Abstract
BACKGROUND AND OBJECTIVES:
Polycystic ovary syndrome (PCOS) is a disorder characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries. Little is known about cardiovascular risk factors in patients with PCOS. We investigated plasma markers of cardiovascular disease in Saudi women with PCOS, with an emphasis on asymmetric dimethylarginine (ADMA) and total homocysteine (tHcy).
PATIENTS AND METHODS:
Fifty Saudi women with PCOS diagnosed by the Rotterdam criteria (mean age [SD] 30.2 [3.0] years) and 40 controls without PCOS (mean age 29.3 [2.5] years) had measyrements taken of clinical, metabolic, and hormonal parameters, including plasma ADMA, tHcy, lipoprotein (a) ([Lp(a)], and serum high sensitivity C-reactive protein (hs-CRP), nitric oxid, and fibrinogen. Insulin resistance was calculated by the homeostasis model assessment (HOMA-IR).
RESULTS:
Women with PCOS had significantly higher fasting insulin, HOMA-IR, and luteinizing hormone (LH) levels than healthy controls (P<.001). Lipid profile, free androgen index (FAI), ADMA, tHcy, hsCRP, and Lp(a) were significantly higher in women with PCOS compared with healthy controls (P<.001). The women with PCOS had significantly lower nitric oxide and high-density lipoprotein cholesterol (HDL-C) levels compared with healthy controls (P<.001).
CONCLUSION:
Our study revealed that Saudi women with PCOS had a significantly different levels of plasma markers of cardiovascular disease compared with normal controls. Therefore, clinicians who manage women with PCOS should follow up on these markers to reduce the risk of cardiovascular disease.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of women of reproductive age and is associated with long-term health risks, including type 2 diabetes mellitus (T2DM) and coronary artery disease.1,2 Most women with PCOS also exhibit features of the metabolic syndrome, including insulin resistance (IR), obesity, and dyslipidemia.3,4 In 2001, Paradisi et al5 first reported that PCOS is characterized by endothelial dysfunction and IR. IR, hyperandrogenism, and dyslipidemia are likely to be the major risk factors for cardiovascular disease (CVD) in women with PCOS.6,7
Several biochemical markers have been identified as risk factors for CVD, including elevated levels of serum low-density lipoprotein cholesterol (LDL-C), total cholesterol, triglycerides (TG), total homocysteine (tHcy), high-sensitivity C-reactive protein (hsCRP), and IR, as well as reduced levels of high density lipoprotein cholesterol (HDL-C). Decreased nitric oxide bioavailability and hyperhomocysteinemia also increase the risk of CVD.6
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase.8 ADMA is considered an indicator for endothelial dysfunction9 and a sensitive marker for cardiovascular risk.10,11 An inverse correlation between endothelium-dependent vasodilatation and ADMA has also been demonstrated after fat intake and experimental hyperhomocysteinemia in humans.9 Hyperhomocysteinemia is another important risk factor for the development of CVD.12 Homocysteine is a sulfur-containing amino acid formed during the metabolism of methionine.11 It has been reported that high insulin levels are associated with increased plasma levels of tHcy in patients with preeclampsia.13
Also, hsCRP has been shown to be a good predictor of vascular events.14 In addition to being a marker of inflammation, there is evidence that hsCRP may have a direct role in atherogenesis via adhesion molecule expression, complement activation, and mediation of LDL uptake by macrophages.15 Similarly, raised fibrinogen concentration has been associated with an increased risk of ischemic heart disease and atherosclerosis. Fibrinogen may promote CVD by a variety of mechanisms, including increased blood viscosity, thrombus formation, or platelet aggregation.14 The aim of the present study was to investigate the plasma markers of CVD in Saudi women with PCOS. Special emphasis was given to ADMA and homocysteine.
