Abstract
Background
Low tumour expression levels of thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD) and thymidine phosphorylase (TP) have been linked with improved outcome for colorectal cancer (CRC) patients treated with 5-fluorouracil (5-FU). It is unclear whether this occurs because such tumours have better prognosis or they are more sensitive to 5-FU treatment.
Patients and methods
Associations between TS, DPD and TP levels, determined by tissue microarrays and immunohistochemistry, and survival was evaluated in 945 CRC patients according to treatment status.
Results
Low TS and DPD expression associated with worse prognosis in stage II [hazard ratio (HR) = 1.69, 95% confidence interval (CI) (1.09–2.63) and HR = 1.92 (95% CI 1.23–2.94), respectively] and stage III CRC patients treated by surgery alone [HR = 1.39 (95% CI 0.92–2.13) and HR = 1.49 (95% CI 1.02–2.17), respectively]. Low TS, DPD and TP associated with trends for better outcome in stage III patients treated with 5-FU [HR = 0.81 (95% CI 0.49–1.33), HR = 0.70 (95% CI 0.42–1.15) and HR = 0.66 (95% CI 0.39–1.12), respectively].
Conclusion
Low TS and DPD expression are prognostic for worse outcome in CRC patients treated by surgery alone, whereas low TS, DPD and TP expression are prognostic for better outcome in patients treated with 5-FU chemotherapy. These results provide indirect evidence that low TS, DPD and TP protein expression are predictive of good response to 5-FU chemotherapy.
Keywords: colorectal cancer, fluorouracil, predictive, prognostic, thymidylate synthase
introduction
The antimetabolite, 5-fluorouracil (5-FU), is one of the most common anticancer drugs in use today and remains the mainstay of chemotherapy for colorectal cancer (CRC) [1]. Adjuvant treatment with 5-FU has been shown to improve the absolute survival rate of stage III colon carcinoma patients by ~10%–15% [2]. Increasing evidence indicates that stage II CRC patients also gain benefit from 5-FU-based therapies [3]. Because only a relatively small proportion of patients appear to benefit from this treatment, considerable effort has been directed towards finding biomarkers that can accurately predict tumour response [1, 4]. These have included molecular factors such as TP53 mutation [5], microsatellite instability [5, 6] and chromosomal deletions [7]. There is, however, currently insufficient evidence to justify the incorporation of these or any other candidate predictive markers into routine clinical practice for the selection of CRC patients to receive 5-FU [8]. Furthermore, direct relevance to the mechanism of 5-FU action remains to be clearly established for many of the markers studied to date.
Inhibition of thymidylate synthase (TS) by the 5-FU metabolite fluorodeoxyuridine monophosphate (FdUMP) has been identified as the major mechanism of 5-FU action [9]. FdUMP binds TS and CH2FH4 in an irreversible ternary complex, thereby disrupting the nucleotide pool and inhibiting DNA synthesis. The level of TS expression is thus a strong candidate marker for the prediction of 5-FU response [10]. A second potential marker is expression of dihydropyrimidine dehydrogenase (DPD), the rate-limiting enzyme in 5-FU catabolism [11]. The nucleoside cleavage enzyme, thymidine phosphorylase (TP), is involved in the regulation of intracellular thymidine levels and has also been implicated as a potential 5-FU-predictive factor [12]. Due to their involvement in nucleotide and fluoropyrimidine metabolism, the expression and activity levels of TS, DPD and TP are therefore potentially important not only as predictive markers for response to 5-FU but also as prognostic factors [13, 14].
