Abstract
The relative impact of two community-based vector control strategies on house infestation by Triatoma infestans and Trypanosoma cruzi infection in bugs, domestic dogs and cats was assessed in two neighboring rural areas comprising 40 small villages and 323 houses in one of the regions most endemic for Chagas disease in northern Argentina. The prevalence and abundance of domestic infestation were 1.5- and 6.5-fold higher, respectively, in the area under pulsed, non-supervised control actions operating under the guidelines of the National Vector Control Program (NCVP) than in the area under sustained, supervised surveillance carried out jointly by the UBA research team and NCVP. The prevalence of infestation and infection varied widely among village groups within each area. In the pulsed control area, the prevalence of infection in bugs, dogs and cats was two- to three-fold higher than in the area under sustained surveillance, most of the infected animals qualified as autochthonous cases, and evidence of recent transmission was observed. Infection was highly aggregated at the household level and fell close to the 80/20 rule. Using multiple logistic regression analysis clustered by household, infection in dogs was associated positively and significantly with variables reflecting local exposure to infected T. infestans, thus demonstrating weak performance of the vector surveillance system. For high-risk areas in the Gran Chaco region, interruption of vector-mediated domestic transmission of T. cruzi requires residual insecticide spraying that is more intense, of a higher quality and sustained in time, combined with community participation and environmental management measures.
Keywords: Chagas disease, Dogs, Cats, Risk factors, Surveillance, Sentinel, Trypanosoma cruzi, Triatoma infestans, vector control
1. Introduction
Triatoma infestans, the main vector of Chagas disease in the southern cone of South America, has been the target of various vector control programs for several decades. The Southern Cone Initiative, a regional effort to eliminate T. infestans and to interrupt transmission of Trypanosoma cruzi, was launched in 1991 (Dias et al., 2002). At present, vector-mediated transmission to humans has been interrupted in Chile, Uruguay, Brazil and parts of Argentina and Paraguay (Schofield et al., 2006; WHO, 2002). However, transmission was not interrupted in the Gran Chaco, a natural landscape unit extending over northern Argentina, Bolivia, Paraguay and southwestern Brazil, where 4 million people live in a subsistence economy (Gürtler, in press).
Chagas disease vector control programs have typically not achieved the desired levels of vector control or suppression mainly because of interruption in provision of funding and resources, and due to reduced political will (Dias et al., 2002). In Argentina, the National Vector Control Program (NVCP) of T. infestans was implemented with a vertical structure in the early 1960s (Segura, 2002). Following disorganized and incomplete decentralization in the early 1980s, the Argentine NVCP adopted in 1992 a new vector control strategy (“Plan Ramón Carrillo”). This program was based on training of local villagers and primary health care agents in insecticide spraying activities and in monitoring house reinfestation (Segura, 2002). Community leaders and primary health care agents were provided with manual compression sprayers and pyrethroid insecticides, and kept records of bug notifications and insecticide treatments over time. Vector control activities comprised an initial attack phase, in which all houses in a village were scheduled to be sprayed twice over a six-month period, and a surveillance phase, in which all houses were scheduled for monitoring of reinfestation and selective sprays. Over 1993–2001, all of the 961,500 rural houses in the endemic area were reported to have been sprayed with insecticides during the attack phase, and 85% of the houses were considered to be under surveillance (Segura, 2002). This community-based vector control program was gradually discontinued during the late 1990s, and the reported number of Chagas acute cases subsequently increased, most notably in Santiago del Estero and other neighboring provinces in the Argentine Chaco. Between 1998 and 2004, insecticide spraying rates have remained low at some 60,000 houses per year, and since then vector control actions have been rather erratic and mainly depended on the political will, knowledge and budget of each province. The actual impact of cumulative control actions on domestic and peridomestic infestation with T. infestans and T. cruzi transmission has not been assessed.
As part of a longitudinal study aimed at modeling the transmission dynamics and control of T. cruzi in a well-defined rural area in Santiago del Estero, all houses from five neighboring villages (the core area, centered in Amamá village) have been regularly monitored for reinfestation over a decade after community-wide residual application of insecticides in 1992 (Gürtler et al., 1999; Cohen and Gürtler, 2001). The vector surveillance program was based on community participation and professional focal spraying in 1993–1995, and on selective insecticide treatments conducted by householders and community leaders between 1996 and 2004 (Cecere et al., 1999, 2002). The long-term impact of such actions on domestic and peridomestic infestation with T. infestans and T. cruzi transmission was assessed by means of entomological indices and by using domestic dogs as natural sentinels of transmission (Cardinal et al., 2006a) to avoid regular screening surveys of the local human populations. The prevalence of T. cruzi infection in dogs decreased from 65.1% at baseline to 4.7% at 10 years after sustained vector surveillance, whereas the average annual force of infection dropped 260-fold from 72.7 per 100 dog-years at baseline to <0.3% in 2002 (Gürtler et al., 2005; Cardinal et al., 2006a).
In this study we assessed the relative impact of two community-based vector control strategies on infestation and transmission of T. cruzi to T. infestans, domestic dogs and cats in two neighboring rural areas. The core area was under sustained, community-based surveillance supervised jointly by the University of Buenos Aires (UBA) research team and NCVP since 1992. The peripheral area, operating under the guidelines of the “Plan Ramón Carrillo” program between 1993 and 1999 and variations of it thereafter, experienced pulsed, non-supervised, community-based insecticide applications promoted by NCVP. Because of the fast recovery rate of local T. infestans populations and of domestic transmission in the absence of sustained surveillance (Gürtler et al., 2005), we hypothesized that domestic infestation and transmission would be more intense in the area under pulsed, non-supervised vector control actions, and in those households having infected dogs or cats.