PATIENTS AND METHODS
The study group comprised 50 Saudi women (aged 24 to 31 years) with primary infertility, who had been diagnosed as having PCOS (study group, aged 24 to 31 years) and were attending the outpatients infertility clinic at the Department of Obstetrics and Gynecology of Safa Al-Madinah Hospital, Al-Madinah, Saudi Arabia, from September 2008 to March 2009. Also, 40 healthy Saudi women volunteers (control group, aged 25 to 30 years) formed the control group in for this study. Women in the control group were healthy volunteers. The diagnosis of PCOS was made according to the Rotterdam European Society of Human Reproduction and Embryology (ESHRE)/American Society for Reproductive Medicine (ASRM)-sponsored PCOS Consensus Workshop Group guidelines.16 Exclusion criteria included hypertension, smoking, or endocrine disorders and those taking fertility drugs, oral anti-diabetic agents, or oral contraceptive pills, and endocrine disorders.
Venous blood samples were collected from an antecubital vein between 08:00 AM and 09:00 AM after an overnight fast. The samples were centrifuged, aliquoted and immediately frozen and stored at –80°C for biochemical analysis. A full physical examination was performed, including measurement of weight, height and waist and hip circumferences. Weight was measured with the subject wearing light clothing without shoes, and height was measured using a stadiometer. Body mass index (BMI) was calculated by using the formula: weight (in kg)/height (in meters).2 Waist circumference (WC) was measured with the patient standing, at a point midway between the lower costal margin and the iliac crest in the mid-axillary line. Blood pressure was measured manually with a sphygmomanometer. Before onset of the study, all women had a full physical examination and were asked to complete a general questionnaire. The study was approved by the Institutional Review Board of the Taibah University and supported partially by funds from the Deanship of Scientific Research (Project No. 73/427) in Taibah University, Al-Madinah, Saudi Arabia. Written informed consent was obtained from all women at the study entry.
Plasma glucose, total cholesterol, triglycerides (TG), HDL-C, and LDL-C levels were measured by the enzymatic method by using the specific commercial kits (Roche/Hitachi 902; Roche Diagnostics GmbH, Manheim, Germany). Plasma insulin, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone-binding globulin (SHBG), 17-hydroxy progesterone (17-OHP), and total testosterone, were determined by enzyme-linked immunosorbent Assay assay (ELISA) kits (R and D Systems, Minneapolis, USA). The concentration of Lp(a) was determined by immunoturbidometry with the Roche instrument and the Cobas Miro Mira Analyzer (Basel, Switzerland). The concentration of fibrinogen was determined using an immunoenzymatic set of markers (Sysmex CA540, Dade-Behring, Germany). The hsCRP concentration was determined using an immunoturbidimetric method (Randox, Mauguio, France). Plasma ADMA and tHcy concentrations were determined by competitive ELISA assay (DLD Diagnostica GmbH, Hamburg, Germany). Nitric oxide concentration was measured using a nitrite/nitrate colorimetric assay kit (Griess reaction, Cat No. 780001; Cayman Chemical, Ann Arbor, MI). All chemicals were of the highest available purity available and were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The free androgen index (FAI)17 and HOMA-IR18 were calculated.
All statistical analysis was performed using the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The t test was used for comparison of means. Data are expressed as mean and standard error of the mean. P values <.05 were considered statistically significant.
RESULTS
There were no significant differences in age, weight, height, body mass index (BMI), and systolic and diastolic blood pressures (BP) between the PCOS and control groups (Table 1). BMI was lower in healthy controls than in patients with PCOS, but these this differences did not achieve statistical significance. Compared with healthy women, patients with PCOS had significantly higher levels of fasting insulin, HOMA-IR, triglycerides, total cholesterol, LDL-C, and VLDL-C (P<.001) (Table 2). On the other hand, patients with PCOS had significantly lower levels of HDL-C (P<.001) and a lower glucose/insulin ratio compared with subjects in the control group (Table 2). In PCOS women, total testosterone, LH, LH/FSH ratio, and 17-OHP were significantly increased (P<.001) compared with those values in the control group. As expected, FSH was lower in PCOS women than in the control group, but these differences did not achieve statistical significance. Plasma SHBG level was also significantly lower in the PCOS patients group compared with control group. Free androgen index was significantly higher in the PCOS group (6.63%) compared with the control group (2.08%). Patients with PCOS had significantly higher concentrations of ADMA, tHcy, hsCRP, Lp(a), and fibrinogen (Figure 1). Nitric oxide was significantly lower in patients with PCOS.
