Abstract
Edotecarin (J-107088), a novel inhibitor of topoisomerase I has an additive effect on colon cell lines (HCT-116) when combined with 5-fluorouracil (5-FU). We conducted a phase I study to determine the maximum tolerated dose and recommended a phase II dose of edotecarin in combination with infusional 5-FU/leucovorin (LV) in patients with advanced solid tumors. Patients and cohorts of three to six patients were sequentially enrolled at progressively higher dose levels of edotecarin administered as a 1-h intravenous (IV) infusion every 2 weeks. The edotecarin starting dose was 6mg/m2, followed by 200mg/m2 LV IV infusion administered over 2 h, then 400mg/m2 bolus dose of 5-FU before the start of 2400mg/m2 5-FU continuous infusion for a further 46 h. Patients were evaluated for safety, pharmacokinetics, and tumor response according to the Response Evaluation Criteria in Solid Tumors criteria. Fourteen patients (10 male; four female) received a total of 90 cycles (range 3–18). Dose-limiting toxicities were observed in five of the 14 patients treated in the study. All dose-limiting toxicities were related to neutropenia. Only the 6 and 8mg/m2 edotecarin dose levels were explored; however, no maximum tolerated dose was declared. One confirmed complete response in a patient with hepatocellular carcinoma and seven stable disease responses were achieved in the 14 treated patients. Pharmacokinetic analysis showed that edotecarin achieved and maintained apparent steady-state plasma concentrations during the IV administration in both the cycles. The administration of edotecarin in combination with infusional 5-FU/LV once every 14 days, even without the 5-FU bolus, did not permit adequate time for recovery from neutropenia.
Keywords: edotecarin, J-107088, maximum tolerated dose, pharmacokinetics, solid tumor, topoisomerase I inhibitor
Introduction
Edotecarin (J-107088) is a derivative of NB-506, an indolocarbazole anti-tumor agent, and is a novel inhibitor of topoisomerase I (Topo I) that induces single-strand DNA cleavage more effectively than NB-506 or camptothecin and at different DNA sequences (Fig. 1) [1]. The DNA-Topo I complexes induced by edotecarin are more stable than those occurring after exposure to camptothecin or NB-506. The anti-tumor activity of edotecarin is less cell-cycle dependent than other Topo I inhibitors [1,2]. In addition, edotecarin does not form any active metabolites and is not a substrate for in vitro P450-mediated metabolism.
Fig. 1.
Structure of edotecarin.
The anti-tumor activity of edotecarin has been tested in vitro and in vivo. Its activity is schedule independent and maximum inhibition of tumor growth has been observed in the breast, cervix, pharynx, lung, prostate, colon (colorectal cancer, CRC), stomach and liver cancer models [1–6]. Edotecarin is effective on cells that have acquired resistance related to P-glycoprotein and is highly effective in inhibiting proliferation of liver micrometastases in mice [1]. Thus, edotecarin seems to have a broad spectrum of anti-tumor activity with a wide therapeutic index. In vitro, edotecarin was tested in combination with cisplatin, 5-fluorouracil (5-FU), etoposide, paclitaxel, doxorubicin, vincristine, camptothecin and gemcitabine on HCT-116 colon, SCC-25 tongue, A427 NSCL and J82 human carcinoma cell lines [1,4–6]. The combination of edotecarin with 5-FU showed an additive effect on a variety of cell lines (e.g. colon, non-small cell, lung, tongue and bladder) [2]. In-vivo studies showed that when edotecarin was tested against a human CRC model (HCT-116) in combination with 5-FU (concurrent treatment), it showed efficacy superior in combination than with the drugs as single agents (with no added toxicity)[1,2].
Three earlier phase I trials had assessed various single-agent regimens. Two of these trials were published earlier [7,8]. Results from these single-agent phase I trials supported using the 6 mg/m2 edotecarin starting dose. The primary objective of this trial was to determine the maximum tolerated dose (MTD) and recommended phase II dose of the Topo I inhibitor edotecarin when given in combination with infusional 5-FU/leucovorin (LV) akin to folinic acid, 5-FU, and irinotecan (FOLFIRI) [9] in patients with advanced/metastatic solid tumors.
Patients and methods
Patient eligibility
Patients eligible for this study had radiographic or clinical evidence of an active solid tumor malignancy for which no curative therapy was available. Other inclusion criteria permitted measurable or nonmeasurable disease according to the Response Evaluation Criteria in Solid Tumors criteria [10], Eastern Cooperative Oncology Group performance status of ≤2, absolute neutrophil count (ANC) ≥1500/mm3, platelet count ≥100 000/mm3, hemoglobin ≥9 g/dl, serum creatinine ≤1.5 mg/dl, serum total bilirubin ≤1.5×upper limit of normal (ULN), lactase dehydrogenase and alkaline phosphatase ≤5.0×ULN, aspartate transaminase ≤2.5×ULN or ≤5.0×ULN if liver involvement, serum albumin ≥3.0 g/dl and patients ≥18 years of age with a life expectancy of at least 12 weeks. Patients were permitted to have received up to two earlier chemotherapy regimens, including adjuvant or metastatic therapy; however, past treatment could not include any Topo I inhibitor, high-dose chemotherapy requiring bone marrow rescue, or irradiation to >25% of the bone marrow reserve. Patients could not enter the study if they had known dihydropyrimidine dehydrogenase deficiency, brain or leptomeningeal disease, any significant vascular or cardiac event in the past 12 months, ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria grade ≥2, or there was evidence of an active infection, such as HIV. Patients should have recovered from toxicities from earlier therapies to grade ≤1 (except alopecia). The study was conducted with full institutional review board approvals and all patients provided written informed consent before study enrollment.