2. Materials and methods
2.1. Study area
Field studies were carried out in several villages around Amamá (27°12′33″S, 63°02′10″W), Moreno department, in the Province of Santiago del Estero, Argentina. The Moreno department reported the highest number of human acute cases of Chagas disease notified in Argentina. The study area has been described elsewhere (Ceballos et al., 2006, Cecere et al., 2002). Briefly, two areas were visited: i) the core, including Amamá, Trinidad, Mercedes, Villa Matilde and Pampa Pozo, with 137 houses, and ii) the periphery surrounding the core area, which includes 33 villages and 2 isolated settlements with 186 houses (La Celestina, San Ramón, San Isidro, El Rosario, San Salvador, Fracción El Rosario, Armonía, San Ignacio, San Pedro, Santos Lugares, San Roque, Santo Domingo, Gondra, Cañada Central, Central Dolores, Esperanza, La Dormida, Laprida, Las Cañitas, Villa Isabel, San Pablo, San Luis, Barrio Pampa Pozo, La Curva, Lote S, Dolores, Escudero, La Favorita, La Nueva Fortuna, Tacoyura, Santa Ana, Santa María, Santo Domingo de los Cisneros, Turquía and Villa Napenay). In the peripheral area, official vector control activities have been conducted mostly in two pulses (in 1993–1996 under “Plan Ramón Carrillo”, and in 2000–2001, when the recent occurrence of symptomatic acute cases of Chagas disease prompted a new round of residual spraying with insecticides. As part of the current investigation, in April 2004 all houses were sprayed with pyrethroid insecticides by NVCP personnel supervised by the research team and monitored for reinfestation thereafter.
2.2. Triatomine surveys
Two teams of four people each visited a total of 186 houses of the peripheral area in October-November 2002. Nearly all (93.4%) of the 137 houses from the core area had been surveyed in October and the results were reported (Cardinal et al., 2006a). A questionnaire inquiring on householders’ practices of vector control and perception of triatomine infestation was completed for each household as described elsewhere (Cecere et al., 1998). Briefly, villagers were asked when the last insecticide spraying of the house was done, who did it, which structures were sprayed, which insecticide was applied and the amount of insecticide used.
Skilled bug collectors from NVCP searched for triatomine bugs in all bedrooms (one person) and in peridomestic areas (two persons) of all compounds in which householders were present (n = 176) using a dislodgant spray (0.2% tetramethrin, Icona, Buenos Aires, Argentina) for 30 minutes per compound (Gürtler et al., 1999). Peridomestic structures included corrals for goats, sheep, cows, horses and pigs, chicken coops, trees where chicken roosted, storerooms, kitchens and other possible refuges for triatomines within the area of human activity. Householders were encouraged to collect invading triatomines, and were given plastic bags to keep them until our next visit. All bugs were later identified to species and stage at the field laboratory and counted per site. All live or moribund third to fifth instars and adults of T. infestans were individually examined for T. cruzi infection within 10 days of capture; Triatoma guasayana and Triatoma garciabesi bugs were separately examined in pools of ≤3 insects from the same site. Bug feces were diluted with saline solution and microscopically examined at 220–400× magnification. Minicircle-DNA based polymerase chain reaction applied to fecal lysates from T. infestans detected T. cruzi in 91% of microscope-positive bugs and only in 3.4% of microscope-negative bugs, but not in the other triatomine species collected (Marcet et al., 2006; Schijman et al., 2006).
2.3. Domestic animal surveys
A subset of the villages (58%) inspected for infestation and less distant from Amamá was chosen for the domestic animal surveys. A house-to-house census of all dogs and cats was undertaken in three surveys (November 2002, March and July 2003) totaling 103 houses from 17 villages and 2 isolated settlements of the periphery. A standard demographic questionnaire was completed for each animal (Cardinal et al., 2006a). Villagers were asked the name, age, sex and place of birth of each dog and cat they owned; the name of the animal’s mother, the animal’s history of visiting or residing in villages outside the study area, the animal’s main function in the household and other reproductive and behavioral details.
A total of 221 (85%) of 260 dogs and 61 (76%) of 80 cats was examined for infection. All animal and blood sample processing were conducted according to the Institutional Animal Care and Use Committee protocol No. 04223 at University of Illinois at Urbana-Champaign as reported elsewhere (Cardinal et al., 2006a). Thirty dogs and 18 cats aged < 3 months were examined only by xenodiagnosis, whereas older animals were bled by venipuncture and diagnosed serologically; a subset of the latter was also examined by xenodiagnosis (124 dogs and 30 cats). Five dogs and 10 cats older than 3 months that could not be bled were examined only by xenodiagnosis. Six seropositive dogs were examined by xenodiagnosis in March or July 2003 to confirm T. cruzi infection and to isolate parasites; one seropositive dog died before being examined by xenodiagnosis.
2.4. Serodiagnosis and xenodiagnosis
Sera from dogs were tested for antibodies to T. cruzi by indirect hemagglutination assay (IHA; Polychaco, Buenos Aires, Argentina), indirect immunofluorescence test (IFAT) and enzyme-linked immunosorbent assay (ELISA) using standardized procedures and criteria reported elsewhere (Lauricella et al., 1998). Sera from cat were tested for antibodies to T. cruzi by IHA (Polychaco, Buenos Aires, Argentina), IFAT (total antigamma LID, Laboratorio Inmunodiagnóstico, Buenos Aires, Argentina) and ELISA (goat anti-cat IgG HRP, Santa Cruz Biotechnology, Santa Cruz, California). Titers ≥1:16 (IHA and IFAT) or an optical absorbance ≥0.2 (ELISA) were used as cutoff values for dogs and cats (Lauricella et al., 1998). Xenodiagnosis was performed using 20 laboratory-reared, third- or fourth-instar nymphs of T. infestans per animal for 25 minutes (Gürtler et al., 2007). Pools of feces from 5 bugs each that fed on a given animal were examined for T. cruzi infection at 400× magnification 30 and 60 days after feeding. Bugs from each positive pool were reexamined individually. ‘Seropositive’ refers to samples reactive by at least two different serologic tests among ELISA, IHA or IFAT. ‘Infected’ means animals having positive xenodiagnosis and/or being seropositive to T. cruzi. The results of serodiagnosis and xenodiagnosis were combined to calculate the composite prevalence of T. cruzi infection.
2.5. Data analysis
Fisher’s exact tests were used to compare proportions of age classes, dog-to-human ratios, infestation or infection between interventions areas, or proportions of infection between dogs and cats. Mann-Whitney tests were used to compare mean host numbers per house and mean number of visits from the NVCP personnel between intervention areas. Villages within each intervention area less distant than 2 km. apart were grouped by vicinity for comparisons.