A landmark publication in this field was the observation that low messenger RNA (mRNA) levels for TS, DPD and TP were predictive of tumour response to 5-FU [15]. Although several other studies have been published since this report, particularly on TS expression, there is still no consensus regarding the clinical utility of marker enzymes from the fluoropyrimidine pathway [10]. Indeed, the American Society of Clinical Oncology 2006 recommendations for the use of tumour markers in gastrointestinal cancer concluded: ‘there is insufficient evidence to recommend the use of TS, DPD or TP as predictors of response to therapy’ [16]. Confusion has arisen because of indiscriminate use of the terms prognostic and predictive. The former relates to tumour aggressiveness, while the latter relates to tumour response to therapy. A review of the literature on TS, DPD and TP has identified the need for more studies to evaluate both the prognostic and predictive values of these markers in CRC [1]. Some of the major issues identified were the standardisation of immunohistochemical (IHC) assessments for protein localisation (cytoplasmic versus nuclear), the method of scoring (intensity versus extent of staining) and the cut-off values used to define positive staining.
In the current study, we used tissue microarrays (TMAs) of a large and well-characterised series of stages II and III CRCs [17] to evaluate the prognostic values of TS, DPD and TP protein expression in patients treated with or without 5-FU chemotherapy. Our results highlight the importance of investigating patient groups that are homogeneous with respect to adjuvant treatment when evaluating the prognostic significance of molecular-based markers.
patients and methods
patients
Patients were diagnosed with CRC during the period 1990–1999 at the PathCentre pathology service, Sir Charles Gairdner Hospital, Western Australia. Information on patient demographics (sex and age) and tumour features (stage, grade and anatomical site) were obtained from the pathology records for each case. Tumours were classified as originating proximal or distal to the splenic flexure. Information on disease-specific survival was obtained from the West Australian State Cancer Registry. The median follow-up time was 69.7 months for patients with stage II disease and 52.4 months for patients with stage III CRC. Information on the use of adjuvant chemotherapy with 5-FU/leucovorin-based regimens was obtained from hospital records. Adjuvant chemotherapy was progressively introduced for advanced CRC during the 1990s in Western Australia, thus accounting for the relatively low percentage of stage III patients who received this treatment (139/358, 39%). At the end of the study period, 313 of 967 (32%) patients had died of disease recurrence and 257 (27%) from other causes. The protocols for this study were approved by the Human Research Ethics Committee of the Sir Charles Gairdner Hospital.
tissue microarrays
Sections from TMA blocks containing 967 CRC (stages II and III) and matching normal tissue samples were obtained from the West Australian Research Tissue Network, Department of Radiation Oncology, Sir Charles Gairdner Hospital. Construction of the TMAs and the tumour and patient characteristics have been described elsewhere [17].
IHC staining and scoring
Five micron sections were cut from the TMA blocks, mounted on silanated slides and subsequently dewaxed and rehydrated using xylene and graded alcohol washes. IHC for TS protein was carried out using the TS106 antibody (Invitrogen, Carlsbad, CA) and for DPD and TP with the anti-DPD and anti-TP kits, respectively (Roche Applied Sciences, Mannheim, Germany). The manufacturer’s instructions were followed in each case, except that antigen retrieval for all three proteins comprised incubation for 20 min at 98°C in a T/T Mega microwave oven (Milestone, Sorisole, Italy) in place of the steaming-based method described in the anti-DPD and anti-TP kits.
IHC staining was scored by a single pathologist (NS) following determination of the scoring criteria by two pathologists (NS and MST). The parameters scored for all three proteins were staining intensity (graded 0–4) and extent of staining (0%–100% of tumour cell compartment) in both the cytoplasm and nucleus, amounting to 12 scores for each tumour. For the current analysis, a low level of expression was defined as cytoplasmic staining with an intensity score of 0, 1 or 2 and high-level expression as a score of 3 or 4. The evaluation of TS, DPD and TP expression levels was carried out blind to clinical outcomes. IHC results were obtained for TS expression in 945 cases, DPD in 858 cases and TP in 942 cases.