Table 1.
Clinical features of women with polycystic ovary syndrome and healthy controls.
| Variables | Control (n=40) | PCOS (n=50) | P value |
|---|---|---|---|
| Age (years) | 29.3 (2.5) | 30.2 (3.0) | .412 |
| Weight (kg) | 57.8 (3.0) | 61.3 (3.3) | .222 |
| Height (cm) | 162.3 (6.4) | 166.7 (4.6) | .284 |
| Waist circumference (cm) | 87.3 (5.0) | 93.5 (7.0) | .246 |
| Hip circumference (cm) | 105.0 (8.0) | 117.0 (10) | .184 |
| Waist/Hip ratio | 0.83 (0.05) | 0.79 (0.03) | .238 |
| BMI (kg/m2) | 25.7 (2.3) | 29.3 (3.1) | .187 |
| SBP (mm Hg) | 128.0 (3.0) | 135.0 (4.0) | .091 |
| DBP (mm Hg) | 73.0 (2.0) | 77.0 (3.0) | .147 |
Data are expressed as mean (SEM); BMI: body mass index; WHR: waist-to-hip ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Table 2.
Metabolic, lipid, and hormonal profiles in women with polycystic ovary syndrome and healthy controls.
| Variables | Control (n=40) | PCOS (n=50) |
|---|---|---|
| Fasting plasma glucose (mg/dL) | 77.3 (3.2) | 82.0 (3.6) ns |
| Fasting insulin (pIU/mL) | 14.2 (1.2) | 25.3 (1.3)a |
| Glucose/insulin ratio | 4.9 (0.22) | 3.3 (0.13)a |
| HOMA-IR | 2.7 (0.11) | 5.1 (0.14)a |
| Triglycerides (mg/dL) | 70.6 (5.3) | 117 (6.4)a |
| Total cholesterol (mg/dL) | 170.3 (8.0) | 198.2 (6.0)a |
| HDL-C (mg/dL) | 46.5 (2.2) | 40.3 (1.4)a |
| LDL-C (mg/dL) | 100.6 (4.1) | 135 (4.3)a |
| VLDL-C (mg/dL) | 21.4 (1.0) | 26.6 (1.1)a |
| Luteinizing hormone (IU/L) | 5.6 (1.02) | 9.8 (1.05)a |
| Follicle stimulating hormone (IU/L) | 6.12 (1.3) | 4.7 (1.2) ns |
| Luteinizing hormone/Follicle stimulating hormone ratio | 0.91 (0.11) | 2.08 (0.14)a |
| Testosterone (ng/dL) | 33.2 (3.1) | 76.9 (5.3)a |
| SHBG (nmol/L) | 55.3 (2.8) | 40.2 (2.6)a |
| Free androgen index (%) | 2.08 (0.12) | 6.63 (1.3)a |
| 17-OHP (mg/dL) | 2.1 (0.13) | 3.2 (0.11)a |
Data are expressed as means (SEM).
PCOS: polycystic ovary syndrome; HDL: high high density lipoprotein; LDL: low low density lipoprotein; VLDL: very very low density lipoprotein;
SHBG: Sex hormone-binding globulin; 17-OHP: 17-hydroxy progesterone.
HOMA-IR: [glucose (in mg/dL×0.05551)×insulin (in μmIU/mL)l]/22.5; FAI=testosterone (nmol/L)/SHBG (nmol/L)×100.
Significantly different from healthy control (P< .01); ns: non-significant.
Figure 1.
