Abstract
A close association between brain metal dishomeostasis and the onset and/or progression of Alzheimer's disease (AD) has been clearly established in a number of studies, although the underlying biochemical mechanisms remain obscure. This observation renders chelation therapy an attractive pharmacological option for the treatment of this disease. However, a number of requirements must be fulfilled in order to adapt chelation therapy to AD so that the term “metal targeted strategies” seems now more appropriate. Indeed, brain metal redistribution rather than brain metal scavenging and removal is the major goal of this type of intervention. The most recent developments in metal targeted strategies for AD will be discussed using, as useful examples, clioquinol, curcumin, and epigallocatechin, and the future perspectives will also be outlined.
Keywords: Alzheimer's disease, clioquinol, cuprizone, metal dishomeostasis, metal ions, nanomedicine, Parkinson's disease, polyphenols
Introduction
Despite an enormous increase in the understanding of the neuropathological and neurochemical events taking place in Alzheimer's disease (AD) as well as in other neurodegenerative diseases, the etiopathogenesis of progressive and mental cognitive dysfunction concomitant with aging remains largely obscure [1]. So far, there are no effective drugs that can be prescribed to reverse or reduce, safely, the mental decline of affected patients.
Metal ions have been shown to abnormally accumulate in the brain with aging as well as in the course of several neurodegenerative disorders including AD [2– 7]. Particularly, the interplay of metal-protein interactions with oxidative stress was recently highlighted by several laboratories [4,8–11]. Accordingly, metal chelation therapy may now be considered as a promising clinical approach to AD [1,12–16].
There is compelling evidence that the etiology of AD involves, among other features, characteristic amyloid-β (Aβ) deposition, severe oxidative stress, and metal-Aβ interactions [14,17,18]. Recent studies have implicated physiological transition metals such as copper (Cu), iron (Fe), and zinc (Zn) and prooxidant non-physiological elements, such as aluminum (Al), as key factors in the pathophysiology of AD [3,7,19]. Al was very much in the public eye for a long time before the turn of this century with scares over its standard utilization as a flocculant in the purification of drinking water. So far, however, there has been no direct attributable connection between AD and Al [20]. Nonetheless, several studies have documented build up of Al in patients with AD [3,21,22], but the results remain rather controversial due to the complexity of Al chemistry in biological systems. It was also shown that there is a high focal increase of Al in the core and around amyloid plaques and neurofibrillary tangles in AD [23]. However, the discovery that clioquinol (CQ), which is a specific Cu-Zn chelator, can inhibit Aβ accumulation has led to the shift in the focus, in our opinion rather imprudently, from Al to Cu and Zn as key players in AD [24]. Recent controversial clinical and experimental results concerning the therapeutic use of CQ reversed the first mechanistic hypothesis stating that the efficacy of CQ essentially arises from its ability to remove metal ions from the brain [25,26]. This underlines the necessity to improve the basic studies in order to better understand the biochemical properties of metal chelators and optimize their use in neurodegenerative therapies.
As the demand for new and more effective drugs for AD treatment continues to grow, pharmacological strategies aimed at lowering brain metal ions and targeting Aβ/metal ions interactions might offer a large potential to chelation therapy. In spite of the conspicuous theoretical basis for chelation therapy in AD, there is still a substantial lack of relevant and reliable data as well as definitive conclusions regarding the clinical advantages of chelation in neurodegenerative conditions. Indeed, the studies concerning the application of chelating agents in neurodegeneration that have appeared so far mainly considered the bioinorganic aspects of the metal complexation reactions; in contrast very scarce interest has been directed to both preclinical and clinical experimentation. However, as there is yet no effective treatment for many neurological diseases, the therapeutic use of effective chelators remains a well grounded option that requires strong interdisciplinary investigation. In this connection, we propose that differential biodistribution and intracellular sub-accumulation of metals concomitantly with the progression of disease severity has to be elucidated and understood precisely before administering any metal chelation therapy [27].
Limited studies exist on metal levels in different phases of AD pathology. We reported data on different elemental levels with respect to the severity of the disorder [3]. In the present paper we advocate that the approach of metal chelation should be furtherly developed and optimized through an understanding of the gradual metal accumulation during disease progression. In addition it must be paid attention to the use of specific chelators and to the time and dosage of administration.
Metal Dishomoestasis in Alzheimer's Disease
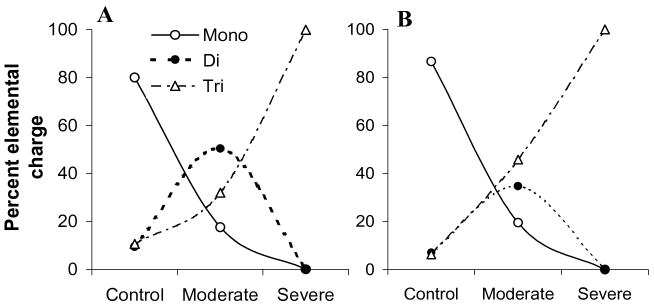
Metal ions have been shown to be involved in cell-cell communication and signal transduction, as well as in influencing transcription and translation via metal responsive regulators. Neurodegeneration in AD is characterized, among other features, by a marked accumulation of metals, mainly Cu, Zn, Fe, and Al, in various regions of the brain [2,3,28–30] and by disruption in the metabolism of these metals leading to their altered transport and accumulation in senile plaques and other topological sites [31]. Indeed, very high levels of Cu (400 μM) and Zn (1 mM) were found in amyloid plaques and AD neuropil regions in comparison to healthy brain (70 μM Cu and 350 μM Zn) [2,32]. A progressive accumulation of metals in AD brain during disease progression from moderate to severe AD was also observed [3]. Remarkably, this latter study revealed that divalent cations increase in the early phase of AD, while trivalent metal ions start increasing significantly in the later phase of AD, mainly in frontal cortex and hippocampus (Fig. 1) [3]. This displacement of divalent metals by Al in the severe AD is a likely result of differences in the metal-ligand affinity exchange rates [3]. In general, it seems that a common trait to many processes underlying neurodegeneration in AD is a (direct or indirect) perturbation in the homeostasis of Cu, Zn, Fe, Al, calcium (Ca) etc. (metal dishomeostasis). Nonetheless, the overall brain metal burden is never dramatically increased in AD as it occurs, instead, in specific alterations of brain metal metabolism on a genetic base (e.g., Wilson's disease, neuroferritinopathy, etc.) [33,34].