statistical analysis
Comparison of the expression of TS, DPD and TP in matched tumour and normal tissue samples was carried out by Wilcoxon signed rank test. Correlations in expression levels of the three enzymes were evaluated by Spearman’s correlation coefficient. Correlations between IHC scores and clinicopathological characteristics were evaluated by chi-square analysis. Associations with survival were assessed by Kaplan–Meier and Cox regression analysis. Only cancer-related deaths were considered as events. P values of <0.05 was considered statistically significant.
results
expression patterns for TS, DPD and TP
TS and TP protein expression were observed in both the cytoplasm and nucleus (Figure 1). DPD staining was predominantly cytoplasmic, with nuclear staining observed in only a minority (<5%) of cases. Cytoplasmic and nuclear expression of all three proteins was significantly higher in tumours compared with matching normal tissue (Wilcoxon signed rank analysis, all P < 0.001). Cytoplasmic and nuclear expression levels in individual tumours were significantly correlated for all three proteins (Spearman’s rho analysis, all P < 0.001).
Figure 1.
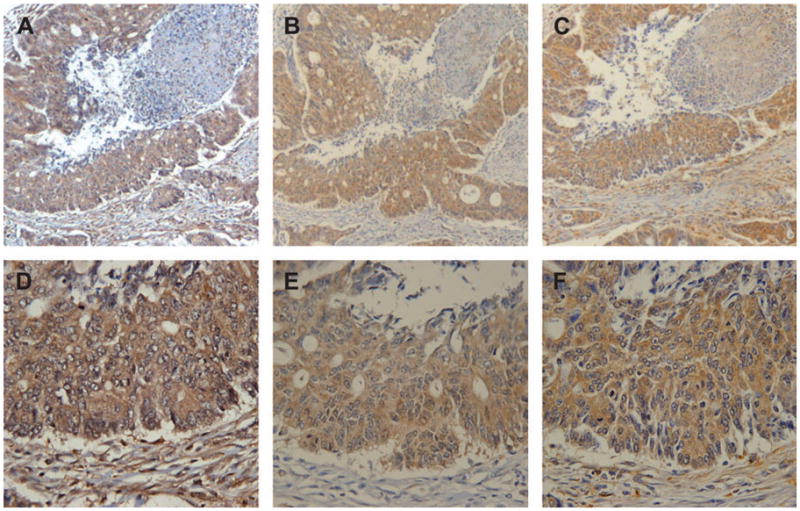
Representative images of thymidylate synthase (A and D), dihydropyrimidine dehydrogenase (B and E) and thymidine phosphorylase (C and F) immunohistochemical stains in colorectal cancer tissue arrays at ×5 (A–C) and ×40 (D–F) magnification.
TS, DPD and TP expression and clinicopathological and molecular characteristics
Low expression levels for TS, DPD and TP were observed in 61%, 34% and 39% of tumours, respectively (Table 1). Low expression of TS was associated with higher stage and left-sided origin, while low DPD expression was also observed more frequently in stage III tumours. No significant differences in TS, DPD and TP expression were observed according to gender, age or histological grade.
Table 1.
Associations between low TS, DPD and TP expression and clinicopathological and molecular features of stages II and III CRC
| Feature | Tumours with low expression levels (%) |
||
|---|---|---|---|
| TS | DPD | TP | |
| Total | 581/945 (61) | 296/858 (34) | 367/942 (39) |
| Male | 296/473 (63) | 145/426 (34) | 186/465 (40) |
| Female | 285/472 (60) | 151/432 (35) | 181/477 (38) |
| <70 years | 288/455 (63) | 156/413 (38) | 177/451 (39) |
| ≥70 years | 293/490 (60) | 140/445 (30) | 190/491 (39) |
| Stage II | 344/587 (59) | 138/506 (27) | 233/584 (40) |
| Stage III | 237/358 (66) | 158/352 (45) | 134/358 (37) |
| P = 0.020 | P < 0.0001 | ||
| Distal colon/rectum | 338/509 (66) | 156/466 (33) | 213/511 (42) |
| Proximal colon | 215/390 (55) | 125/357 (35) | 139/388 (36) |
| P = 0.0005 | |||
| Well differentiated | 95/156 (61) | 45/132 (34) | 60/159 (38) |
| Moderately differentiated | 237/388 (61) | 110/341 (32) | 152/381 (40) |
| Poorly differentiated | 46/88 (52) | 27/73 (37) | 35/89 (39) |
CRC, colorectal cancer; TS, thymidylate synthase; DPD, dihydropyrimidine dehydrogenase; TP, thymidine phosphorylase.