To evaluate the relationship between potential risk factors and T. cruzi infection in dogs (n = 221) and cats (n = 61) from the pulsed control area, unadjusted odds ratios (OR) and 95% confidence intervals (CI) for univariate analyses were calculated by Woolf’s method. The demographic variables considered were age (in months, a surrogate of length of exposure); sex; permanent local residence (two levels: natives without history of travel outside of the study villages, i.e., animals with permanent residence; rural or urban immigrants or natives with history of travel); the number of T. cruzi-infected dogs or cats the animal cohabited with (0 to 4), and hunting habit (two levels). Reference levels were the lowest age group, males, impermanent local residence, not cohabiting with an infected animal, and no hunting habit. Vector control variables were derived from NVCP records and from householders’ answers to the questionnaire, and included the occurrence of insecticide spraying at the animal’s household during the animal’s lifetime (two levels). Variables computed for each household were intensity of insecticide spraying (three levels: houses sprayed 3, 2 or ≤ 1 times during 1994–2002); spraying operator (two levels: professional or not (i.e., community leader, householder)); insecticide spray coverage (four levels: house compound completely sprayed, house compound partially sprayed (i.e., at least one peridomestic structure not sprayed), only domestic structures sprayed, and no spray at all), and years since last insecticide spray (three levels: sprayed during the previous 4 years, 5–9 years, and ≥10 years or never sprayed). The entomological variables, derived from timed manual searches and householders’ bug collections at each compound, included the occurrence (two levels) and the proportion of T. cruzi-infected T. infestans among bugs caught in all structures at the animal’s compound; the relative abundance of T. infestans (three levels: no bugs, 1–9 bugs, and ≥10 bugs per 0.5 person-hour) captured at the animal’s compound (nearby peridomestic sites, which included domestic areas, kitchens, storerooms and ovens, and other peridomestic sites, mainly corrals and chicken coops) in each of two strata defined according to previous evidence on the occurrence of infected T. infestans, and the occurrence of T. guasayana or T. garciabesi at domestic or peridomestic areas at the animal’s compound (two levels). Adjusted OR and CI were estimated from maximum-likelihood logistic multiple regression analysis clustered on dog’s compound to provide robust standard errors (Stata 9.0, College Station, TX). Backward and forward stepwise procedures were used to obtain the most parsimonious model that retained independent variables at the 5% nominal significance level. Interaction terms were then added to this model and tested for significance.
3. Results
A total of 1,408 people was registered in the study area (Table 1). The mean number of people per house (4.8-4.6 persons) and the percentage younger than 15 years of age (40%–45%) did not differ significantly between areas. House construction materials were similar between areas, and wall plasters were very cracked or nonexistent in 40%–48% of the houses. The area under pulsed control actions had significantly more thatched roofs, peridomestic sites and chickens per house than the area under sustained surveillance. A higher ratio of dogs to humans and mean numbers of goats per house were also recorded in the area under pulsed control actions. According to NVCP records, no houses were sprayed in the pulsed control actions area during 1996–1998; spray coverage was low during 1999 (8%), peaked in 2000 (44%), decreased in 2001 (7%), and no spraying actions were recorded thereafter. However, in the area under sustained surveillance over the five-year period 1997–2002, selective insecticide spraying of domiciles was always >0% and ranged from 6% to 27%. Villages under pulsed control actions experienced significantly less visits from NVCP personnel between 1993 and 2002 (3.5 visits) than villages under sustained surveillance (9.4 visits) (Table 1).
Table 1.
Characteristics of two neighboring rural areas under different community-based vector control strategies in Santiago del Estero, October 2002.
| Area under sustained surveillance | Area under pulsed control actions | Significance test | |
|---|---|---|---|
| Total population (% <15 years) | 595 (45%) | 813 (40%)b | P = 0.07 |
| Mean number of people per house (SD) | 4.8 (2.5) | 4.6 (3.0)a | P > 0.25 |
| Dog-to-human ratio (total number of dogs) | 0.47 (279) | 0.58 (475)b | P < 0.0001 |
| Houses with wall plaster very cracked or nonexistent | 40% | 48%b | P = 0.23 |
| Houses with thatched roof | 87% | 96%b | P = 0.006 |
| Mean number of peridomestic sites per house (SD) | 4.2 (2.1) | 5.4 (3.0)a | P < 0.001 |
| Mean number of chickens per house (SD) | 17.6 (16.1) | 20.2 (14.9)a | P < 0.05 |
| Mean number of goats per house (SD) | 6.2 (9.3) | 8.2 (11.7)a | P > 0.15 |
| Mean number of visits from NVCP personnel (SD) | 9.4 (1.1) | 3.5 (1.5)a | P < 0.001 |
Mann-Whitney test,
Fisher’s test.
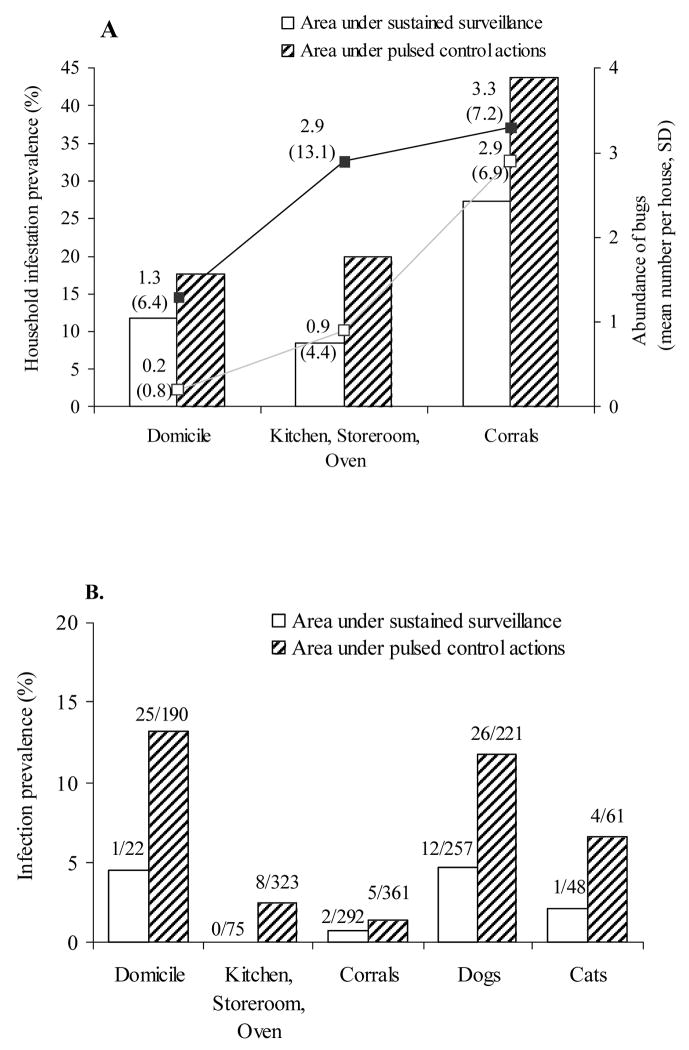
The prevalence and abundance of domestic infestation were 1.5- and 6.5-fold higher, respectively, in the area under pulsed control actions than in the area under sustained, supervised surveillance (Fig. 1A). Infestations in nearby peridomestic sites (P = 0.009) and corrals (P = 0.004) in the pulsed control area were significantly higher than in the area under sustained surveillance. Chicken coops and goat or pig corrals were the peridomestic structures most frequently found infested and with the highest bug abundance in each area.