Study design and treatment
This was a phase I, single-arm, open-label, two-center dose-escalation trial in sequential cohorts of patients with advanced/metastatic solid tumors. Edotecarin was administered through a central line as an intravenous (IV) 1-h infusion every 2 weeks, followed by a 2-h 200 mg/m2 LV IV infusion, followed by a 46-h 2400 mg/m2 5-FU continuous infusion. Patients were assigned to a dose cohort at enrollment. The edotecarin starting dose (6 mg/m2 every 2 weeks) selected for this trial was based on the results of the single-agent, phase I trials [8,9], and the recommended starting doses for 5-FU/LV were the same as those that have been established earlier for use in combination regimens with irinotecan [10]. In the original protocol, the treatment regimen included a 400 mg/m2 IV bolus dose of 5-FU before the start of the 46-h infusion. However, two of the first six patients who received this treatment experienced dose-limiting toxicities (DLTs) because of neutropenia; therefore, the protocol was amended and the bolus dose was removed from the treatment regimen at the suggestion of the investigators. Edotecarin, 5-FU, and LV were administered as described in Tables 1 and 2. The drugs used in the study are listed in the order of administration. The dose of each drug was based on the baseline body surface area.
Table 1.
Edotecarin dose levels
| Dose level | Edotecarin (mg/m2) | |
|---|---|---|
| Initial dose level | 1 | 6 |
| 2 | 8 | |
| 3 | 11 | |
| 4 | 13 | |
| 5 | 15 | |
| 6 | 17 |
Table 2.
Edotecarin/5-FU/LV treatment regimen
| Drug | Starting dose and route | Dosing schedule | |
|---|---|---|---|
| Edotecarin | X mg/m2 IV over 1 h | Day 1 | Every 2 weeks |
| LV | 200 mg/m2 IV over 2 h | Day 1 | Every 2 weeks |
| 5-FU | 2400 mg/m2 IV over 46 h | Day 1–2 | Every 2 weeks |
5-FU, 5-fluorouracil; IV, intravenous; LV, leucovorin.
X Edotecarin dose level was determined from Table 3 and was assigned by the sponsor when a patient was registered for the study.
A standard phase I dose-escalation design was used, with three to six patients per dose level, to determine the MTD. The definition of MTD will be based on the tolerability observed in the first cycle (i.e. 2 weeks) of treatment. If any of the first three patients treated at a given dose level experienced DLT, an additional three patients were treated at that level. The MTD was defined as the dose at which 0 of six or one of six patients experienced DLT with the next higher dose having at least two of three or two of six patients encountering DLT during the first treatment cycle. Once the MTD was reached, at least six patients were to be treated at the immediately preceding dose, which would be defined as the recommended phase II dose. Patients continued treatment every 2 weeks in the absence of DLT and tumor progression. Within each cohort, the first patient was treated and observed for 2 weeks or until recovery before subsequent patients were enrolled at that same dose level, and the last patient was treated and observed for 2 weeks or until recovery before subsequent patients were enrolled at the next higher dose level. If a patient discontinued treatment before completing cycle 1 (for reasons other than toxicity), an additional patient would be enrolled to replace the discontinued patient at that dose level.
Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria, version 2.0 [11]. DLT was defined by events in cycle 1, as follows: (i) grade 4 neutropenia; (ii) febrile neutropenia, defined as grade 3 or 4 neutropenia (ANC <1000/mm3) and fever ≥38.5°C; or (iii) neutropenic infection, defined as grade 3 or 4 neutropenia with ≥grade 3 infection; or (iv) grade 4 thrombocytopenia; or (v) grade 3 thrombocytopenia and ≥grade 3 with hemorrhage; or (vi) grade ≥3 nausea or vomiting despite maximal anti-emetic therapy; or (vii) grade ≥3 diarrhea despite maximal loperamide support; or (viii) grade 3 or 4 toxicities attributable to the study drug; or (ix) failure to recover to ≤grade 1 toxicity (except alopecia) after delaying the initiation of the next cycle by >1 week. Doses reduced for drug-related toxicity were not re-escalated, even if there was minimal or no toxicity with the reduced dose. If, in ≥cycle 3, despite supportive measures and dose reduction, the patient experienced grade 3–4 toxicity, further dose reduction was discussed with the sponsor.
A new cycle of treatment could begin when the ANC was ≥1500/mm3, the platelet count was ≥75 000/mm3, and nonhematologic toxicities (except for alopecia) were ≤grade 1. If these conditions were not met, the treatment was delayed for 1 week to allow for recovery. If after a 1-week delay, all toxicities (except for alopecia) were ≤grade 1, the treatment proceeded as outlined in the dose modification (Tables 3 and 4) if the patient had experienced excessive toxicity. If recovery did not occur by day 21, the sponsor and the investigator assessed the risk-benefit of continuing the treatment. Adjustment of edotecarin and 5-FU was made independently based on the specific types of toxicities that were observed. The LV dose remained the same throughout the treatment. Intrapatient dose escalation of edotecarin was not permitted.
Table 3.