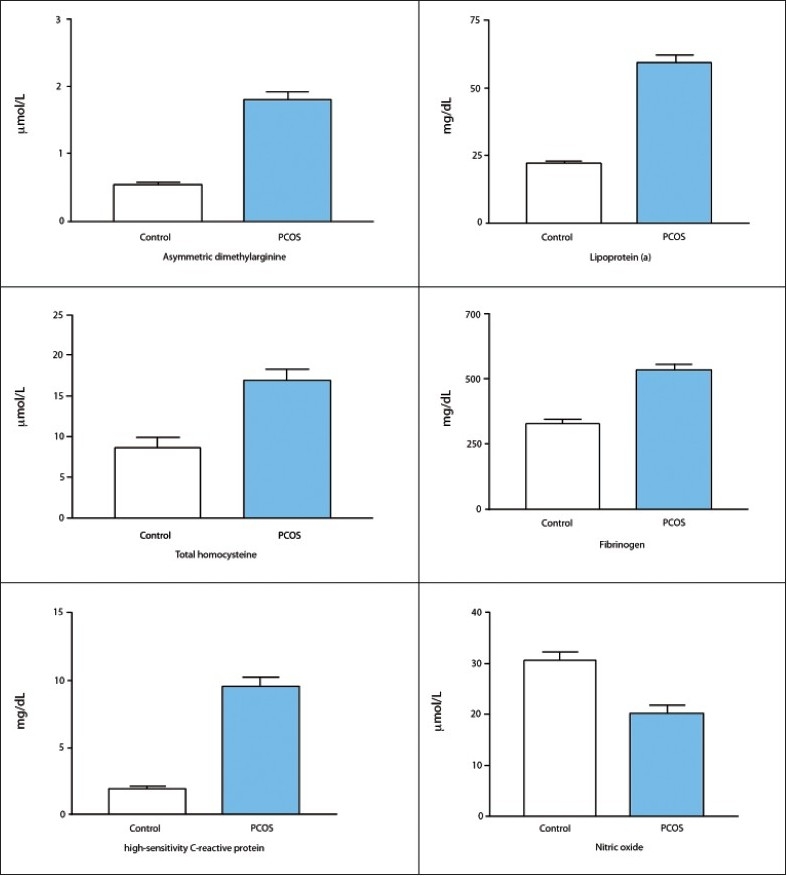
Plasma markers of cardiovascular disease in Saudi women with PCOS vs controls. All differences statistically significant (P<.001)
DISCUSSION
The present study shows that circulating ADMA, tHcy, hsCRP, Lp(a), and fibrinogen were higher in women with PCOS than in healthy controls. Our study confirms a positive association between these parameters and both IR and free androgen index. These findings are consistent with the results of several recent investigations.2,7,19 Hyperinsulinemia is a predictor of coronary artery disease, and IR has been proposed as a key factor linking linked to hypertension, glucose intolerance, obesity, lipid abnormalities, and coronary heart disease.4 IR may, in part, contribute indirectly to cardiovascular risk in PCOS by amplifying androgen excess. As a consequence of IR, PCOS patients often have an abnormal lipid profile and an increased incidence of cardiovascular risk factors.6 High levels of total cholesterol and LDL-C, and low levels of HDL-C are the most frequent forms of dyslipidemia, observed in insulin resistance states. Lower HDL-C level is another independent of risk factor for CVD associated with PCOS.20 As expected, in our study, we observed a significant increase in total cholesterol, LDL-C, and TG and a reduction in HDL-C in women with PCOS. Our results are in accordance with recent studies by Macut et al19 and Valkenburg et al20 who reported that lipid profile parameters were increased in patients with PCOS, suggesting that women with PCOS have a higher risk for CVD.
The results of the current study suggest that hyperandrogenemia in patients with PCOS has the strongest association with the presence of IR. This finding is supported by several other studies.19,21 IR, with elevated circulating insulin levels, induces unfavorable changes in lipid metabolism and increased androgen production from theca cells.22 At the same time, androgen excess may support the presence of an unfavorable metabolic state and lead to dyslipidemia. The mechanism by which hyperandrogenemia might affect vascular reactivity is still unknown. In experimental models, testosterone influences vasocontractile responses, and impairs endothelium-dependent relaxation in hypercholesterolemic rabbits and monkeys.22,23 Moreover, androgens may act synergistically with IR and inflammatory cytokines on vascular endothelial function.1
Elevated hsCRP was reported as a proinflammatory agent and a risk factor for the CV events.14 The present study demonstrated that circulating hsCRP levels were higher in women with PCOS compared with controls. Our results are in agreement with those reported by Heutling et al24 who documented higher hsCRP in women with PCOS than in healthy controls. Our data strengthen the idea that there is an increased risk of CVD events in women with PCOS.