Fig. 1.
A, Prevalence and abundance of Triatoma infestans and B, prevalence of Trypanosoma cruzi infection in Triatoma infestans, dogs and cats from two neighboring rural areas under different vector control strategies in Santiago del Estero, October 2002–July 2003. The mean abundance of bugs and (standard deviation) are shown beside squares (A). Numbers on top of bars are individuals infected/examined for infection (B).
The prevalence of T. infestans infected with T. cruzi in domiciles was 2.9-fold higher in the area under pulsed control actions (13.2%) than in the area under sustained surveillance (4.5%) (P = 0.04) (Fig. 1B). Bug infection prevalence was highest in domiciles in both areas, lower in nearby peridomestic sites in the area under pulsed control actions (2.5%), and rare in corrals (<1.4%). The bug infection prevalence for all peridomestic structures pooled together was 1.8% (n = 737) for T. infestans. No T. garciabesi (n = 151) or T. guasayana (n = 25) were found infected with T. cruzi. In the area under sustained surveillance, T. cruzi infection was only detected in 2.5% of T. infestans males (n = 120); no infection was found among third or fourth instars (n = 74), fifth instars (n = 113) and females (n = 82). In the pulsed control area, infection increased from 0% in third or fourth-instars (n = 175) to 3.3% in fifth instars (n = 243), and to 5.6% in males (n = 288) and 8.3% in females (n = 168).
The composite prevalence of T. cruzi infection in the pulsed control area relative to the sustained surveillance area was 2.5-fold higher in all dogs (11.8% vs. 4.7%, P = 0.006) and 3.1-fold higher in all cats (6.6% vs. 2.1%, P = 0.38) (Fig. 1B). In the pulsed control area, dogs (11.8%) had a 1.8-fold higher prevalence of T. cruzi than cats (6.6%), but this difference was also not statistically significant (P = 0.35).
Within each intervention area, the prevalence of infested houses and of T. cruzi-infected bugs, dogs and cats varied widely among village groups (Table 2). However, the area under pulsed control actions showed the highest prevalence of infestation in domiciles (83%) and peridomestic sites (100%), and the highest prevalence of infection in bugs (12.7%), dogs (20.0%) and cats (16.7%).
Table 2.
Prevalence of infestation by Triatoma infestans and infection with Trypanosoma cruzi in Triatoma infestans, dogs and cats from two neighboring rural areas under different community-based vector control strategies in Santiago del Estero, October 2002– July 2003; nd, no data available.
| Prevalence of infestation (%) | Prevalence of infection (number examined) | |||||
|---|---|---|---|---|---|---|
| Village group | Number of houses | Domestic | Peridomestic | Bugs | Dogs | Cats |
| Area under pulsed control actions b | ||||||
| Celestina | 7 | 0 | 57 | 0.0 (39) | nd | nd |
| Central Dolores | 48 | 9 | 57 | 0.4 (254) | 0.0 (26) | 0.0 (11) |
| Escudero | 22 | 10 | 40 | 3.1 (96) | nd | nd |
| Gondra | 6 | 0 | 20 | 0.0 (1) | 0.0 (14) | 0.0 (1) |
| Laprida | 20 | 5 | 63 | 1.5 (137) | nd | nd |
| La Curva | 6 | 83 | 100 | 2.7 (73) | 20.0 (20) | 0.0 (4) |
| Pampa Pozo Barrio | 2 | 0 | 0 | - | 0.0 (7) | - |
| Rosario | 39 | 37 | 49 | 8.5 (235) | 17.1 (76) | 16.7 (18) |
| San Pablo | 8 | 0 | 25 | 0.0 (17) | 6.3 (16) | 0.0 (6) |
| Santo Domingo | 26 | 23 | 46 | 12.7 (71) | 13.6 (59) | 5.0 (20) |
| Total | 186 | 17.6 | 50.7 | 4.0 (923) | 11.8 (221) | 6.6 (61) |
| Mean (SD)a | 18.5 (25.7) | 50.7 (22.1) | 3.6 (4.6) | 8.1 (8.7) | - | |
| Area under sustained surveillance | ||||||
| Amamá | 66 | 12 | 35 | 1.1 (176) | 5.6 (144) | 0.0 (22) |
| Mercedes | 34 | 6 | 30 | 0.0 (117) | 5.7 (53) | 0.0 (12) |
| Pampa Pozo | 9 | 0 | 33 | 0.0 (41) | 0.0 (17) | 0.0 (7) |
| Trinidad | 28 | 23 | 23 | 1.8 (56) | 2.3 (44) | 14.3 (7) |
| Total | 137 | 11.7 | 31.3 | 0.8 (390) | 4.7 (258) | 2.1 (48) |
| Mean (SD)a | 9.0 (9.9) | 19.3 (11.6) | 0.7 (0.9) | 3.4 (2.8) | - | |
Only villages with ≥6 houses or with ≥10 T. infestans or dogs examined for infection were considered when calculating mean infestation or infection prevalence.