prognostic significance of TS, DPD and TP expression in patients treated by surgery alone
The prognostic significance of TS, DPD and TP expression was first examined in stages II and III CRC patient groups treated by surgery alone, thus excluding any possible interaction between chemotherapy and survival. Low TS expression was associated with worse prognosis in stage II CRC and a trend for worse survival in stage III CRC (Table 2). Low DPD expression was associated with significantly worse survival in both stages II and III CRC treated by surgery alone, whereas low TP expression showed no prognostic significance in either group.
Table 2.
Prognostic significance of TS, DPD and TP cytoplasmic expression level in different CRC stage and treatment groups
| Stage and treatment | No. of cases (low, high) | HR | 95% CI | P |
|---|---|---|---|---|
| Stage II (surgery alone) | ||||
| TS low versus TS high | 302, 216 | 1.69 | 1.09–2.63 | 0.019 |
| DPD low versus DPD high | 121, 323 | 1.92 | 1.23–2.94 | 0.003 |
| TP low versus TP high | 199, 318 | 1.16 | 0.78–1.75 | 0.449 |
| Stage III (surgery alone) | ||||
| TS low versus TS high | 147, 78 | 1.39 | 0.92–2.13 | 0.124 |
| DPD low versus DPD high | 97, 124 | 1.49 | 1.02–2.17 | 0.038 |
| TP low versus TP high | 87, 133 | 1.05 | 0.71–1.54 | 0.812 |
| Stage III (5-FU chemotherapy) | ||||
| TS low versus TS high | 83, 42 | 0.81 | 0.49–1.33 | 0.415 |
| DPD low versus DPD high | 58, 65 | 0.70 | 0.42–1.15 | 0.160 |
| TP low versus TP high | 44, 86 | 0.66 | 0.39–1.12 | 0.129 |
TS, thymidylate synthase; DPD, dihydropyrimidine dehydrogenase; TP, thymidine phosphorylase; CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval; 5-FU, 5-fluorouracil.
prognostic significance of TS, DPD and TP expression in stage III patients treated with 5-FU chemotherapy
We next examined the prognostic significance of TS, DPD and TP expression in stage III patients treated with chemotherapy. Low TS expression showed no prognostic significance in these patients; however, low DPD and TP expression levels were associated with trends for better survival (Table 2). The above results indicate that low TS, DPD and TP expression have different prognostic values depending on whether patients were treated with or without 5-FU chemotherapy. An obvious explanation for the change in association from worse to better prognosis is that CRCs with low TS, DPD and TP expression are more responsive to 5-FU.
discussion
The present study is one of the largest to investigate the prognostic value of three major enzymes involved in the fluoropyrimidine metabolism. The intensity of cytoplasmic staining was used to provide a semi-quantitative estimate of TS, DPD and TP protein expression levels. For TS, this has been validated as an accurate reflection of protein levels [18]. Colorectal tumours were derived from a population-based cohort with long patient follow-up periods and well-documented pathological and clinical information, including the use of 5-FU-based adjuvant chemotherapy [17]. This allowed us to determine the prognostic value of TS, DPD and TP expression in clearly defined stage and treatment groups (Table 2).