Dose modification levels
| Edotecarin (mg/m2) | 5-FU (mg/m2)a 46-h infusion |
|
|---|---|---|
| 17 | Adjustment of edotecarin and 5-FU was made independently based on the specific types of toxicities that were observed | 3000a |
| 15 | 2400 | |
| 13 | 2000 | |
| 11 | 1600 | |
| 8 | ||
| 6 |
5-FU, 5-fluorouracil.
In cycle 3 and beyond, the infusional dose of 5-FU could have been increased to 3000 mg/m2 providing that there were no toxicities ≥grade 1 in the preceding cycle.
Table 4.
Dose modifications for day 1 of new cycle based on worst type toxicity observed in the earlier cycle
| Dose changes are relative to the day 1 dose of the previous cycle | ||
|---|---|---|
| Toxicity (NCI CTC grade)a | Edotecarin | Infusional 5-FU |
| Hematologic | ||
| Grade 4 neutropenia at any time during the 2-week cycle | ||
| Febrile neutropenia [grade 3 or 4 neutropenia (ANC < 1000/mm3 with fever ≥38.5°C)] | ||
| Neutropenic infection: grade 3 or 4 neutropenia with ≥grade 3 infection | ↓ 1 dose level | ↓ 1 dose level |
| Grade 4 thrombocytopenia | ||
| Grade 3 thrombocytopenia and ≥grade 3 hemorrhage | ||
| Diarrhea | ||
| Grade 3 or 4 diarrhea despite maximal loperamide support | ↓ 1 dose level | ↓ 1 dose level |
| Mucositis | ||
| Grade 3 or 4 mucositis | Maintain dose level | ↓ 1 dose level |
| Vomiting | ||
| Grade ≥3 vomiting despite maximal oral antiemetic therapy | ↓ 1 dose level | ↓ 1 dose level |
| Palmar-plantar erythrodysesthesia | ||
| Grade 3 palmar-plantar erythrodysesthesia | Maintain dose level | ↓ 1 dose level |
| Other nonhematologic toxicities | ||
| Grade 3 or 4 toxicities attributable to study drug | ↓ 1 dose level | ↓ 1 dose level |
| Failure to recover | ||
| Failure to recover to grade ≤1 toxicity (except alopecia) after delaying the initiation of the 2nd cycle by > 7 days (i.e. >21 days from the start of the current cycle) | Investigator to discuss with Pfizer | Investigator to discuss with Pfizer |
| All toxicities (except alopecia) ≤grade 1 | ||
| At Cycle 3 and beyond – if no drug-related adverse events (except alopecia) ≥grade 1 in the preceding cycle | Maintain dose level | ↑ infusional 5-FU only to 3000 mg/m2 |
5-FU, 5-fluorouracil; ANC, absolute neutrophil count; CTC, Common Toxicity Criteria; NCI, National Cancer Institute.
NCI CTC (Version 2.0).
Routine prophylactic use of granulocyte colony-stimulating factor (G-CSF; filgrastim, Neupogen) was not recommended. However, prophylactic administration of G-CSF in a patient who was experiencing recurrent difficulties with neutropenia in the subsequent cycles, or its therapeutic use in patients with serious neutropenic complications such as tissue infection, sepsis syndrome, fungal infection, etc., were considered for treatment at the investigator’s discretion, consistent with the American Society of Clinical Oncology guidelines. G-CSF was not given concurrent with either edotecarin or 5-FU/LV infusion. The use of erythropoietin was permitted at the discretion of the treating physician.
Edotecarin was supplied by Pfizer, Inc. (Nerviano, Italy) as a 5 mg/ml solution in a 25 mg vial (5 ml/vial) which required dilution in 5% glucose or dextrose before administration. Before receiving chemotherapy, the patients were administered prophylactic anti-emetics of 1 mg IV granisetron or 32 mg IV ondansetron, with 20 mg IV dexamethasone on day 1, followed by 1 mg twice daily (BID) po granisetron or 8 mg BID po ondansetron with 4mg BID po dexamethasome permitted on days 2–4.
Patients were monitored for safety (vital signs, hematology, serum chemistry, and urinalysis) during each cycle of therapy. Physical examinations were performed before the treatment, weekly during cycle 1, and before drug administration on day 1 of each subsequent cycle. Adverse events were monitored throughout the study. Clinical and radiographic tumor assessments were performed before the first treatment and after every third cycle of therapy (i.e. 6 weeks) for four assessments, and subsequently after every six cycles (i.e. 12 weeks). The overall tumor response was categorized using the Response Evaluation Criteria in Solid Tumors criteria [11]. Complete or partial responses were to be confirmed at least 4 weeks and no more than 6 weeks after the first documentation of response, with a CT scan or MRI as the preferred method for tumor assessment. The patients continued in the study until disease progression, patient refusal, or unacceptable toxicity occurred.
Pharmacokinetic sampling schedule
Blood samples were collected from all the patients during cycles 1 and 2 for evaluation of edotecarin and 5-FU pharmacokinetics (PK) at the times outlined in Table 5. Plasma samples were assayed for edotecarin concentrations using the high-performance liquid chromatography method. In brief, edotecarin and its stable isotope-labeled internal standard, PHA-848205 (13C6), were isolated from a 250-μl aliquot of human plasma, containing sodium heparin, after addition of 250 μl of pH 7.0 phosphate buffer, by solid phase extraction using a Varian BondElut CH (100 mg) 96-well extraction plate. The eluate was evaporated under a nitrogen stream, and the remaining residue was reconstituted with 400 μl of reconstitution solution. The sample extract was analyzed by LC/MS/MS (Sciex API 3000, Triple quadrupole) using negative ion Turbo IonSpray with multiple reaction monitoring ion detection. The monitored mass transitions were m/z 607.2–355.1 for edotecarin and m/z 613.2–355.1 for PHA-848205 (13C6). The chromatographic separation was achieved on a Waters XTerra RP18 column using a methanol/water mobile phase gradient containing 2.0m/mol ammonium hydroxide in both the components, and a flow rate of 0.200 ml/min. The retention time for edotecarin and its internal standard was approximately 1.5 min. The dynamic range of the assay was 0.1–100 ng/ml. Samples containing edotecarin concentrations above 100 ng/ml were diluted with sufficient control plasma and re-assayed in a separate analytical run. The 5-FU samples were not analyzed.