Excludes two isolated settlements where no T. infestans were found (San Luis and Lote S). No infection was found in 3 dogs and 1 cat examined from the latter.
Of 103 households surveyed for domestic animals, 94% owned at least one dog and 61% at least one cat. The mean number of dogs and cats per household was 2.6 (SD = 1.5) and 0.8 (SD = 0.8), respectively. Dogs were 3.3 times more abundant than cats (260 vs. 80, respectively) and nearly all of them were mongrels. The sex ratio between males and females (M:F) was significantly biased toward males in dogs (M:F = 5.8, χ2 = 128.4, degrees of freedom [df] = 1, P < 0.0001) but not in cats (M:F = 1.4, χ2 = 2.5, df = 1, P > 0.1). Both populations were very young, with median ages of 2.0 years (first and third quartiles [Q1–Q3] = 1–5) for dogs and 1.8 years (Q1–Q3 = 0–4) for cats. Cats (75%) were born locally significantly more frequently than dogs (57%) (P = 0.004). Most dogs and cats were unrestrained; 40% of the dogs were used for hunting; 50% of the dogs and 68% of the cats were reported to sleep indoors.
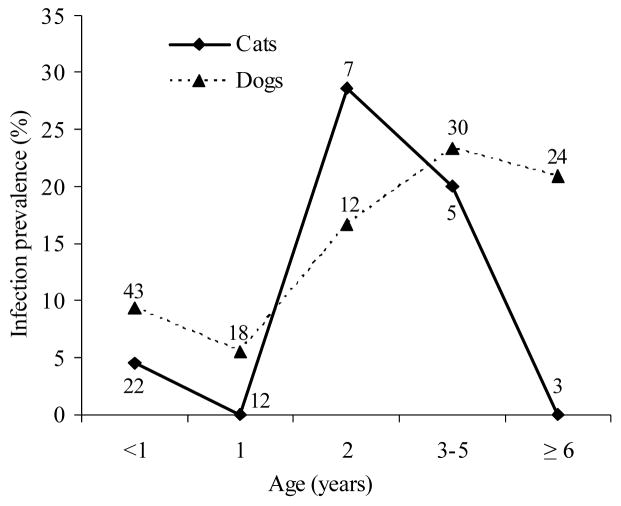
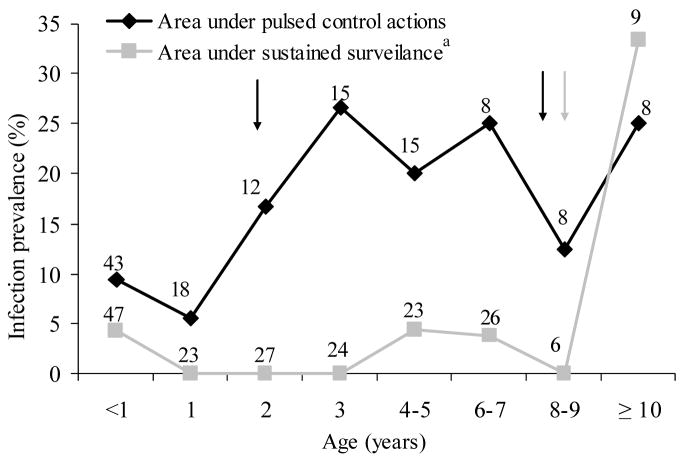
The age-prevalence curves in native cats and dogs from the area under pulsed control actions were very similar up to 5 years of age (Fig. 2). When host age was divided in two classes (≤1 and > 1 year), multiple logistic regression analysis showed no significant effects on host T. cruzi infection of host type (dog or cat) (OR = 1.4, CI = 0.5–4.3) but significant effects of host age (OR = 3.6, CI = 1.6–8.1) (Wald χ2 = 13.4, 2 df, P < 0.005). Among native dogs from the area under pulsed control actions, the age-prevalence curve rose steadily from 5–10% at ages ≤1 year to 27% at age 3 years, and fluctuated between 13 and 25% in older ages (Fig. 3). In contrast, for the sustained surveillance area, the age-prevalence curve of native dogs younger than 10 years of age fluctuated below 5% and rose steeply to 35% only among dogs aged ≥10 years – the dogs that were alive before the 1992 insecticide spraying campaign (Cardinal et al., 2006a). In the pulsed control area, the prevalence of T. cruzi in immigrant dogs (7.5%, n = 94) was half of that in native dogs (15.0%, n = 127) and peaked at age 2 (22%) and >6 (17–25%) years old (data not shown). Most of the infected animals (77%) in the pulsed control area were natives with permanent residence, and therefore qualified as autochthonous cases.
Fig. 2.
Age-specific prevalence of Trypanosoma cruzi infection in native dogs and cats from the area under pulsed control actions in Santiago del Estero, November 2002–July 2003. Numbers close to data points represent the numbers of animals examined for infection. Figure excludes seven dogs and three cats of unknown age.
Fig. 3.
Age-specific prevalence of Trypanosoma cruzi infection in native dogs from two neighboring rural areas under different community-based vector control strategies in Santiago del Estero, November 2002–July 2003. Numbers close to data points represent the numbers of dogs examined for infection. Figure excludes 16 dogs of unknown age. Arrows indicate the main insecticide spraying campaigns.
a Data taken from Cardinal et al. (2006a)
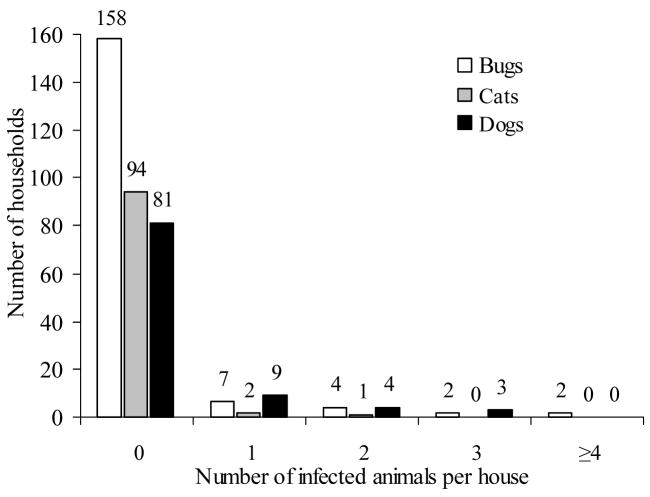
The 30 infected dogs and cats detected were in 16 (15.5%) households from nine villages. Ten of these houses (63%) harbored T. cruzi-infected T. infestans, three harbored uninfected bugs, two had no bugs detected, and from one house no entomologic data were collected. Only a very small fraction of the households surveyed harbored infected animals (9% for T. infestans, 3% for cats, 16% for dogs) (Fig. 4), resulting in high household aggregation of T. cruzi infection (test of variance, χ2 = 228.4, df = 96, P < 0.0001). All infected cats were found cohabiting with infected dogs (P = 0.004). One household had three infected dogs and two infected cats.