Fig. 1.
Percentage of elemental charge distribution in control and AD brains. A, frontal cortex; B, hippocampus [3].
Chelation Therapy Definition and Specificity
Chelation therapy has been proposed as the appropriate treatment for reducing the abnormal accumulation of essential heavy metals, such as Fe, Cu, and Zn, or nonessential and poisonous metals, such as lead (Pb), mercury (Hg), cadmium (Cd), and Al [35–37]. Typically, chelators bind to metal ions enhancing their urinary and fecal excretion and causing a progressive decrease of their body concentrations. Chelation therapy became a popular alternative treatment, in spite of its still controversial clinical results [38,39], when ethylenediaminetetraacetic acid (EDTA) turned out to be effective in chelating and removing toxic metals from blood. Following the introduction of EDTA in clinical practice, other suggestions were made that a treatment based on a metal chelator might benefit patients with atherosclerosis as the hardened arteries could be “softened” due to removal of Ca from artery walls [40]. This was the earliest recorded report on the use of chelation therapy and since then metal ion chelators have been suggested as potential therapies for diseases involving metal ion imbalance as well as metal poisoning. However, regarding the effectiveness of chelation therapy, it is important to note that positive results obtained from some laboratories are by no means unanimous and contradictory evidence counterbalance these claims [41–44]. Thus, EDTA therapy in cardiology has been considered by the Food and Drug Administration (FDA) as a highly controversial and questionable issue. In contrast, the use of chelating agents to treat acute metal poisoning is now well established.
Effective chelation treatments of metal poisoning require a precise understanding of the pharmacodynamic and pharmacokinetic of the administered chelator which, in turn, depends on the physical and chemical characteristics of metals and chelators such as ionic radius, solvation sphere size and deformability, hardness/softness of electron donors and acceptors, chemical stability, administration route, bioavailability, metabolism, organ and intra/extra cellular specific compartmentalization, and, of course, natural excretion [36].
Hydrophilic chelators enhance renal excretion, but their mainly extracellular localization limits activity only to extracellular metal pools. Conversely, lipophilic chelators might decrease intracellular stores, but may also redistribute toxic metals to more vulnerable organs, e.g., the brain. The metal selectivity of chelators is very important, due to the risk of essential metal depletion. Moreover, in chronic metal induced disease, necessitating long-life chelation, toxicity and side effects of the chelator may drastically limit the time of treatment. Hence, development of new and safer chelators suited to long-term oral administration to remove metal deposits still remains an important research challenge. In addition, a significant teratogenic potential has been demonstrated for most chelators due to induced trace element deficiencies [45], and hence mineral supplementation during treatment is recommended. Improved chelator design should aim at enhancing selectivity, affinity, stability, renal clearance and oral activity, while maintaining a low toxicity and also a low cost. Finally, it must be remembered that adaptation of chelation therapy to neurodegenerative conditions is a very complex task. Upon critical consideration of the studies on CQ, metal redistribution rather than metal removal seems to be the most important therapeutic goal for the treatment of these pathologies. From this perspective, it may be preferred to denote these kinds of therapeutic approaches as metal targeted strategies for neurodegenerative diseases.
Chelators for Alzheimer's Disease: CQ and Related Compounds
Researchers have postulated that metal chelation might promote beneficial results in AD patients by inhibiting Al [46] and/or Cu, Zn deposition in the brain and/or preventing Fe from catalyzing the formation of toxic hydroxyl radicals [47–49]. Few case studies and animal experimentation have been reported in this area [50–52]; however, no clinical evidence has been provided so far to support the use of chelating agents as an adjunctive treatment for AD or other neurodegenerative disorders with similar etiology. Accordingly, neurodegeneration represents an excellent target for exploiting the metal chelator approach to therapeutics. However, in the light of recent experience deriving from the several studies on CQ, a United States Pharmacopoeia (USP) antibiotic, (5-chloro-7-iodo-8-hydroxyquinoline, MW 305.5) [13,24,53–56], a very different point of view has emerged as detailed below. In contrast to the direct chelation approach developed for metal overload disorders and aimed at removing excess metals, the main goal in AD seems to be a better and more suitable modulation of metal ion homeostasis and of metal-Aβ interactions, aimed at restoring broken ionic balance. Known chelators that have been clinically tested include desferrioxamine (DFO) [46]; rasagiline, an Fe chelator approved by the FDA in 2005; and CQ [50–52], an antibiotic banned for internal use in the USA since 1971 that appeared to block the genetic action of Huntington's disease in mice and in cell culture [57]. DFO is a chelator of tripositive metals still used against Al overloading in chronic dialysis treatment and in the treatment of Fe overload conditions, but no longer being pursued clinically for AD. Conversely, CQ has completed a first Phase II clinical trial, however, with rather controversial results [25,52,58] and has been recently withdrawn from human experimentation. In any case the story of CQ remains emblematic and very instructive. After Cherny and colleagues [24] first reported that CQ treatment is beneficial in a mouse model of AD, many researchers have focused on its potential promise in AD. CQ selectively binds Cu and Zn with a far higher affinity than Ca and magnesium (Mg) [k1(Zn) = 7.0, k1(Cu) = 8.9, k1(Ca) = 4.9, and k1(Mg) = 5.0] [24,26]. CQ is hydrophobic and freely crosses the blood-brain barrier (BBB) [59]; hence it possesses the prototypic properties for a potential therapeutic