Table 5.
Timings for PK collection
| Edotecarin PK | Pre-edotecarin infusion | During edotecarin infusion | Post-end edotecarin infusion |
|---|---|---|---|
| Cycle 1 (12 samples) | Within 30 min before start of infusion | 15, 30 and 50 min after start of infusion | 5, 15, 30 min and 1, 1.25, 1.5, 2, 3, 4, 6, 8, 24, 48, 72 and 96 h |
| Cycle 2 (10 samples) | Within 30 min before start of infusion | 50min after start of infusion | 5min and 1, 2, 4, 24, 48, and 72 h |
| 5-FU PK | During 5-FU infusion | Post-end 5-FU infusion | |
| Cycle 1 (5 samples) | 3 h, 24 h, and 45 h+ 50 min, after start of infusion | 10 min and 1 h | |
| Cycle 2 (5 samples) | 3 h, 24 h, and 45 h+ 50 min, after start of infusion | 10 min and 1 h | |
5-FU, 5-fluorouracil; PK, pharmacokinetics.
Each blood sample was placed on ice immediately after collection and, within 60 min for edotecarin samples and 30 min for 5-FU samples, centrifuged for harvesting of plasma. Centrifugation was done either at room temperature or at 4°C. Plasma was stored frozen (at or below −20°C).
Statistical and pharmacokinetic analysis methods
Summary statistics for the determination of the MTD and the recommended phase II dose were based on data from cycle 1, while the PK results were based on data collected from cycle 1 and cycle 2. Edotecarin plasma concentration–time data were analyzed by noncompartmental methods using WinNonlin V.3.2 (Pharsight, Mountain View, California, USA).
Results
Patient characteristics
Fifteen patients were enrolled to the study between May 2003 and May 2004 at two sites (nine in France; six in the USA). One patient randomized to the 8 mg/m2 dose cohort died after randomization but before receiving any study treatment and therefore was not included in the analysis. Most of the patients were male (10 of the 14 patients), aged from 38 to 73 years, and had Eastern Cooperative Oncology Group performance scores of 0 or 1 at baseline. Demographic and baseline characteristics are summarized in Table 6. All the patients had metastatic disease, and all but four patients had experienced at least two recurrences of their disease. The primary diagnoses included pancreatic adenocarcinoma (three); adrenal carcinoma (one); clear cell renal cell carcinoma (two); lung carcinoma (one); hepatocarcinoma (HCC; one); esophageal carcinoma (one); neuroendocrine carcinoma (two); gastric cancer (one); ampullary adenocarcinoma (one); and duodenal adenocarcinoma (one). Sites of metastases included liver (six), lung (six), bone (one), lymph nodes (three) and other sites (four). Thirteen of the 14 evaluable patients had received chemotherapy earlier, seven patients had received radiation earlier, and 11 patients had undergone surgery in the past. A total of 90 cycles of treatment were administered in the study (range 3–18).
Table 6.
Demographic and baseline characteristics
| Demographic/baseline variable | Number of patients |
|
|---|---|---|
| Edotecarin +5-FU/LV N=8a |
Edotecarin + 5-FU (w/bolus)/LV N=6b |
|
| Sex | ||
| Male | 7 | 3 |
| Female | 1 | 3 |
| Race | ||
| White | 3 | 2 |
| Black | 0 | 1 |
| Not allowed to ask | 5 | 3 |
| ECOG performance status | ||
| 0 | 4 | 4 |
| 1 | 4 | 2 |
| Age (years) | ||
| Mean (SD) | 60.25 (8.84) | 51 (8.39) |
| Range | 48–73 | 38–61 |
| Weight (kg) | ||
| Mean (SD) | 82.21 (12.95) | 69.13 (14.31) |
| Range | 61–99 | 57–90 |
| Body surface area (ratio) | ||
| Mean (SD) | 1.965 (0.179) | 1.8.01 (0.175) |
| Range | 1.631–2.173 | 1.616–2.017 |
5-FU, 5-fluorouracil; ECOG, Eastern Cooperative Oncology Group; LV, leucovorin; SD, standard deviation.
5 patients assigned to edotecarin dose of 6 mg/m2, 3 patients assigned to edotecarin dose of 8 mg/m2.
Edotecarin 6 mg/m2, 5-FU administered as bolus and infusion.
Safety
Overall, a total of 90 cycles of treatment were administered. No patients died within 30 days of the last treatment dose; four patients were discontinued from the study because of adverse events (AEs; neutropenia), three patients had dose reductions because of AEs (neutropenia), and four patients had serious adverse events (unrelated pyrexia in one patient, unrelated dehydration in one patient, treatment-related febrile neutropenia and lower respiratory tract infection in one patient, and treatment-related neutropenia in one patient).