Fig. 4.
Household distribution of Trypanosoma cruzi infection in Triatoma infestans, domestic dogs and cats from the area under pulsed vector control actions in Santiago del Estero, November 2002–July 2003. Numbers on top of bars are counts of households.
Serologic titers of dogs and cats showed a clear-cut distinction between non-reactive and reactive sera, with nearly perfect concordance between ELISA and IFAT. A total of 191 sera (163 dogs and 28 cats) with ELISA absorbance values <0.15 and IFAT titers ≤1:8 were clearly negative, whereas 25 sera (22 dogs and 3 cats) with ELISA ≥0.25 and IFAT ≥1:64 were clearly positive. The only serologically discordant dog (ELISA <0.15, IFAT ≥64 and IHA ≤1:8) was not examined by xenodiagnosis. Concordance between xenodiagnosis and serodiagnosis was 96% (119/124) in dogs and 100% (n = 30) in cats. Four dogs aged 2–12 years old were seropositive for T. cruzi and xenodiagnosis-negative. Only one dog, a five-month-old native pup with permanent residence, was xenodiagnosis-positive and seronegative by the three tests. This dog most likely was an acute case of infection, as it cohabited with two dogs and one cat all infected with T. cruzi in a house with infected domestic bugs.
The relationship between T. cruzi infection in dogs and significant risk factors by univariate analysis is shown in Table 3. Ages were unknown for 7 dogs and no entomological data were available for 10 dogs. Based on a multiple logistic regression analysis of data clustered by house, T. cruzi infection was associated positively and significantly with age of the dog, sex (being a female), having permanent residence in the pulsed control area, the number of infected dogs or cats the animal cohabited with, the pooled abundance of T. infestans in domestic or nearby peridomestic sites at the dog’s house compound, and the proportion of infected T. infestans caught at the dog’s compound (Wald χ2 = 54.7, 7 df, P < 0.0001) (Table 3). Living in houses that had only been sprayed twice was not a significant risk factor in the regression analysis. In univariate analyses, infection in dogs was not significantly associated with having a hunting habit, type of spraying operator, insecticide spray coverage, years since last insecticide spray, abundance of T. infestans in corrals, or with the capture of T. guasayana or T. garciabesi at the dog’s compound (data not shown). Among 33 dogs for which the mother’s name and serostatus was known, three infected dogs (37.5%) had seropositive mothers, whereas only two infected dogs (8%) had seronegative mothers.
Table 3.
Prevalence of Trypanosoma cruzi infection and potential risk factors in 221 dogs from the area under pulsed control actions in Santiago del Estero, November 2002–July 2003.
| Factor | % infected (n) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Age (in months) | − (214) | 1.0 (1.00–1.02) | 1.0 (1.01–1.04)** |
| Sex | |||
| Male | 9.5 (189) | ||
| Female | 25.0 (32) | 3.1 (1.2–8.1) | 4.7 (1.1–21.0)* |
| Permanent residence in the study villages | |||
| No | 7.1 (99) | ||
| Yes | 15.6 (122) | 2.4 (1.0–6.0) | 3.8 (1.2–12.5)* |
| No. of infected dogs or cats the dog cohabited with | |||
| − (221) | 2.5 (1.7–3.5) | 2.0 (1.4–2.9)** | |
| Intensity of insecticide spraying (1994–2002) | |||
| Three times | 7.6 (119) | - | |
| Twice | 18.1 (83) | 1.1 (1.1–6.5) | - |
| Once or never | 10.5 (19) | 1.4 (0.3–7.2) | - |
| No. of T. infestans caught in domestic or nearby peridomestic sites at the dog’s compound | |||
| 0 | 2.6 (117) | ||
| 1–9 | 17.0 (53) | 7.8 (2.0–30.0) | 6.0 (1.4–24.9)* |
| ≥ 10 | 31.7 (41) | 17.6 (4.7–66.2) | 6.7 (1.1–39.2)* |
| Proportion of infected T. infestans at the dog’s compound | |||
| − (211) | 23.0 (4.2–126.3) | 5.1 (1.5–17.4)* | |
n, number of dogs examined for infection.
P < 0.05,
P < 0.01.
In cats, T. cruzi infection was associated positively and significantly with cohabiting with at least one infected dog or cat (OR = 58.2, CI = 2.8–1194.2), the abundance of T. infestans in nearby peridomestic sites at the cat’s compound (OR = 46.0, CI = 3.6–587.2), and the occurrence of infected T. infestans at the cat’s compound (OR = 103.0, CI = 4.8–2233.4). None of the studied variables in cats were significant in multiple regression analysis.
4. Discussion
Our study shows that active transmission of T. cruzi occurred in villages under pulsed, non-supervised, community-based vector control actions operating under NCVP guidelines for a decade, but not in the neighboring villages that were under sustained, supervised, community-based surveillance during the same period of time. The prevalence of T. cruzi infection in T. infestans, dogs and cats was two- to three-fold higher in the area under pulsed control actions. Domestic infestations were also more frequent and abundant in the pulsed control area. Weak performance of the vector surveillance system in this area, including lack of full coverage of insecticide spraying, absence of supervision and irregular insecticide supply, led to a rapid recovery of the bug population after spraying and to renewed parasite transmission within 2–3 years of domestic reinfestation. This pattern has been shown for a single village of the core area in the late 1980s (Gürtler et al., 2005). In the peripheral area, vector control actions were highly heterogeneous among villages and were mostly performed in two pulses. A recent analysis of the Argentinean vector control program in Moreno department (where the study villages are located) found that control actions during the attack phase lacked full coverage of rural villages and that reinfestation was highly clustered in space (Vazquez-Prokopec et al., unpublished results).