High TS expression levels have generally been associated with worse overall survival in CRC [1]. A recent meta-analysis found an hazard ratio (HR) of 1.35 for high TS expression in 2610 patients with localised CRC [19], although evidence of heterogeneity and possible publication bias was observed. The adjuvant treatment status of patients in many studies has not always been well defined, leaving open the possibility that 5-FU might differentially influence the outcome of low or high TS expression groups. In the present study, we found that low TS expression was a prognostic marker for worse survival in stage II CRC patients treated by surgery alone (Table 2). The same trend was also apparent for stage III cases. In contrast, TS expression showed no prognostic significance in stage III CRC patients treated with 5-FU. The latter result concurs with several other recent studies on 5-FU-treated CRC patient cohorts [20–22]. One explanation for these results may be that low TS expression is associated with a more aggressive tumour phenotype and hence the worse prognosis observed for patients treated by surgery alone. If low TS tumours, however, are more sensitive to 5-FU than high expressing tumours, patients with these tumours would derive preferential benefit, thus explaining the lack of prognostic value observed for TS in 5-FU-treated patients (Table 2).
In vitro results with tumour cell lines have indicated that low expression or activity of TS was associated with good response to 5-FU [23, 24]. This was subsequently confirmed by several clinical studies [15, 25–28]; however, others have reported discordant results [29–32]. The results of the current study on the prognostic significance of TS expression in CRC patients treated with or without chemotherapy (Table 2) indirectly support the contention that low TS expression level is a predictive marker for survival benefit from 5-FU chemotherapy. Although patients in this study were not randomly allocated to the study, exploratory analysis revealed the HR associated with 5-FU chemotherapy was 0.70 [95% confidence interval (CI) 0.48–1.02] for stage III patients with low TS expression compared with 1.2 (95% CI 0.70–2.05) for those with TS high expression.
In comparison to TS, considerably less work has been carried out on the prognostic and predictive values of DPD and TP expression in CRC. The present results demonstrate that low DPD expression was associated with worse survival of both stages II and III CRC treated by surgery alone (Table 2). This concurs with the strong correlation observed here between low DPD expression and more advanced tumour stage (Table 1), as well as previous work showing that low DPD expression was associated with worse prognosis for stages II and III CRC patients treated by surgery alone [33]. Most published studies have reported no significant associations between DPD expression and prognosis [1], although many of the findings are difficult to interpret because study groups were not clearly defined for adjuvant treatment status. Similar to TS expression, exploratory analysis revealed that the use of 5-FU chemotherapy was associated with good survival for stage III CRC patients with low DPD expression (HR = 0.54, 95% CI 0.34–0.86) but not for those with high DPD expression (HR = 1.16, 95% CI 0.76–1.76). These results support those of a recent study using oral 5-FU-based chemotherapy in stages II and III CRC [33].
There is some evidence in the literature to indicate that high TP expression is associated with worse prognosis [1]. This was not supported by the current results, however, where the study design was aimed at investigating prognostic significance in the absence of 5-FU chemotherapy. In contrast to TS and DPD, low expression of TP did not have significant prognostic value in either stage II or stage III CRC treated by surgery alone (Table 2). Low TP expression was similar to low TS and DPD expression in that it was associated with a trend for better survival in stage III CRC treated with 5-FU, again indicative of response to chemotherapy in these patients. This was confirmed by the finding that chemotherapy was associated with good survival in patients with low TP expression (HR = 0.59, 95% CI 0.34–1.01) but not in those with high TP expression (HR = 0.93, 95% CI 0.63–1.35).
In summary, the major finding of the present study was that low TS and DPD protein expression were prognostic markers of poor survival in stages II and III CRC patients treated by surgery alone. The trends observed here for association of low TS, DPD and TP expression with better survival in patients receiving 5-FU indicates that they are also predictive markers of good response to this treatment. Confirmation of this was obtained by exploratory analyses that compared the survival of patients treated with and without 5-FU in low and high expression groups. The current results support the findings of Salonga et al. [15] who used tumour response as the end point rather than disease-specific survival and who evaluated mRNA rather than protein levels. Together, these results indicate that assessment of protein or mRNA levels for TS, DPD or TP may allow improved targeting of 5-FU-based treatments towards patients who are most likely to benefit. Validation of their clinical utility will, however, require further prospective trials that are designed specifically to evaluate the predictive significance of these markers [4]. Since TS, DPD and TP are involved in the mechanism of response to 5-FU, any predictive value associated with these markers should not be affected by the simultaneous use of additional agents that do not target the fluoropyrimidine pathway.
