neurosurg 1995;82:911–912. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol 2005;4:653–661. [DOI] [PubMed] [Google Scholar]
- 5.Flisser A. Taeniasis and cysticercosis due to Taenia solium. Prog Clin Parasitol 1994;4:77–116. [PubMed] [Google Scholar]
- 6.Escobar A. The pathology of neurocysticercosis. In: Palacios E, Rodriguez-Carbajal J, Taveras JM, eds. Cysticercosis of the Central Nervous System. Springfield: Charles C. Thomas; 1983:27–54. [Google Scholar]
- 7.Dixon HB, Lipscomb, FM. Cysticercosis: An Analysis and Follow-up of 450 cases. London: Medical Research Council; 1961. [Google Scholar]
- 8.Dixon HBF, Hargreaves WH. Cysticercosis (Taenia solium): a further ten years' clinical study, covering 284 cases. Q J Med 1944;13:107–121. [Google Scholar]
- 9.Dixon HBF, Smithers DW. Epilepsy in cysticercosis (Taenia solium): a study of seventy-one cases. Q J Med 1934;3:603–616. [Google Scholar]
- 10.Gonzalez AE, Falcon N, Gavidia C, et al. Time-response curve of oxfendazole in the treatment of swine cysticercosis. Am J Trop Med Hyg 1998;59:832–836. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld EA, Byrd SE, Shulman ST. Neurocysticercosis among children in Chicago. Clin Infect Dis 1996;23:262–268. [DOI] [PubMed] [Google Scholar]
- 12.Rajshekhar V. Etiology and management of single small CT lesions in patients with seizures: understanding a controversy. Acta Neurol Scand 1991;84:465–470. [DOI] [PubMed] [Google Scholar]
- 13.Singh MK, Garg RK, Nath G, Verma DN, Misra S. Single small enhancing computed tomographic (CT) lesions in Indian patients with new-onset seizures: a prospective follow-up in 75 patients. Seizure 2001;10:573–578. [DOI] [PubMed] [Google Scholar]
- 14.Chandy MJ, Rajshekhar V, Ghosh S, et al. Single small enhancing CT lesions in Indian patients with epilepsy: clinical, radiological and pathological considerations. J Neurol Neurosurg Psychiatry 1991;54:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Brutto OH. Solitary cysticercal granuloma in Latin America. In: Rajshekhar V, Chandy MJ, eds. Solitary Cysticercus Granuloma: The Disappearing Lesion. Chennai: Orient Longman; 2000:153–166. [Google Scholar]
- 16.Bhargava S, Tandon PN. Intracranial tuberculomas: a CT study. Br J Radiol 1980;53:935–945. [DOI] [PubMed] [Google Scholar]
- 17.Sethi PP, Wadia RS, Kiyawat DP, et al. Ring or disc enhancing lesions in epilepsy in India. J Trop Med Hyg 1994;97:347–353. [PubMed] [Google Scholar]
- 18.Chandy MJ, Rajshekhar V, Prakash S, et al. Cysticercosis causing single, small CT lesions in Indian patients with seizures. Lancet 1989;1:390–391. [DOI] [PubMed] [Google Scholar]
- 19.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004;62:2236–2240. [DOI] [PubMed] [Google Scholar]
- 20.Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004;62:1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montano SM, Villaran MV, Ylquimiche L, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology 2005;65:229–233. [DOI] [PubMed] [Google Scholar]
- 22.Cruz ME, Schantz PM, Cruz I, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol 1999;28:799–803. [DOI] [PubMed] [Google Scholar]
- 23.Del Brutto OH, Santibanez R, Idrovo L, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia 2005;46:583–587. [DOI] [PubMed] [Google Scholar]
- 24.Medina MT, Duron RM, Martinez L, et al. Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama Study. Epilepsia 2005;46:124–131. [DOI] [PubMed] [Google Scholar]
- 25.De Aluja AS, Villalobos NM, Nava G, et al. Therapeutic capacity of the synthetic peptide-based vaccine against Taenia solium cysticercosis in pigs. Vaccine 2005;23:4062–4069. [DOI] [PubMed] [Google Scholar]
- 26.Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 2004;350:249–258. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Allan C, Martinez N, Flisser A, Aluja A, Allan JC, Craig PS. Immunocharacterization of Taenia solium oncosphere and metacestode antigens. J Helminthol 1996;70:271–280. [DOI] [PubMed] [Google Scholar]
- 28.Verastegui M, Gilman RH, Garcia HH, et al. Prevalence of antibodies to unique Taenia solium oncosphere antigens in taeniasis and human and porcine cysticercosis. Am J Trop Med Hyg 2003;69:438–444. [PubMed] [Google Scholar]
- 29.Correa D, Medina E. Host-parasite immune relationship in Taenia solium taeniosis and cysticercosis. In: Garcia HH, Martinez SM, eds. Taenia solium Taeniasis/Cysticercosis, 2nd ed. Lima: Ed. Universo; 1999:15–24. [Google Scholar]
- 30.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy: Cysticercosis Working Group in Peru. J Infect Dis 1997;175:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajshekhar V, Chandy MJ. Solitary Cysticercus Granuloma: The Disappearing Lesion. Chennai: Orient Longman; 2000. [Google Scholar]
- 32.Garcia HH, Gonzalez AE, Gilman RH, et al. Short report: transient antibody response in Taenia solium infection in field conditions: a major contributor to high seroprevalence. Am J Trop Med Hyg 2001;65:31–32. [DOI] [PubMed] [Google Scholar]
- 33.Singh G. Neurocysticercosis in South-Central America and the Indian subcontinent: a comparative evaluation. Arq Neuropsiquiatr 1997;55:349–356. [DOI] [PubMed] [Google Scholar]