agent that might solubilize Zn/Cu-assembled Aβ deposits in vivo and inhibit Aβ aggregation [60] and redox toxicity. The findings that CQ reverses Cu and Zn induced Aβ aggregates and solubilizes, postmortem, Aβ deposits in AD-affected brain tissue [24], supported by the observation that CQ complexes with Zn in the brain [61], argue in favor of this drug. After showing that CQ can reduce plaque load in transgenic mouse models of AD, Ritchie et al. further reported that CQ lowered plasma Aβ42 levels [52]. However, it is unclear whether this was a result of attenuated Aβ/metal ion interactions or of some other mechanism. Patients with mild disease had a lowering of plasma Aβ42 but no significant cognitive benefit, while those with severe disease appeared to have a cognitive benefit but no change in plasma Aβ42 [52]. They also noted that the study is subject to the usual caveats associated with small-scale trials. Recently, Treiber and coworkers [25] reported that oral treatment with CQ significantly elevated brain Cu and Zn uptake by 19% and 13% respectively. These results led to the conclusion that CQ does not induce a loss in over-accumulated metal ions (as it was originally believed) but acts in the opposite way with respect to the action of metal chelators. However, CQ is apparently able to reduce Aβ deposition in the brain [25] acting, at least potentially, as an anti-amyloidogenic molecule but not as a metal chelator. Notably, two recent studies have reported some apparently favorable effects for another hydroxyquinolne ligand (PBT2), structurally related to CQ, in a mouse model of AD and in a Phase IIa, double bind trial [62,63]. In the 12-weeks randomized trial PBT2 shows to possess a safe profile and the ability to significantly reduce cerebrospinal fluid (CSF) Aβ42 concentrations compared with patients on placebo [62]. Moreover, the unchanged plasma concentrations of Aβ42 and Aβ40 highlight the ability of PBT2 to selectively affect brain but not peripheral Aβ. Adlard and colleagues hypothesized that PBT2 can capture metal ions from oligomerized Aβ thus favoring peptide clearance [63]. In addition, PBT2 was found to decrease insoluble and soluble phosphorylated tau in APP/PS1 mice and insoluble total tau in Tg2576 mice [63].
Nevertheless, other investigations as well as larger clinical trials are necessary to draw firm conclusions about the clinical efficacy of PBT2 [64].
Challenges to be Overcome with Metal Chelation Therapy
Beyond being a metal ligand, CQ may also act as an anti-aggregating agent, but this aspect needs to be better explored. Recent reports describe successful treatment using Cu chelation therapy in neurodegenerative animal models. However, the success claimed for chelation therapy in neurodegenerative diseases is still rather controversial. Recently Zatta and collaborators utilized cuprizone, a very sensitive and selective Cu-chelating agent with well-known neurotoxic properties, as a relevant chemical model in mice [11]. Upon cuprizone treatment, mice developed a pronounced astrocytosis, with brain edema and spongiosis characterized by vacuolizations of the neuropil predominantly in the white matter. In addition, cuprizone treatment severely altered Cu and Zn homeostasis in the central nervous system (CNS) as well as in all other examined tissues, leading to a generalized increasing of metal ion concentrations, particularly in the CNS. Concomitant with this increase in the Cu and Zn brain concentration, metallothionein-I and -II were also highly immunoreactive in astrocyte, consistent with the astrocytosis and demyelination that were observed.
As stated above, there is a differential and sequential build up of metals during disease progression in AD [3]. In early or moderate AD patients, the divalent metals mainly comprising of Fe, Cu, and Zn are elevated (see Fig. 1). Closer examination of this study indicates that a potential divalent metal chelator may be useful only in patients within the early phase of AD and in patients with severe AD, thus a chelator specific to a trivalent metal ion like Al may be a more relevant option. Moreover, the use of divalent chelators like CQ in the severe AD case may further deplete the essential divalent metals that are already in lower amounts than required and thus worsen the AD pathology. Hence, we suggest that the progressive deposition of various metals in relation with the severity of AD needs to be more systematically established which would dictate the suitability of a particular chelator for the given case.
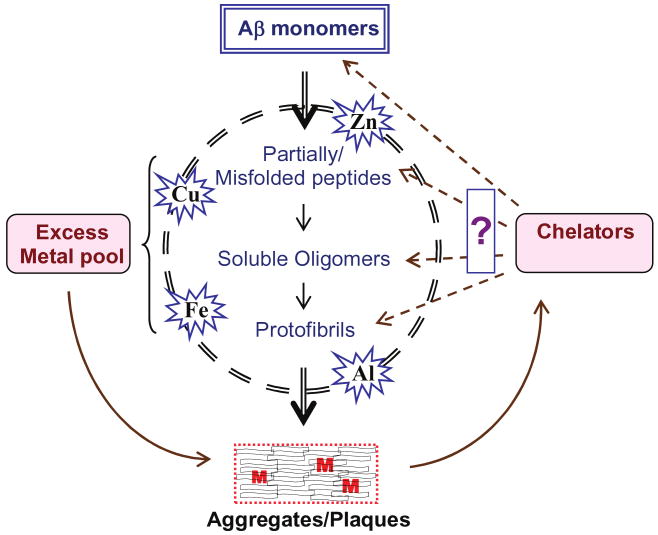
It is well known that Aβ has the ability to bind transition metal ions, which play an enhancing role in Aβ toxicity. Metals have also been shown to potentiate Aβ aggregation in vitro [16,65,66]. In the Aβ aggregation pathway, from monomers to plaques, several definite intermediates have been identified such as partially/misfolded peptides, soluble oligomers, and protofibrils (see Fig. 2). Presently, the biological significance of the monomeric, misfolded isoforms, soluble Aβ-oligomers and the fibrillar aggregates is not clear. For a variety of technical reasons, biophysical studies on the conformational diversity of different forms of Aβ and the conformational conversion between the normal cellular form and the pathological conformations have failed to provide us with a clear picture of these events in AD. However, recent studies advocate that the soluble oligomers and protofibrils of Aβ and not the fibrils are the active species that lead to neurodegeneration and dementia [67]. We have shown that the Aβ oligomers are more toxic than the aggregates in terms of their ability to damage DNA [68,69]. Studies carried out by Cherny and colleagues have shown that the metal chelator CQ can reduce Aβ plaques by solubilizing them [24]. But it is not known whether CQ can reverse the plaques to monomers or to any of the intermediate forms. If the aggregates are to be dissolved to soluble oligomers by chelators, then as for the current concept of toxicity of soluble forms of Aβ, the benefits of this need to be reviewed. The above questions create a renewed need to clarify how metals deposit with the progression of AD and which form of Aβ is generated by solubilizing the plaques with metal chelators.