Of the 14 patients treated in this study, five (36%) patients experienced DLTs. All DLTs were related to neutropenia (100%). In the first cohort, patients received the 400mg/m2 5-FU bolus and 2400 mg/m2 5-FU infusion in addition to the LV and 6 mg/m2 edotecarin. Two of the six patients enrolled to this first cohort experienced a failure to recover to at least grade 1 neutropenia by day 21, conforming DLT. One patient had a maximum grade 3 neutropenia, whereas the other patient experienced a maximum grade 4 neutropenia. In the 8 mg/m2 edotecarin cohort (no 5-FU bolus), two of the three patients experienced grade 4 neutropenia (66%). No DLTs were seen in the first three patients enrolled to the 6 mg/m2 edotecarin cohort without the 5-FU bolus. However, when the cohort was expanded with two more patients, one patient had grade 3 neutropenia, which failed to recover to at least a grade 1 neutropenia by day 21, thus resulting in a DLT.
The most frequently reported AEs overall were nausea (10 patients; 71%), fatigue (nine patients; 64%), neutropenia (eight patients; 57%), pyrexia (seven patients; 50%), and vomiting (six patients; 42%). The occurrence of treatment-related neutropenia of ≥grade 3 severity after treatment with the edotecarin+5-FU (with bolus)/LV dose resulted in the discontinuation of three patients from the treatment and revision of the treatment regimen. The AEs of nausea, fatigue, pyrexia, and vomiting were of ≤grade 2 severity; nausea, fatigue, and vomiting were generally considered to be related to the treatment, and pyrexia was generally considered to be related to the underlying disease (tumor fever).
The most frequently reported AE after edotecarin+ 5-FU/LV 6 mg/m2 treatment was nausea (five of the five patients; 100%); after edotecarin+5-FU/LV 8mg/m2 treatment was back pain (three of the three patients; 100%); and after edotecarin+5-FU (with bolus)/LV treatment was fatigue (five of the six patients; 83%). Of these most frequently reported AEs, nausea and fatigue were generally considered to be related to treatment.
The most frequently reported AEs during cycle 1 were neutropenia (seven patients; 50%), nausea (seven patients; 50%), and fatigue (five patients; 35%). The most frequently reported AE in the first cycle of edotecarin+ 5-FU/LV 6 mg/m2 treatment was nausea (four of the five patients; 80%), and in the first cycle of edotecarin+5-FU/LV 8 mg/m2 treatment was neutropenia (two of the three patients; 66%). The most frequently reported AEs in the first cycle of edotecarin+5-FU (with bolus)/LV treatment were neutropenia, nausea, and fatigue (three of the six patients for each).
Beyond the first cycle of treatment, the most frequently reported AEs were generally the same as those reported during cycle 1, with the exception of a more frequently reported vomiting (eight patients; 57%).
Tumor response
A summary of tumor response by the patients is provided in Table 7. The best overall response was considered by the investigator to be complete response in one patient diagnosed with HCC, stable disease in seven patients, and progressive disease in six patients. The patient with HCC was initially diagnosed in April 1998 and had metastasis to the lungs and stomach diagnosed in January 2003. By cycle 5 on edotecarin/5-FU/LV, this patient had achieved a confirmed complete response and discontinued further study treatment after the administration of cycle 12 as a complete responder. The patients with stable disease received an average of nine cycles of the study treatment (range 3–18 cycles). One patient with pancreatic cancer who had progressed earlier on two past chemotherapy regimens, achieved stable disease by cycle 3 and continued to receive a total of 18 cycles of treatment, the maximum number of cycles of treatment administered in the study.
Table 7.
Summary of final response status assessments by patient
| Primary diagnosis | Assigned treatment | Completed cycles of treatment | Best overall overall responsea |
|---|---|---|---|
| Renal cell carcinoma | Edotecarin + 5-FU/LV | Cycle 3 | PD |
| Pancreatic cancer | Edotecarin + 5FU/LV | Cycle 18 | SD |
| Adenocarcinoma of pancreas | Edotecarin + 5-FU/LV | Cycle 3 | PD |
| Epidermoid lung carcinoma | Edotecarin + 5-FU/LV | Cycle 3 | PD |
| 1/3 esophagus carcinoma | Edotecarin + 5-FU/LV | Cycle 9 | SD |
| Adrenal carcinoma | Edotecarin + 5-FU/LV | Cycle 3 | SD |
| Pancreatic adenocarcinoma | Edotecarin + 5-FU/LV | Cycle 3 | PD |
| Hepatocarcinoma | Edotecarin + 5-FU/LV | Cycle 12 | CR |
| Moderately differentiated adenocarcinoma duodenum | Edotecarin + 5-FU (w/bolus) +LV | Cycle 7 | SD |
| Gastric carcinoid | Edotecarin + 5-FU (w/bolus) +LV | Cycle 9 | SD |
| Ampullary cancer | Edotecarin + 5-FU (w/bolus) +LV | Cycle 6 | SD |
| Small cell neuroendocrine carcinoma liver metastases | Edotecarin + 5-FU (w/bolus) +LV | Cycle 3 | PD |
| Bronchus carcinoma | Edotecarin + 5-FU (w/bolus) +LV | Cycle 8 | SD |
| Superior pole of left kidney cancer | Edotecarin + 5-FU (w/bolus) +LV | Cycle 3 | PD |
5-FU, 5-fluorouracil; CR, complete response; LV, leucovorin; PD, progression of disease; SD, stable disease.