Like other risk factors of CVD, hyperhomo-cysteinemia is associated with endothelial dysfunction, an early and reversible event of atherogenesis. Endothelial dysfunction results in reduced nitric oxide bioavailability and increased lipid peroxidation and the formation of atherogenic oxidized low density lipoprotein.25 High homocysteine levels are a risk factor for CVD because of the increased oxidative stress in the vascular endothelium and activation of platelet aggregation.26 In the present study, women with PCOS had significantly higher tHcy and lower nitric oxide levels, compared controls. Our data are in agreement with those reported by Badawy et al27 who also documented higher tHcy concentrations in women with PCOS.
In the present study, the plasma concentration of ADMA was significantly higher in women with PCOS compared with controls. The possible mechanisms of elevation of ADMA in hyperhomocysteinemia include reduced renal excretion28 and decreased activity of the hydrolase enzyme, which metabolizes ADMA.29
The correlations found in this study between nitric oxide bioavailability, hyperhomocysteinemia, chronic inflammation, IR, and hyperandrogenemia support the concept of interplay among these agents on the vascular bed in the pathophysiology of CVD in women with PCOS. Therefore, since impaired nitric oxide bioavailability is one of the first steps in atherogenesis, further evaluation of ADMA, tHcy, Lp(a), and hsCRP as potential markers in this these high-risk patients may have clinical relevance.
In conclusion, plasma ADMA, tHcy, Lp(a), and fibrinogen concentrations were elevated in Saudi women with PCOS compared to healthy women. Plasma nitric oxide and HDL-cholesterol were not significantly different between the two groups. This finding highlights the clinical importance of the assessment of the above mentioned markers in women with PCOS to reduce the risk of CVD.
Acknowledgments
The study was supported partially by funds from the Deanship of Scientific Research (Project No. 73/427) in Taibah University, Al-Madinah, KSA. We thank all participants in our research and the biochemical laboratory personnel.
REFERENCES
- 1.Christakou CD, Diamanti-Kandarakis E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Womens Health (Lond Engl) 2008;4:583–94. doi: 10.2217/17455057.4.6.583. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran S, Willoughby SR, Chan WP, Liberts EA, Heresztyn T, Saha M, et al. Polycystic ovary syndrome is associated with severe platelet and endothelial dysfunction in both obese and lean subjects .Atherosclerosis. 2009;204:509–14. doi: 10.1016/j.atherosclerosis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Chae SJ, Kim JJ, Choi YM, Hwang KR, Jee BC, Ku SY, et al. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod. 2008;23:1924–31. doi: 10.1093/humrep/den239. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzo A, Amato MC, Giordano C. Insulin resistance and polycystic ovary syndrome. Nutr Metab Cardiovasc Dis. 2008;18:511–8. doi: 10.1016/j.numecd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 6.Giallauria F, Orio F, Palomba S, Lombardi G, Colao A, Vigorito C. Cardiovascular risk in women with polycystic ovary syndrome. J Cardiovasc Med (Hagerstown) 2008;9:987–92. doi: 10.2459/JCM.0b013e32830b58d4. [DOI] [PubMed] [Google Scholar]
- 7.Moran LJ, Hutchison SK, Meyer C, Zoungas S, Teede HJ. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Sci (Lond) 2009;116:761–70. doi: 10.1042/CS20080218. [DOI] [PubMed] [Google Scholar]
- 8.Sydow K, Mondon CE, Cooke JP. Insulin resistance: Potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med. 2005;10:S35–43. doi: 10.1177/1358836X0501000106. [DOI] [PubMed] [Google Scholar]