Fig. 2.
Schematic representation of Aβ aggregation pathway. The Aβ monomers and the intermediate forms bind the excess metal ions (M) giving rise to higher-order structures. There is a need to understand which form of the peptide is generated by the solubilization of plaques by metal chelators. (Colours are visible in the electronic version of the article at www.iospress.nl.)
Recent Challenges in Nanomaterials
Nanomedicines are systems that exhibit similarity in size and structure to natural carriers like viruses and serum lipoproteins, and offer multi-faceted specific properties, useful in applications for delivery of imaging and therapeutic agents to CNS. Recently, Liu et al. reviewed the potential role of nanoparticles as chelation agents in relation to AD [70]. Nanomaterials synthesized from natural or artificial polymers, ranging in sizes of about or less than 300 nm have been shown to possess the ability to cross the BBB. Nanoparticles conjugated to metal chelators have shown a unique ability to cross the BBB, chelate metals, and exit through the BBB bound to the metal ion [70,71]. This method would be safe and effective in reducing metal burden in the brain. However, the long term toxicity of nanoparticles needs to be ascertained. It is assumed that specific biocompatible nanoparticles have low drug toxicity, efficient biodistribution, and therapeutic potential [72]. Nanoparticles mimic low density lipoproteins (LDL) and interact with the LDL receptor, thereby resulting in their uptake by brain endothelial cells [73]. The transferrin transcytosis mechanism may play a key role in nanoparticles drug delivery system. Scientists can bind metal chelators (Fe, Al, and Pb) covalently on nanoparticles as drug delivery vehicles to deliver the chelator to the brain. For example, hydrophilic hexadentate Fe chelators, with a large molecular weight, have been attached to nanoparticles and delivered to the brain [74]. Recent studies have reported the synthesis of a series of Fe chelators with functional side chains, which can be conjugated to nanoparticles and increase their efficiency in entering the brain [75–77]. Early studies from Liu's group showed that these nanochelators can effectively remove Fe from tissue sections of AD brain and also from ferritin (important Fe storage protein). Further, they also showed that these particles are more effective than DFO. Thus, nanotechnology is very helpful to deliver even hydrophilic compounds to the brain. Scientists can develop biopolymers as nanotools for conjugating metal chelators, because these nanoparticles are biodegradable. Liposomes, nanospheres, nanotubes, nanogels, polymeric micelles, and dendrimers pose some interesting and important challenges in their potential for clinical application [78]. Further work is needed for clinical validation and for assessing the safe and efficient use of these unique particles. In addition, to design useful means of chelation therapy based on specific metal levels in the moderate or severe phase of the disease, future studies should focus on the safer chelators targeting such as nanoparticle-linked chelators, which could also be useful for a wide range of other metal-toxicity disorders. Furthermore, it is important to study the surface chemistry of the chelator and nanoparticle complex, enhancing the desired characteristics by modulating linkages, coating materials, etc. These modifications will help to synthesize optimized nanometal chelators which can enter the brain and remove metals without being toxic. After all these basic data have been developed, then it is necessary to understand the clinical validation of these unique particles. In addition, to design useful means of chelation therapy based on specific metal levels in the moderate or severe phase of the disease, future studies should focus on the safer chelators such as nanoparticle-linked chelators, which could also be used for a wide range of other metal-related disorders.
Natural Compounds as Iron Chelators: Curcumin and Epigallocatechins
Beyond the case of CQ and of the few metal ligands described above, some other molecules were recently reported to show promising effects in AD, probably mediated by direct interactions with brain metals. We describe here the case of two natural compounds, curcumin and epigallocatechin, that were found to significantly affect brain Fe level and contrast AD progression.
Curcumin, the wonder molecule
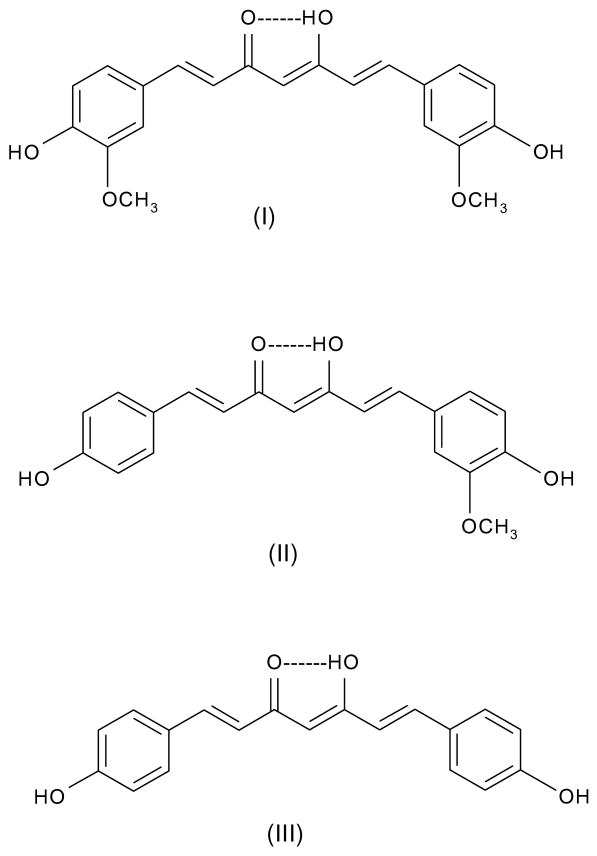
Curcumin is abundantly used in cuisine of several Asian countries. India, for example, is a major producer of a variety of spices and herbs which constitutes an excellent source for natural molecules, which not only can impart odor, color, and flavor to food, but also possesses several interesting preservative as well as medicinal properties. Curcumin (Fig. 3-I) is the major curcuminoid of the three found in tumeric, which also contains minor amounts of demethoxycurcumin (Fig. 3-II) and bisdemethoxycurcumin (Fig. 3-III).