As determined by the investigator.
Pharmacokinetics
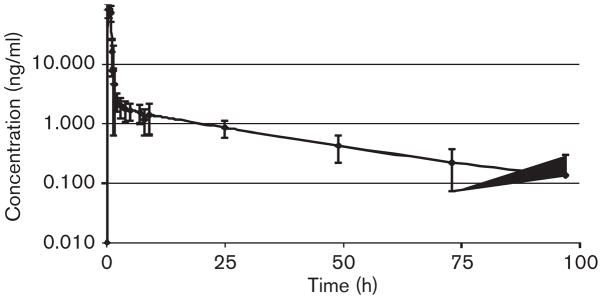
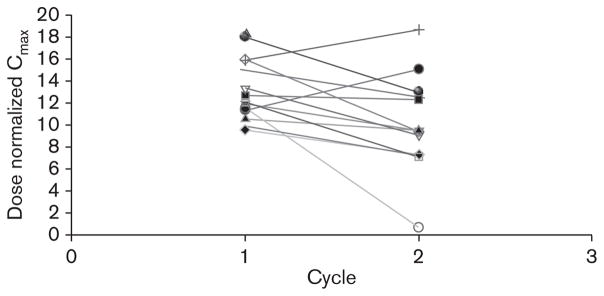
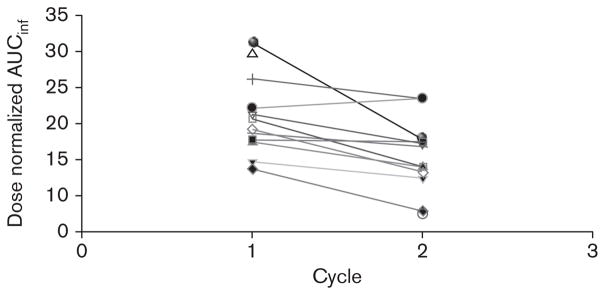
Edotecarin rapidly achieved and maintained apparent steady-state plasma concentrations during IV administration in both the cycles. After the completion of the IV administration, edotecarin exhibited bi-exponential disposition with a very rapid initial decline in plasma concentrations followed by a much slower reduction in plasma concentrations. Figure 2 depicts the mean concentration-time profile of edotecarin after cycle 1. Figures 3 and 4 show the relationship between cycle 1 and cycle 2 for the dose-normalized edotecarin Cmax and area under the plasma concentration–time curveinf, respectively. In general, the systemic exposure values were consistent between the cycles with a trend towards lower exposures in cycle 2, which may be partially because of the different initial sampling schedules between cycle 1 and cycle 2. Table 8 summarizes the PK parameters of edotecarin during cycle 1 and cycle 2. Edotecarin seems to be rapidly cleared with systemic clearance approaching hepatic blood flow. The volume of distribution varied greatly between the patients, especially during cycle 2, and a trend towards a larger value in cycle 2 was observed. The terminal half-life was approximately 25 h during cycle 1 and 20 h during cycle 2.
Fig. 2.
Mean (±SD) plasma concentration–time profiles of edotecarin in cancer patients when administered as a 1-h intravenous infusion (cycle 1).
Fig. 3.
Comparison of dose normalized Cmax between cycle 1 and cycle 2.
Fig. 4.
Comparison of dose normalized AUC (0-inf) between cycle 1 and cycle 2.
Table 8.
Mean (±SD) of pharmacokinetic parameters of edotecarin after 1-h
| Parameter | Edotecarin |
|
|---|---|---|
| Cycle 1 | Cycle 2 | |
| Cmax (ng/ml)a | 79.0 ± 17.6 | 59.2± 27.3 |
| Dose normalized Cmax (ng/ml)b | 13.2 ± 2.9 | 10.1 ± 4.4 |
| Tmax (h) | 0.48 ± 0.22 | 0.83 ± 0.0 |
| AUC (0–24) (ng/h/ml)a | 91.0 ± 21.6 | 65.3 ± 22.9 |
| Dose normalized AUC (0–24) (h/ng/ml)b | 15.3 ± 3.6 | 11.1 ± 3.7 |
| AUC (0-inf) (ng/h/ml)a | 124.5 ± 34.7 | 91.7 ± 31.9 |
| Dose normalized AUC (0-inf) (ng/h/ml/)b | 20.7 ± 5.4 | 15.4 ± 5.1 |
| CL (l/h/m2) | 51.3 ± 12.4 | 73.3± 29.7 |
| Vss (l/m2) | 884.4 ± 296.0 | 1408.7± 999.7 |
| T (h) | 25.3 ± 6.4 | 20.3 ± 4.2 |
AUC, area under the plasma concentration–time curve; CL, total body-clearance; Cmax, peak plasma concentration; t, half-life; tmax, time to maximum concentration.
Parameter following the 6 mg/m2 dose only.
Normalized to a 1 mg/m2 dose.
The average concentration obtained at steady state for 5-FU during cycle 1 was 470.6±186.8 and 455.9±222.8 ng/ml during cycle 2.