- 9.Lentz SR, Rodionov RN, Dayal S. Hyperhomocysteinemia, endothelial dysfunction, and cardiovascular risk: The potential role of ADMA. Atheroscler Suppl. 2003;4:61–5. doi: 10.1016/s1567-5688(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 10.Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. ADMA, cardiovascular disease and diabetes. Diabetes Res Clin Pract. 2008;82:S122–6. doi: 10.1016/j.diabres.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. 2009;35:120–9. doi: 10.1002/biof.17. [DOI] [PubMed] [Google Scholar]
- 13.Malek-khosravi S, Kaboudi M, Kaboudi B, Atefi G. Plasma homocysteine concentrations and insulin resistance in preeclampsia. Hypertens Pregnancy. 2009;28:13–22. doi: 10.1080/10641950802233049. [DOI] [PubMed] [Google Scholar]
- 14.Tekin IO, Pocan B, Borazan A, Ucar E, Kuvandik G, Ilikhan S, et al. Positive Correlation of CRP and Fibrinogen Levels as Cardiovascular Risk Factors in Early Stage of Continuous Ambulatory Peritoneal Dialysis Patients. Ren Fail. 2008;30:219–25. doi: 10.1080/08860220701813350. [DOI] [PubMed] [Google Scholar]
- 15.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–7. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 17.Clark AF, Marcellus S, deLory B, Bird CE. Plasma testosterone free index: A better indicator of plasma androgen activity? Fertil Steril. 1975;26:1001–5. [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostatic model assessment: Insulin resistance and _-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Macut D, Panidis D, Glisi_ B, Spanos N, Petakov M, Bjeki_ J, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. 2008;86:199–204. doi: 10.1139/Y08-014. [DOI] [PubMed] [Google Scholar]
- 20.Valkenburg O, Steegers-Theunissen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, et al. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: A case-control study. J Clin Endocrinol Metab. 2008;93:470–6. doi: 10.1210/jc.2007-1756. [DOI] [PubMed] [Google Scholar]
- 21.Lindholm A, Andersson L, Eliasson M, Bixo M, Sundström-Poromaa I. Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;102:39–43. doi: 10.1016/j.ijgo.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo M, Rini GB, Carmina E. Androgen excess and cardiovascular risk. Minerva Endocrinol. 2007;32:67–71. [PubMed] [Google Scholar]
- 23.Hutchison SJ, Sudhir K, Chou TM, Sievers RE, Zhu BQ, Sun YP, et al. Testosterone worsens endothelial dysfunction associated with hypercholesterolemia and environmental tobacco smoke exposure in male rabbit aorta. J Am Coll Cardiol. 1997;29:800–7. doi: 10.1016/s0735-1097(96)00570-0. [DOI] [PubMed] [Google Scholar]
- 24.Heutling D, Schulz H, Nickel I, Kleinstein J, Kaltwasser P, Westphal S, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab. 2008;93:34–6. doi: 10.1210/jc.2007-0842. [DOI] [PubMed] [Google Scholar]
- 25.Romerio SC, Linder L, Nyfeler J, Wenk M, Litynsky P, Asmis R, et al. Acute hyperhomocysteinemia decreases NO bioavailability in healthy adults. Atherosclerosis. 2004;176:337–44. doi: 10.1016/j.atherosclerosis.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–56. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 27.Badawy A, State O, El Gawad SSh, El Aziz OA. Plasma homocysteine and polycystic ovary syndrome: The missed link. Eur J Obstet Gynecol Reprod Biol. 2007;131:68–72. doi: 10.1016/j.ejogrb.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, et al. Asymmetric Dimethylarginine and Reduced Nitric Oxide Bioavailability in Young Black African Men. Hypertens. 2007;49:873–7. doi: 10.1161/01.HYP.0000258405.25330.80. [DOI] [PubMed] [Google Scholar]
- 29.Fleck C, Schweitzer F, Karge E, Busch M, Stein G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in patients with chronic kidney diseases. Clin Chim Acta. 2003;336:1–12. doi: 10.1016/s0009-8981(03)00338-3. [DOI] [PubMed] [Google Scholar]