Fig. 3.
Molecular structures of curcumin (I), demethoxycurcumin (II), and bisdemethoxycurcumin (III).
Studies on neuronal degeneration in the brains of patients with AD show that hippocampus is one of the primary regions affected during the early stages of the disease [79]. Neurotoxic heavy metals like Pb [80] and Cd [81] are known to disrupt structural features of the cells also in this region of the brain. Recent studies suggest that curcumin significantly reduces Pb- and Cd-induced neurotoxicity in rat hippocampal neurons [82, 83] and increased hippocampal neurogenesis in chronically stressed rats [84]. The ability of curcumin to bind toxic metals and to form tight and inactive complexes could be a plausible pathway by which curcumin offers protection to the brain. The anti-inflammatory property of curcumin could also contribute to the reduced amount of swelling observed within neuronal cells [85]. The use of phytochemical products as an alternative strategy for the reduction or amelioration in neurotoxic mechanisms has been gaining a lot of attention recently. Curcumin has the phenolic hydoxyls, the enolic proton, the keto moieties and the methylene group alpha to the two carbonyl groups as the potential reactive centers. Of these, the phenolic and the enolic hydroxyls have the ability to form metal complexes [86]. The ability of curcumin to bind effectively with redox-active metals like Cu and Fe and the enhanced radical scavenging efficacy of the curcumin-metal complexes, indicate a possible potent neuroprotective role for curcumin [87]. The interaction of curcumin with Cu and Fe reaches half-maximum at approximately 3–12 and 2.5–5 μM levels respectively [87]. In fact, the ability of curcumin to bind to several metals [82,86,88] and thereby potentially reduce their toxicity is well documented. Curcumin, in addition to providing protection to the brain by direct binding and complex formation with toxic metals, could also afford protection against oxidative damage and inflammation [89,90].
Polyphenols
Polyphenols are natural compounds that are largely present in plant products. Polyphenols, that are abundant in natural compounds such as green tea, curcumin flavanoids, and blueberry extracts, have been shown to reveal pronounced antioxidant, metal chelating, and anti-inflammatory properties [91]. These compounds have also been demonstrated to exert neuroprotective activities. Flavanoids are the largest group of polyphenols, which include anthocyanins and anthoxanthins, the latter further subdivided into flavones, isoflavones, flavanols, and flavans. Dried leaves of the plant Camellia sinesis are used to prepare the well-known beverage green tea. The ingredients of the green tea have been proposed for the treatment of various cardiovascular, inflammatory, and certain neurodegenerative diseases [92,93]. The major green tea component is the polyphenolic compound (-)-epigallocatechin-3-gallate (EGCG) [94–100]. The green tea polyphenols act as relatively potent metal chelators, binding to metal ions such as Fe and Cu. It has been proposed that AD pathogenesis is associated with the accumulation of Fe and hence tea polyphenols and curcumin may gain significance in the metal chelation treatment of AD. However, the efficacy of natural product chemistry for metal chelation properties may still be further explored.
Curcumin and polyphenols for AD: potential iron scavengers
Mandel and colleagues [96] in a recent review proposed that either curcumin or green tea EGCG might be conveniently employed for AD treatment. Indeed, both these substances have been reported to have easy access to the brain and to possess multifunctional activities such as metal chelation, radical scavenging, anti-inflammation, and neuroprotection. Interest in the use of these molecules is further motivated by the fact that they are relatively safe and are usually very abundant in the diet. It was specifically hypothesized that these two substances might act primarily as Fe chelators, again implying an important role for Fe in the etiopathogenesis and progression of AD. Remarkably clinical studies are currently underway to define more precisely whether the use of natural, non-toxic, neuroprotective compounds with brain access could offer potential therapeutic benefits in order to reduce Fe burden in those brain areas where it preferentially accumulates and causes severe neuronal damage.
Conclusions and Perspectives
We have critically reviewed here a number of chelation therapy approaches applied to AD during the last two decades. AD is most likely a multifactorial disease with a complex and still largely unknown origin. However, as it is increasingly evident that there are close links between brain metal dishomeostasis and AD onset and/or progression, a therapeutic strategy aimed at targeting brain metals is theoretically well grounded and justified. As it is also evident that brain metal alterations in AD patients predominantly consist of both a perturbed distribution and an anomalous metal protein interactions rather than of a large metal overload, metal targeting approaches for AD should not be aimed at removing metals but rather at favoring their redistribution and disrupting pathologically relevant metal-protein interaction. Thus, metal targeted therapies based on intermediate strength ligands are to be preferred to aggressive chelation strategies. From this perspective, the case of CQ has contributed significantly to our basic knowledge. Despite several controversies, it seems that CQ and some related hydroxyquinoline ligands are of some significant benefit to AD patients as they may slow down cognitive decline. Further work is thus needed in this direction and other molecules behave as good drug candidates. We refer in particular to the case of two natural compounds, curcumin and EGCG, that are known to be appreciable Fe ligands and were reported to favorably affect the AD neurodegenerative processes. Given that they are safe, they can be straightforwardly proposed for combination therapies. Other strategies have been described based on the use of functionalized nanoparticles. Based on the above arguments, it is evident that metal targeted strategies, either alone or in combination with other drugs, may play a significant role in the medical treatment of AD as they have the potential to attenuate metal mediated effects. A synergism with antioxidant substances capable of counteracting metal induced oxidative stress could be expected.