Age-prevalence curves reflected the history of major vector control actions, despite the fact that activities were not homogeneous and simultaneous across all the villages and houses studied. In native dogs from the area under pulsed control actions, the prevalence of T. cruzi rose steadily from 5–10% at ages ≤1 year (born in 2001–02) to nearly 25% at age 3 years or more (mostly born after the first round of insecticide spraying in 1993–1996). This curve reflects the massive vector control actions conducted in most of the study villages in two pulses; the 2000–01 control pulse was prompted by the occurrence of symptomatic acute cases of human Chagas disease. This rapid recovery of domestic transmission of T. cruzi may be explained by the combined effects of fast domestic reinfestation, presence of infected dogs and cats resting indoors, their high incidence of infection and high infectiousness to bugs, and frequent blood meals on them (Cecere et al., 2006, Gürtler et al., 2007).
The area under pulsed control actions was more rural (included more peridomestic sites, goats, chickens and dogs) and slightly inferior housing quality than the core area under sustained, supervised surveillance. Whether these small differences, in addition to marked differences in insecticide spraying rates between areas, would modify domestic transmission remains unknown and requires further research. Lifestyle and animal management practices were very similar in the two intervention areas. Unlike the area under sustained surveillance where native dogs with permanent residence had a significantly lower prevalence of infection (5%) than immigrant dogs (11%) (Cardinal et al., 2006a), in the pulsed control area most of the infected animals were natives with permanent residence. This pattern is consistent with the lower risk of infection for dogs residing in or immigrating from the area under sustained surveillance in comparison to native dogs residing in or emigrating from the pulsed control area. The finding of a likely acute case of T. cruzi infection in a pup inhabiting an infested house with infected bugs, dogs and cats provides further evidence of vector-mediated transmission occurring locally at the time of the survey. Interestingly, a similar prevalence of T. cruzi infection in dogs (15.1%) was recorded in a rural area under irregular control actions located some 200 km away (but in the same ecoregion) in Chaco province (Diosque et al., 2004).
Infection in dogs was associated positively and significantly with variables reflecting local exposure to infected bugs (Table 3), which suggests that the most likely route of infection was vector-mediated within the dog’s compound. T. infestans was the only species found infected with T. cruzi even after 10 years of vector control actions in both areas. Vertical transmission may possibly account for some of the very young infected dogs. The occurrence of a xenodiagnosis-positive pup born to a seropositive, xenodiagnosis-positive female dog was most likely due to vertical transmission because the pup was only three days old, which is less than the latent period of vector-mediated infection. Cohabiting with at least one infected dog or cat was also significantly associated with T. cruzi infection in dogs and cats, which reflects household aggregation of infection. Cats were probably involved in the same vector-borne domestic transmission cycle as were dogs, as suggested by the association between infestation and cat infection and by the similar age-prevalence curves of native dogs and cats (Gürtler et al., 2007). Further evidence in support of this hypothesis was obtained by molecular typing of T. cruzi isolates from infected dogs, cats and domestic T. infestans of the study area; most of them shared sublineage TCIIe (Cardinal et al., unpublished results).
The evidence here provided gives further support to the use of dogs as natural sentinels of domestic and peridomestic transmission of T. cruzi (Gamboa, 1967; Gürtler et al., 1990; Castañera et al, 1998; Cardinal et al. 2006a). Several studies are showing that infected dogs constitute a major domestic reservoir of T. cruzi and a risk factor for domestic transmission throughout Latin America (Gürtler et al., 2005, 2007; Crisante et al., 2006; Estrada-Franco et al., 2006). Consequently, the regular screening of domestic dog populations for T. cruzi infection can be used to identify houses or clusters with infected dogs and high risk of transmission. A highly sensitive and specific immunochromatographic dipstick test may be used for this purpose (Cardinal et al., 2006b) and for in situ implementation of appropriate strategies, such as insecticide-impregnated collaring (Reithinger et al., 2006), parasiticidal drug treatment, culling, or selective spraying of the house.
Our study is the first to show household aggregation of T. cruzi infection in dogs and cats, so far only reported for humans (Mott et al., 1976). Host infection was concentrated in a small fraction of households (15.5%) and fell close to the 80/20 rule; this rule describes the disproportionate contribution to infection prevalence (80% or more) made by a small fraction (20% or less) of the households (Woolhouse et al., 1997). High aggregation of T. cruzi infection in dogs and cats also occurred in the area under sustained surveillance, where all the infected animals (12 dogs and 1 cat) were in 8.4% of houses. Household aggregation of T. cruzi infection is expected to increase the basic reproductive number (Ro) of infection and the intensity of control actions needed to interrupt transmission. However, taking this heterogeneity into consideration may increase the effectiveness of vector control activities if effective targeting of the key households that concentrate infected reservoir hosts and transmission is achieved.
In the history of control actions against vector-mediated transmission of T. cruzi, there is a clear trend from vertically structured programs toward more horizontal strategies in which the community plays a crucial role during the surveillance phase (Bryan, 1994). In this study we show that a community-based strategy can indeed be quite succesful in reducing or controling domestic T. cruzi transmission (not in eliminating T. infestans) provided it is accompanied by timely sustained support and external supervision is ensured in time. It is however, this temporal synchronicity that is so difficult to achieve in endemic areas where the rural population is sparsely distributed and access through dirt roads is limited, particularly during the rainy season. For highly endemic areas in the Gran Chaco region such as Santiago del Estero, interruption of vector-mediated domestic transmission of T. cruzi requires residual insecticide spraying that is more intense, of a higher quality, sustained in time, and combined with community participation and environmental management measures.