Acknowledgments
funding
Academic Research Fund (PS2040222); National Medical Research Council (0874/2004); Singapore Cancer Syndicate, Singapore (BU51); Cancer Council of Western Australia; National Institutes of Health, USA (CA85381).
Footnotes
For permissions, please journals.permissions@oxfordjournals.org
References
- 1.Soong R, Diasio RB. Advances and challenges in fluoropyrimidine pharmacogenomics and pharmacogenetics. Pharmacogenomics. 2005;6:835–847. doi: 10.2217/14622416.6.8.835. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Midgley R, Kerr DJ. Adjuvant chemotherapy for stage II colorectal cancer: the time is right! Nat Clin Pract Oncol. 2005;2:364–369. doi: 10.1038/ncponc0228. [DOI] [PubMed] [Google Scholar]
- 4.Sargent DJ, Conley BA, Allegra C, et al. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 5.Elsaleh H, Powell B, McCaul K, et al. P53 alteration and microsatellite instability have predictive value for survival benefit from chemotherapy in stage III colorectal carcinoma. Clin Cancer Res. 2001;7:1343–1349. [PubMed] [Google Scholar]
- 6.Ribic CM, Sargent DJ, Moore MJ, et al. Tumour microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barratt PL, Seymour MT, Stenning SP, et al. DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: a molecular study. Lancet. 2002;360:1381–1391. doi: 10.1016/s0140-6736(02)11402-4. [DOI] [PubMed] [Google Scholar]
- 8.Graziano F, Cascinu S. Prognostic molecular markers for planning adjuvant chemotherapy trials in Dukes’ B colorectal cancer patients: how much evidence is enough? Ann Oncol. 2003;14:1026–1038. doi: 10.1093/annonc/mdg284. [DOI] [PubMed] [Google Scholar]
- 9.Van Triest B, Pinedo HM, Giaccone G, et al. Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann Oncol. 2000;11:385–391. doi: 10.1023/a:1008351221345. [DOI] [PubMed] [Google Scholar]
- 10.Allegra C, Sargent D. Molecular diagnostics: assays, tissues, progress, and pitfalls. J Clin Oncol. 2003;21:395–396. doi: 10.1200/JCO.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 11.Diasio RB, Lu Z. Dihydropyrimidine dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol. 1994;12:2239–2242. doi: 10.1200/JCO.1994.12.11.2239. [DOI] [PubMed] [Google Scholar]
- 12.Metzger R, Danenberg K, Leichman CG, et al. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumours is associated with nonresponse to 5-fluorouracil. Clin Cancer Res. 1998;4:2371–2376. [PubMed] [Google Scholar]
- 13.Beck A, Etienne MC, Cheradame S, et al. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer. 1994;30A:1517–1522. doi: 10.1016/0959-8049(94)00216-r. [DOI] [PubMed] [Google Scholar]
- 14.Diasio RB, Johnson MR. The role of pharmacogenetics and pharmacogenomics in cancer chemotherapy with 5-fluorouracil. Pharmacology. 2000;61:199–203. doi: 10.1159/000028401. [DOI] [PubMed] [Google Scholar]
- 15.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumours responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 16.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumour markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 17.Chai SM, Zeps N, Shearwood AM, et al. Screening for defective DNA mismatch repair in stage II and III colorectal cancer patients. Clin Gastroenterol Hepatol. 2004;2:1017–1025. doi: 10.1016/s1542-3565(04)00451-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnston PG, Liang CM, Henry S, et al. Production and characterization of monoclonal antibodies that localize human thymidylate synthase in the cytoplasm of human cells and tissue. Cancer Res. 1991;51:6668–6676. [PubMed] [Google Scholar]