Acknowledgments
The authors wish to thank the CFTRI for his encouragement. MLH and SA thank Council for Scientific and Industrial Research, India for Senior Research Fellowships. KSJ is thankful to DAE for funding a project through BRNS, India. This project has been also supported by the Italian Minister of Research and University (FIRB # RBNEOPX83). KS is thankful for NIH AG023055 grant.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Lovell MA, Robertson JD, Teesadale WJ, Campbell JL, Markesberry WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 3.Rao KSJ, Rao RV, Shanmugavelu P, Menon RB. Trace elements in Alzheimer's disease brain: A new hypothesis. Alz Rep. 1999;2:241–246. [Google Scholar]
- 4.Campbell A, Smith MA, Sayre LM, Bondy SC, Perry G. Mechanisms by which metals promote events connected to neurodegenerative diseases. Brain Res Bull. 2001;55:125–132. doi: 10.1016/s0361-9230(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 5.Zatta P, Lucchini R, van Rensburg SJ, Taylor A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res Bull. 2003;62:15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 6.Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Reviews Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 7.Filiz G, Price KA, Caragounis A, Du T, Crouch PJ, White AR. The role of metals in modulating metalloprotease activity in the AD brain. Eur Biophys J. 2008;37:315–321. doi: 10.1007/s00249-007-0244-1. [DOI] [PubMed] [Google Scholar]
- 8.Zambenedetti P, Giordano R, Zatta P. Metallothionein is highly expressed in astrocytes and microcapillaries in Alzheimer's disease. J Chem Neuroanat. 1998;15:21–26. doi: 10.1016/s0891-0618(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 9.Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem. 2001;8:731–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 10.Zambenedetti P, Schmitt HP, Zatta P. Metallothionein I-II immunocytochemical reactivity in Binswanger's encephalopathy. J Alzheimers Dis. 2002;4:459–466. doi: 10.3233/jad-2002-4602. [DOI] [PubMed] [Google Scholar]
- 11.Zatta P, Raso M, Zambenedetti P, Wittkowski W, Messori L, Piccoli F, Mauri PL, Beltramini M. Copper dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell Mol Life Sci. 2005;62:1502–1513. doi: 10.1007/s00018-005-5073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 13.Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer's disease. J Am Geriatr Soc. 2003;51:1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Ashcroft AE, Korchazhkina OV, Exley C. Metal-mediated formation of fibrillar ABri amyloid. J Inorg Biochem. 2004;98:2006–2010. doi: 10.1016/j.jinorgbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 16.Ricchelli F, Drago D, Filippi B, Tognon G, Zatta P. Aluminum-triggered structural modifications and aggregation of β-amyloid. Cell Mol Life Sci. 2005;62:1724–1733. doi: 10.1007/s00018-005-5141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkitadze MD, Kowalska A. Molecular mechanisms initiating amyloid beta- fibril formation in Alzheimer's disease. Acta Biochim Pol. 2005;52:417–423. [PubMed] [Google Scholar]
- 18.Cuajungco MP, Frederickson CJ, Bush AI. Amyloidbeta metal interaction and metal chelation. Subcell Biochem. 2005;38:235–254. doi: 10.1007/0-387-23226-5_12. [DOI] [PubMed] [Google Scholar]
- 19.Zatta P, Kiss T, Suwalsky M, Berthon G. Aluminium(III) as a promoter of cellular oxidation. Coord Chem Rev. 2002;228:263–270. [Google Scholar]
- 20.Gupta VB, Anitha S, Hegde ML, Gurroto R, Zecca L, Ravid R, Stein R, Shankar KS, Shanmugavelu P, Rao KSJ. ‘Aluminium in Alzheimer's disease: Are we still at a Cross-Road’. Cell Mol Life Sci. 2005;62:143–158. doi: 10.1007/s00018-004-4317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perl DL, Brody AR. Alzheimer's disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science. 1980;208:297–299. doi: 10.1126/science.7367858. [DOI] [PubMed] [Google Scholar]
- 22.Yokel RA. The toxicology of aluminum in the brain: a review. Neurotoxicol. 2000;21:813–828. [PubMed] [Google Scholar]
- 23.Good PF, Perl DP, Bierer LM, Schmeidler J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992;31:286–292. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- 24.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Master CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 25.Treiber C, Simons A, Strauss M, Hafner M, Cappai R, Bayer TA, Multhaup G. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of Alzheimer's disease. J Biol Chem. 2004;279:51958–51964. doi: 10.1074/jbc.M407410200. [DOI] [PubMed] [Google Scholar]
- 26.Di Vaira M, Bazzicalupi C, Orioli PL, Messori L, Bruni B, Zatta P. Clioquinol, a drug for Alzheimer's disease specifically interfering with brain metal metabolism: structural characterization of its Zinc(II) and Copper(II) complexes. Inorg Chem. 2004;43:3795–3797. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- 27.Zatta P. Metal Ions and Neurodegenerative Disorders. World Scientific; Singapore, London: 2003. pp. 1–511. 2003. [Google Scholar]
- 28.Ehman WD, Markesbery WR, Alauddin, Hossain TI, Brubaker EH. Brain trace elements in Alzheimer's disease. Neurotoxicol. 1986;7:195–206. [PubMed] [Google Scholar]
- 29.Speziali M, Orvini E. Metals distribution and regionalization in the brain. In: Zatta P, editor. Metal Ions and Neurodegenerative Disorders. World Sci; Singapore: 2003. pp. 15–65. [Google Scholar]
- 30.Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P. Metals ions and neurodegeneration. Biometals. 2007;20:639–654. doi: 10.1007/s10534-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 31.Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease. Dev Neurosci. 2002;24:184–187. doi: 10.1159/000065696. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Atwood CS, Moir RD, Hartshorn MA, Vonsattel JP, Tanzi RE, Bush AI. Zinc-induced Alzheimer's Abeta 1-40 aggregation is mediated by conformational factors. J Biol Chem. 1997;272:26464–26470. doi: 10.1074/jbc.272.42.26464. [DOI] [PubMed] [Google Scholar]