Acknowledgments
We are grateful to Leonardo A. Ceballos, Gonzalo M. Vazquez-Prokopec, María C. Cecere, Francisco G. Petrocco, Cristina Maidana, Juan P. Hurtado, María L. Colli and Leonardo Lanati for field and laboratory assistance. We also thank Sonia Blanco (deceased May 3, 2005), Cynthia Spillmann and Raúl Stariolo (National Vector Control Program, Argentina) for continuing support. Antonio Cañones Olmo and Nilo Baiocci kindly provided field accommodation. Financial support: This study was supported by awards from the National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to U.K. and R.E.G., the Agencia Nacional de Promoción Científica y Técnica (Argentina), and University of Buenos Aires to R.E.G. Ricardo E. Gürtler is member of Consejo Nacional de Investigaciones Científicas y Técnicas Researcher’s Career.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bryan RT, Balderrama F, Tonn RJ, Pinto Dias JC. Community participation in vector control: lessons from Chagas’ Disease. Am J Trop Med Hyg. 1994;50:61–71. doi: 10.4269/ajtmh.1994.50.61. [DOI] [PubMed] [Google Scholar]
- Cardinal MV, Castañera MB, Lauricella MA, Cecere MC, Ceballos LA, Vazquez-Prokopec GM, Kitron U, Gürtler RE. A prospective study of the effects of sustained vector surveillance on Trypanosoma cruzi infection of dogs and cats in rural northwestern Argentina. Am J Trop Med Hyg. 2006a;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- Cardinal MV, Reithinger R, Gürtler RE. Use of an immunochromatographic dipstick test for rapid detection of Trypanosoma cruzi in sera from animal reservoir hosts. J Clin Microbiol. 2006b;44:3005–3007. doi: 10.1128/JCM.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos LA, Cardinal MV, Vazquez-Prokopec GM, Lauricella MA, Orozco MM, Cortinas R, Schijman AG, Levin MJ, Kitron U, Gürtler RE. Long-term reduction of Trypanosoma cruzi infection in sylvatic mammals following deforestation and sustained vector surveillance in northwestern Argentina. Acta Trop. 2006;98:286–296. doi: 10.1016/j.actatropica.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Gürtler RE, Chuit R, Cohen JE. Factors limiting the domestic density of Triatoma infestans in north-west Argentina: a longitudinal study. Bull World Health Organ. 1998;76:373–384. [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Castañera MB, Canale DM, Chuit R, Gürtler RE. Trypanosoma cruzi infection in Triatoma infestans and other triatomines: long-term effects of a control program in a rural area of northwestern Argentina. Pan Am J Public Health. 1999;5:392–399. doi: 10.1590/s1020-49891999000500003. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Gürtler RE, Canale DM, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerg Infect Dis. 2006;12:1096–1102. doi: 10.3201/eid1207.051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American Trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Crisante G, Rojas A, Teixeira MMG, Añez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006;98:247–254. doi: 10.1016/j.actatropica.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas Disease control in Latin America – a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Diosque P, Padilla A, Cimino RO, Cardozo RM, Sanchez Negrette O, Marco JD, Zaca R, Meza C, Juarez A, Rojo H, Rey R, Corrales RM, Nasser JR, Basombrío MA. Chagaś disease in rural areas of Chaco province, Argentina: epidemiologic survey in humans, reservoirs, and vectors. Am J Trop Med Hyg. 2004;71:590–593. [PubMed] [Google Scholar]
- Estrada-Franco JG, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbosa A, Vazquez-Chagoyan JC, Martinez-Perez MA, Guzman-Bracho C, Garg N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg Infect Dis. 2006;12:624–630. doi: 10.3201/eid1204.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa JC. Evaluación de las medidas antitriatominas por medio de la prevalencia de Schizotrypanum cruzi en perros. Bol Inf Dir Malariol San Amb (Venezuela) 1967;7:321–325. [Google Scholar]
- Gürtler RE, Kravetz FO, Petersen RM, Lauricella MA, Wisnivesky-Colli C. The prevalence of Trypanosoma cruzi and the demography of dog populations after insecticidal spraying of houses: a predictive model. Ann Trop Med Parasitol. 1990;84:313–323. doi: 10.1080/00034983.1990.11812475. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Canale D, Castañera MB, Chuit R, Cohen JE. Monitoring house reinfestation by vectors of Chagas disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/s0001-706x(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Canale D, Castañera MB, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am J Trop Med Hyg. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:1–14. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE. Eco-epidemiología regional de la transmisión vectorial: Enfermedad de Chagas en el Gran Chaco. In: Silveira AC, editor. La enfermedad de Chagas. A la puerta de los 100 años del conocimiento de una endemia americana ancestral. Balance y futuro, 1909–2006. Chagas, hacia el Siglo XXI. Organización Panamericana de la Salud; 2007. in press. [Google Scholar]
- Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Immunodiagnosis of Trypanosoma cruzi (Chagaś Disease) infection in naturally infected dogs. Mem Inst Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based identification of Trypanosoma cruzi lineages in faeces of triatomine bugs from rural northwestern Argentina. Parasitology. 2006;132:1–9. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KE, Lehman JS, Jr, Hoff R, Morrow RH, Muniz TM, Sherlock I, Draper CC, Pugliese C, Guimaraes AC. The epidemiology and household distribution of seroreactivity to Trypanosoma cruzi in a rural community in northeast Brazil. Am J Trop Med Hyg. 1976;25:552–562. doi: 10.4269/ajtmh.1976.25.552. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Ceballos LA, Stariolo R, Davies CR, Gürtler RE. Extinction of experimental Triatoma infestans populations following continuous exposure to dogs wearing deltamethrin-treated collars. Am J Trop Med Hyg. 2006;74:766–771. [PMC free article] [PubMed] [Google Scholar]
- Schijman AG, Lauricella MA, Marcet PL, Duffy T, Cardinal MV, Bisio M, Levin MJ, Kitron U, Gürtler RE. Differential detection of Blastocrithidia triatomae and Trypanosoma cruzi by amplification of 24sα ribosomal RNA genes in faeces of sylvatic triatomine species from rural northwestern Argentina. Acta Trop. 2006;99:50–54. doi: 10.1016/j.actatropica.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Segura EL. El control de la enfermedad de Chagas en la República Argentina. In: Silveira AC, editor. El control de la enfermedad de Chagas en los países del Cono Sur de América. Historia de una iniciativa internacional 1991/2001. PAHO; Argentina: 2002. pp. 45–108. [Google Scholar]
- World Health Organization. Control of Chagas disease: Second report of the WHO Expert Committee. World Health Organization; Geneva: 2002. pp. 82–83. [Google Scholar]
- Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]