Discussion
Topo I is an essential enzyme that produces a DNA single-strand break allowing DNA relaxation for replication. The enzymatic mechanism involves sequential transesterifcations. The breakage and closure reactions generate phosphodiester bonds and similar free energies; therefore, the reaction is reversible. Camptothecin analogs, topotecan, and irinotecan are approved Topo I-targeted drugs. Both have limitations because of the equilibrium between the camptothecin lactone and ring-opened forms. Several strategies are being explored to develop improved Topo I inhibitors. Edotecarin is a novel inhibitor of Topo I that induces single-strand DNA cleavage more effectively than NB-506 or camptothecin and at different DNA sequences [1,2] with more stable DNA-Topo I complexes. Moreover, the anti-tumor activity of edotecarin is less cell-cycle dependent than other Topo I inhibitors and it does not form any active metabolites and is not a substrate for in vitro P450-mediated metabolism [6]. The anti-tumor activity of edotecarin has been tested in vitro and in vivo, including the additive effect when combined with 5-FU on colon cell lines (HCT-116) [1,6].
Since the mid-1990’s, it was established that the Topo I inhibitor, CPT-11 (irinotecan, CAMPTOSAR, CAMPTO), had activity in the therapy of metastatic colorectal cancer. Given CPT-11’s significant second-line, single-agent activity and its different mechanism of action compared with 5-FU, two pivotal phase III trials in patients with untreated metastatic colorectal cancer were conducted to evaluate whether the combination of CPT-11/5-FU/LV would improve tumor control and survival relative to 5-FU/LV alone [10,12], and produced statistically significant improvements in the tumor response rate, time to tumor progression and overall survival compared with 5-FU/LV alone. These significant improvements in efficacy outcomes were observed with both the bolus and infusional combination regimens. On the basis of these data, CPT-11/5-FU/LV has been registered in countries all over the world and is now considered to be the standard of care for patients with advanced disease. Diarrhea and myelosuppression are common DLT-associated with both CPT-11 and 5-FU single-agent and combination therapies. Evaluation of the safety profile from the two pivotal trials with CPT-11/5-FU/LV indicated that approximately 23% of the patients experienced grade 3 or 4 diarrhea with both the combination regimens, and 9% of the patients experienced grade 4 neutropenia with infusional CPT-11/5-FU/LV versus 24% with bolus combination therapy. Diarrhea represents a particularly bothersome toxicity for patients, and, when severe, can increase the risk for infectious complications in patients with concurrent neutropenia. Therefore, the development of a new Topo I inhibitor, such as edotecarin, which can be combined with 5-FU/LV to maintain the tumor control and an improved safety profile with less diarrhea seems very promising. This led us to conduct this phase I study to test the combination in a regimen akin to FOLFIRI or folinic acid, 5-FU, and oxaliplatin (FOLFOX) using an infusional 5-FU regimen [10,13].
In our study, the patients received edotecarin at a starting dose of 6 mg/m2, followed by 200 mg/m2 LV IV infusion administered over 2 h, then 400 mg/m2 bolus dose of 5-FU before the start of 2400mg/m2 5-FU continuous infusion for a further 46 h. Two of the six patients enrolled to this first cohort experienced grade 3 neutropenia and grade 4 neutropenia, resulting in two DLTs. The protocol was amended after the first six patients to remove the 5-FU bolus dose, as bolus 5-FU was considered by the investigators and the sponsor as one of the leading causes of the dose-limiting neutropenia observed at the original dose level 1 [14]. This decision was also based on the rationale of the regimen that may mimic FOLFIRI or FOLFOX and may replace irinotecan caused by less severe diarrhea [15]. On the basis of various regimens of FOLFOX: FOLFOX-6, comprising a higher dose of oxaliplatin added to a new, simplified bimonthly LV–5-FU regimen, oxaliplatin 100mg/m2 and LV 400mg/m2 were administered as 2-h infusions on day 1, followed by a 5-FU bolus of 400 mg/m2 and a 46-h infusion of 5-FU 2400–3000 mg/m2 over days 1 and 2; and FOLFOX-7, consisting of oxaliplatin 130 mg/m2 as a 2-h infusion given concurrently with the 2-h infusion of LV 400 mg/m2, followed by a 5-FU bolus of 400 mg/m2 on day 1 and a 46-h infusion of 5-FU 2400 mg/m2. Even the administration of edotecarin in combination with infusional 5-FU/LV without the 5-FU bolus, did not permit adequate time for recovery from neutropenia. The finding that despite omitting bolus 5-FU, the patients were unable to recover by day 21 from neutropenia associated with combined edotecarin/5-FU treatment has precluded further development of this treatment regimen. However, activity in HCC and pancreatic cancer patients was appreciated. No clinically important treatment-related changes in clinical laboratory parameters, vital signs measurements, or ECG results were observed.
Subsequent studies evaluated edotecarin as a single agent with a 21-day schedule. As compared with these phase I studies [8,9], the DLT was similar: neutropenia. Hurwitz et al. concluded that the recommended phase II dose of edotecarin is 13 mg/m2 once every 21 days. The toxicities in this study were those typical of cytotoxic chemotherapy and less severe than those associated with other Topo I inhibitors. However, as evident, the higher dose was tolerated as a single agent when given every 3 weeks [8]. Similarly, Yamada’s phase I study showed that the MTD of edotecarin administered IV over 2 h every 21 days was 15mg/m2 [9]. The recommended dose for phase II studies with a 3-week schedule (with 1 week permitted for recovery from toxicities, if needed) was 13mg/m2.