- 33.Llanos RM, Mercer JF. The molecular basis of copper homeostasis copper-related disorders. DNA Cell Biol. 2002;21:259–270. doi: 10.1089/104454902753759681. [DOI] [PubMed] [Google Scholar]
- 34.Burn J, Chinnery PF. Neuroferritinopathy. Semin Pediatr Neurol. 2006;13:176–181. doi: 10.1016/j.spen.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Kalia K, Flora SJ. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health. 2005;47:1–21. doi: 10.1539/joh.47.1. [DOI] [PubMed] [Google Scholar]
- 36.Andersen O. Chemical and biological considerations in the treatment of metal intoxications by chelating agents. Mini Rev Med Chem. 2004;4:11–21. doi: 10.2174/1389557043487583. [DOI] [PubMed] [Google Scholar]
- 37.Yokel RA, Ackrill P, Burgess E, Day JP, Domingo JL, Flaten TP, Savory J. Prevention and treatment of aluminum toxicity including chelation therapy: status and research needs. J Toxicol Environ Health. 1996;48:667–683. [PubMed] [Google Scholar]
- 38.Carter JP. If chelation therapy is so good, why is it not more widely accepted? J Advancement Med. 1989;21:213–226. [Google Scholar]
- 39.Knudtson ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, Richardson D, Burkart C, Burgess E. Chelation therapy for ischemic heart disease: a randomized, controlled trial. JAMA. 2002;287:481–486. doi: 10.1001/jama.287.4.481. [DOI] [PubMed] [Google Scholar]
- 40.Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetra acetic acid. Am J Med Sci. 1956;232:654–656. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Guldager B, Jelnes E, Jorgensen SJ, Nielsen JS, Klaerke A, Mogensen K, Larsen KE, Reimer E, Holm J, Ottesen S. EDTA treatment of intermittent claudication : double blind, placebo- control study. J Intern Med. 1992;231:261–267. doi: 10.1111/j.1365-2796.1992.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 42.Ernst E. Chelation therapy for coronary heart disease: An overview of all clinical investigations. Am Heart J. 2002;140:139–141. doi: 10.1067/mhj.2000.107548. [DOI] [PubMed] [Google Scholar]
- 43.Pippard MJ, Wheatherall DJ. Oral chelation therapy for thalassemia: an uncertain scence. Br J Haematol. 2000;111:2–5. doi: 10.1046/j.1365-2141.2000.02406.x. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez DJ, Gomez M, Domingo JL, Lobet JM. Relative efficacy of chelating agents on excretion and tissue distribution of manganese in mice. J Appl Toxicol. 1995;15:285–288. doi: 10.1002/jat.2550150409. [DOI] [PubMed] [Google Scholar]
- 45.Domingo JL. Prevention by chelating agents of metal-induced developmental toxicity. Reprod Toxicol. 1995;9:105–113. doi: 10.1016/0890-6238(94)00060-3. [DOI] [PubMed] [Google Scholar]
- 46.McLachlan DR, Smith WL, Kruck TP. Desferrioxamine and Alzheimer's disease: video home behavior assessment of clinical course and measures of brain aluminum. Ther Drug Monit. 1993;15:602–607. [PubMed] [Google Scholar]
- 47.Smith MA, Nunomura A, Zhu X, Takeda A, Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal. 2000;2:413–420. doi: 10.1089/15230860050192198. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg RN. Metal chelation therapy for Alzheimer's disease. Arch Neurol. 2003;60:1678–1679. doi: 10.1001/archneur.60.12.1678. [DOI] [PubMed] [Google Scholar]
- 49.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer's disease. J Alzheimers Dis. 2004;6:291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 50.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 51.Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjogren M, Wallin A, Xilinas M, Gottfries CG. Treatment of Alzheimer's disease with clioquinol. Dement Geriatr Cogn Disord. 2001;12:408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- 52.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorohydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer's disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 53.Atamna H, Frey HL. A role for heme in Alzheimer's disease: Heme binds amyloid β and has altered metabolism. Proc Natl Acad Sci USA. 2004;101:11153–11158. doi: 10.1073/pnas.0404349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibach B, Haen E, Marienhagen J, Hajak G. Clioquinol treatment in familiar early onset of Alzheimer's disease: a case report. Pharmacopsychiatry. 2005;38:178–179. doi: 10.1055/s-2005-871241. [DOI] [PubMed] [Google Scholar]
- 55.Gouras GK, Beal MF. Metal Chelator decreases Alzheimer β-amyloid plaques. Neuron. 2001;30:641–647. doi: 10.1016/s0896-6273(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 56.Melov S. ‘… .and C is for Clioquinol’ – the AbetaCs of Alzheimer's disease. Trends Neurosci. 2002;25:121–123. doi: 10.1016/s0166-2236(00)02086-5. [DOI] [PubMed] [Google Scholar]
- 57.Bush AI. Metal complexing agents as therapies for Alzheimer's disease. Neurobiol Aging. 2002;23:1031–1038. doi: 10.1016/s0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 58.Richardson DR. Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann N Y Acad Sci. 2004;1012:326–341. doi: 10.1196/annals.1306.026. [DOI] [PubMed] [Google Scholar]
- 59.Padmanabhan G, Becue I, Smith JA. Clioquinol. In: Klauss E, Florey E, editors. analytical profiles of drug substances. Academic Press; New York: 1989. pp. 57–90. [Google Scholar]
- 60.Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, Goto Y, Rao ChM. AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an aamyloid beta-peptide and beta2-microglobulin. Biochem J. 2005;392:573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiraki H. Handbook of clinical Neurology. Vol. 37. Elsevier/North-Holland Biomedical Press; Amsterdam: 1979. [Google Scholar]
- 62.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. PBT2-201-EURO study group. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 63.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Relkin NR. Testing the mettle of PBT2 for Alzheimer's disease. Lancet Neurol. 2008;7:762–763. doi: 10.1016/S1474-4422(08)70168-6. [DOI] [PubMed] [Google Scholar]