- 19.Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 20.Allegra CJ, Parr AL, Wold LE, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002;20:1735–1743. doi: 10.1200/JCO.2002.07.080. [DOI] [PubMed] [Google Scholar]
- 21.Popat S, Chen Z, Zhao D, et al. A prospective, blinded analysis of thymidylate synthase and p53 expression as prognostic markers in the adjuvant treatment of colorectal cancer. Ann Oncol. 2006;17:1810–1817. doi: 10.1093/annonc/mdl301. [DOI] [PubMed] [Google Scholar]
- 22.Westra JL, Hollema H, Schaapveld M, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase protein expression on survival in adjuvantly treated stage III colon cancer patients. Ann Oncol. 2005;16:1646–1653. doi: 10.1093/annonc/mdi316. [DOI] [PubMed] [Google Scholar]
- 23.van Triest B, Pinedo HM, van Hensbergen Y, et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin Cancer Res. 1999;5:643–654. [PubMed] [Google Scholar]
- 24.Amatori F, Di Paolo A, Del Tacca M, et al. Thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer and normal mucosa in patients. Pharmacogenet Genomics. 2006;16:809–816. doi: 10.1097/01.fpc.0000230410.07899.bc. [DOI] [PubMed] [Google Scholar]
- 25.Aschele C, Debernardis D, Tunesi G, et al. Thymidylate synthase protein expression in primary colorectal cancer compared with the corresponding distant metastases and relationship with the clinical response to 5-fluorouracil. Clin Cancer Res. 2000;6:4797–4802. [PubMed] [Google Scholar]
- 26.Aschele C, Debernardis D, Bandelloni R, et al. Thymidylate synthase protein expression in colorectal cancer metastases predicts for clinical outcome to leucovorin-modulated bolus or infusional 5-fluorouracil but not methotrexate-modulated bolus 5-fluorouracil. Ann Oncol. 2002;13:1882–1892. doi: 10.1093/annonc/mdf327. [DOI] [PubMed] [Google Scholar]
- 27.Etienne MC, Chazal M, Laurent-Puig P, et al. Prognostic value of tumoural thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol. 2002;20:2832–2843. doi: 10.1200/JCO.2002.09.091. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa W, Uetake H, Shirota Y, et al. Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expressions in primary tumours as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Clin Cancer Res. 2003;9:786–791. [PubMed] [Google Scholar]
- 29.Takenoue T, Nagawa H, Matsuda K, et al. Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method. Ann Surg Oncol. 2000;7:193–198. doi: 10.1007/BF02523653. [DOI] [PubMed] [Google Scholar]
- 30.Edler D, Glimelius B, Hallstrom M, et al. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol. 2002;20:1721–1728. doi: 10.1200/JCO.2002.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Kornmann M, Schwabe W, Sander S, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression levels: predictors for survival in colorectal cancer patients receiving adjuvant 5-fluorouracil. Clin Cancer Res. 2003;9:4116–4124. [PubMed] [Google Scholar]
- 32.Johnston PG, Benson AB, 3rd, Catalano P, et al. Thymidylate synthase protein expression in primary colorectal cancer: lack of correlation with outcome and response to fluorouracil in metastatic disease sites. J Clin Oncol. 2003;21:815–819. doi: 10.1200/JCO.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji T, Sawai T, Takeshita H, et al. Tumour dihydropyrimidine dehydrogenase in stage II and III colorectal cancer: low level expression is a beneficial marker in oral-adjuvant chemotherapy, but is also a predictor for poor prognosis in patients treated with curative surgery alone. Cancer Lett. 2004;204:97–104. doi: 10.1016/j.canlet.2003.09.030. [DOI] [PubMed] [Google Scholar]