A phase II multicenter study was performed to determine the activity and safety of single-agent edotecarin in patients with irinotecan-naïve patients with CRC [16]. Twenty-four patients received edotecarin at 13 mg/m2. Three patients (13%) had a partial response, and 13 patients (57%) had stable disease (including four minor responses). The median time to tumor progression was 7.1 months. Grade 3/4 AEs were febrile neutropenia in one patient (4.2%). These follow-up studies show that edotecarin is active and safe as a single-agent, second-line therapy for patients with CRC. On account of the low incidence of grade 3/4 vomiting and the absence of grade 4 diarrhea, the investigators suggested that edotecarin may offer an improved therapeutic index over the existing agents and should be developed as a single agent and in combination with other cytotoxics or biologic agents, especially cetuximab or panitumumab, for CRC and other cancers.
In general, gastrointestinal toxicities were minimal, especially diarrhea. However, neutropenia >grade 2 in severity was observed in all the patients treated with the edotecarin+5-FU (w/bolus)/LV regimen. The treatment regimen was altered such that the 5-FU bolus was eliminated whereas the 5-FU continuous infusion was maintained. However, the inability of the patients to recover by day 21 from neutropenia associated with combined edotecarin/5-FU treatment has precluded further development of this treatment regimen. Pfizer Pharmaceuticals, Inc., has suspended the clinical development of this agent and has handed it over to Merck. Future studies of evaluating edotecarin every 3 weeks with a less myelosuppressive antifolate, capecitabine, are warranted.
In summary, the administration of edotecarin in combination with infusional 5-FU/LV once every 14 days, even without the 5-FU bolus, did not permit adequate time for recovery from neutropenia. The observed safety profile and preliminary evidence of clinical benefit in patients with solid tumors warrant further investigation of this drug combination therapy with an extended recovery period between the treatment cycles.
Acknowledgments
This study was sponsored by Pfizer Inc.
References
- 1.Saif MW, Diasio RB. Edotecarin: a novel topoisomerase I inhibitor. Clin Colorectal Cancer. 2005;5:27–36. doi: 10.3816/ccc.2005.n.014. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa H, Morita M, Kodera T, Okura A, Ohkubo M, Morishima H, Nishimura S. In vivo anti-tumor activity of a novel indolocarbazole compound, J-107088, on murine and human tumors transplanted into mice. Jpn J Cancer Res. 1999;90:1163–1170. doi: 10.1111/j.1349-7006.1999.tb00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciomei M, Croci V, Ciavolella A, Ballinari D, Pesenti E. Antitumor efficacy of edotecarin as a single agent and in combination with chemotherapy agents in a xenograft model. Clin Cancer Res. 2006;12:2856–2861. doi: 10.1158/1078-0432.CCR-05-1859. [DOI] [PubMed] [Google Scholar]
- 4.Ciomei M, Croci V, Pesenti E, Kodera T, Monden Y, Ciavolella A, et al. Antitumor efficacy of edotecarin in combination with other cytotoxic drugs. Proc Amer Assoc Cancer Res. 2004:45. Abstract # 2099. [Google Scholar]
- 5.Cavazos CM, Keir ST, Yoshinari T, Bigner DD, Friedman HS. Therapeutic activity of the topoisomerase I inhibitor J-107088 [6–N-(1-hydroxymethyla-2-hydroxyl) ethylamino-12,13-dihydro-13-(beta-D-glucopyranosyl)-5Hindolo( 2,3-a)-pyrrolo(3,4-c)-carbazole-5,7(6H)-dione] against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol. 2001;48:250–254. doi: 10.1007/s002800100347. [DOI] [PubMed] [Google Scholar]
- 6.Ciomei M, Croci V, Stellari F, Amboldi N, Giavarini R, Pesenti E. Antitumor activity of edotecarin in breast carcinoma models. Cancer Chemother Pharmacol. 2007;60:229–235. doi: 10.1007/s00280-006-0365-8. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz HI, Cohen RB, McGovren JP, Hirawat S, Petros WP, Natsumeda Y, Yoshinari T. A phase I study of the safety and pharmacokinetics of edotecarin (J-107088), a novel topoisomerase I inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2007;59:139–147. doi: 10.1007/s00280-006-0267-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Tamura T, Yamamoto N, Shimoyama T, Ueda Y, Murakami H, et al. Phase I and pharmacokinetic study of edotecarin, a novel topoisomerase I inhibitor, administered once every 3 weeks in patients with solid tumors. Cancer Chemother Pharmacol. 2006;58:173–182. doi: 10.1007/s00280-005-0149-6. [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicenter randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J NCI. 2000;95:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute, Cancer Therapy Evaluation Program. CTCAE/CTC Archive. URL: http://ctep.cancer.gov/reporting/ctc_archive.html://ctep.cancer.gov/protocoldevelopment/.../ctcv20_4-30-992.pdf.
- 12.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 13.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 14.Shaib W, Lee V, Saif MW. Bolus 5-fluorouracil as an alternative modality to infusion 5-fluorouracil in a patient with rectal cancer and capecitabine-induced cardiotoxicity. In Vivo. 2009;23:821–826. [PubMed] [Google Scholar]
- 15.Maindrault-Goebel F, Tournigand C, André T, Carola E, Mabro M, Artru P, et al. Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer. Ann Oncol. 2004;15:1210–1214. doi: 10.1093/annonc/mdh305. [DOI] [PubMed] [Google Scholar]
- 16.Nahum K, Shiba D, Padavanija P, Garcia M, Hurwitz H, Mackintosh F, et al. Phase II efficacy and tolerability study of edotecarin (J-107088) in patients with irinotecan-naïve metastatic colorectal cancer (MCRC) Proc Am Soc Clin Oncol. 2003:22. Abstract #1099. [Google Scholar]