- 65.Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel JP, Gusella JF, Beyreuthu K, Masters CL, Tanzi RE. rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 66.Atwood CS, Moir RD, Huang X, Bacarra NME, Scarpa RC, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 67.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of Aβ amyloid protofibrils: An in vitro model for a possible early event in Alzheimer's disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 68.Hegde ML, Anitha S, Rao KSJ. Are monomeroligomer-aggregates of amyloidogenic peptides toxic species in neurodegeneration? A new experimental evidence. Neurobiol Aging (Abstract) 2004;25:170. S2. [Google Scholar]
- 69.Hegde ML, Anitha S, Latha KS, Mustak MS, Stein R, Ravid R, Rao KSJ. First evidence for helical transitions in supercoiled DNA by Amyloid β peptide (1-42) and Aluminium: A new insight in understanding Alzheimer's disease. J Mol Neurosci. 2004;22:19–31. doi: 10.1385/jmn:22:1-2:19. [DOI] [PubMed] [Google Scholar]
- 70.Liu G, Garrett MR, Men P, Zhu X, Perry G, Smith MA. Nanoparticle and other metal chelation therapeutics in Alzheimer's disease. Biochem Biophys Acta. 2005;1741:246–252. doi: 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Cui Z, Lockman PR, Atwood CS, Hsu CH, Gupte A, Allen DD, Mumper RJ. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer's and other CNS diseases. Eur J Pharm Biopharm. 2005;59:263–272. doi: 10.1016/j.ejpb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 73.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 74.Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood – brain barrier. J Pharm Sci. 1998;87:1305–1307. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 75.Liu G, Bruenger FW, Miller SC, Arif AM. Molecular structure and biological and pharmacological properties of 3-hydroxy-2-methyl-1-(beta-d-ribofuranosyl or pyranosyl)-4-pyridinone: potential iron overload drugs for oral administration. Bioorg Med Chem Lett. 1998;8:3077–3080. doi: 10.1016/S0960-894X(98)00569-1. [DOI] [PubMed] [Google Scholar]
- 76.Liu G, Men P, Kenner GH, Miller SC, Bruenger FW. Acyclonucleoside iron chelators of 1-(2-hydroxye-thoxy) methyl-2-alkyl-3-hydroxy-4-pyridinones: potential oral iron chelation therapeutics. Nucleos Nucleot Nucleic Acids. 2004;23:599–611. doi: 10.1081/NCN-120030718. [DOI] [PubMed] [Google Scholar]
- 77.Liu G, Miller SC, Bruenger FW. Synthesis of lipophilic 3-hydroxy-2-methyl-4-pyridinone derivatives. Synth Commun. 1995;25:3247–3253. [Google Scholar]
- 78.Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci. 2007;32:1054–1082. doi: 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seabrook GR, Rosahl TW. Transgenic animals relevant to Alzheimer's disease. Neuropharmacol. 1999;38:1–17. doi: 10.1016/s0028-3908(98)00170-1. [DOI] [PubMed] [Google Scholar]
- 80.Monterio HP, Bechara EJ, Abdalla DS. Free radicals involvement in neurological porphyrias and lead poisoning. Mol Cell Biochem. 1991;103:73–83. doi: 10.1007/BF00229595. [DOI] [PubMed] [Google Scholar]
- 81.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 82.Daniel S, Limson JL, Dairam A, Watkins GM, Daya S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem. 2004;98:266–275. doi: 10.1016/j.jinorgbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Dairam A, Limson JL, Watkins GM, Antunes E, Daya S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem. 2007;55:1039–1044. doi: 10.1021/jf063446t. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 85.Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447–452. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 86.Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S, Zhang HY, Priyadarsini KI. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic Biol Med. 2005;39:811–822. doi: 10.1016/j.freeradbiomed.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimers Dis. 2004;6:367–377. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- 88.Barreto R, Kawakita S, Tsuchiya J, Minelli E, Pavasuthipaisit K, Helmy A, Marotta F. Metal-induced oxidative damage in cultured hepatocytes and hepatic lysosomal fraction: beneficial effect of a curcumin/absinthium compound. Chin J Dig Dis. 2005;6:31–36. doi: 10.1111/j.1443-9573.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 89.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiao Y, Wilkinson J, 4th, Pietsch CE, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81:313S–316S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 92.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandel S, Weinreb O, Amit T, Youdim MBH. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88:1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 94.Weinreb O, Amit T, Youdim MB. The application of proteomics for studying the neurorescue activity of the polyphenol (-)-epigallocatechin-3-gallate. Arch Biochem Biophys. 2008;476:152–160. doi: 10.1016/j.abb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Avramovich-Tirosh Y, Reznichenko L, Mit T, Zheng H, Fridkin M, Weinreb O, Mandel S, Youdim MB. Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multi-functional brain permeable iron-chelating- antioxidants, M-30 and green tea polyphenol, EGCG. Curr Alzheimer Res. 2007;4:403–411. doi: 10.2174/156720507781788927. [DOI] [PubMed] [Google Scholar]
- 96.Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer's disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol. 2007;82:348–360. doi: 10.1016/j.pneurobio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007;43:546–556. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Kalfon L, Youdim MB, Mandel SA. Green tea polyphenol (-)-epigallocatechin-3-gallate promotes the rapid protein kinase C- and proteasome-mediated degradation of Bad: implications for neuroprotection. J Neurochem. 2007;100:992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- 99.Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MB, Weinreb O, Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. J Neurochem. 2006;97:527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 100.Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MB. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res. 2006;50:229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]