Abstract
Length change mutation at the Ms6hm hypervariable mouse minisatellite locus was analyzed in C57BL/6N × C3H/HeN F1 mice and the F1 of the reciprocal cross born to irradiated male parents. Spontaneous mutant frequencies were 8.4% and 9.8% for the paternally derived and maternally derived C3H/HeN alleles, respectively. The mutant frequencies for the paternally derived allele increased to 22% and 19% when the male parents were irradiated with 6 Gy at the postmeiotic spermatozoa stage and the spermatogonia stage, respectively. These increases in the mutant frequency were at least 10 to 100 times higher than those expected from the frequency of hits to the 3- to 4-kb allele, suggesting that the length change mutation at this minisatellite locus was not a targeted event due directly to DNA damage in the region. Further analysis demonstrated that the mutant frequency increased also at the maternally derived C3H/HeN allele to 20% when the male parents were irradiated at the spermatozoa stage. This increase in the maternal allele mutation was not observed in F1 born to irradiated spermatogonia. The present study suggests that introduction of DNA damage by irradiated sperm triggers genomic instability in zygotes and in embryos of subsequent developmental stages, and this genomic instability induces untargeted mutation in cis at the paternally derived minisatellite allele and in trans at the maternally derived unirradiated allele. Untargeted mutation revealed in the present study defines a previously unnoticed genetic hazard to the maternally derived genome by the paternally introduced DNA damage.
Minisatellite sequences consist of medium-size repeat unit of 10 to 60 bp and are distributed widely in a variety of mammalian species (1). Some of the minisatellites in human and mouse are highly unstable and undergo frequent length change mutation in germ cells, sometimes as high as 10−1 per gamete (2, 3). Germline mutation of mouse minisatellite sequences was reported to be sensitive to irradiation of premeiotic stage germ cells (4). In addition, a frequent germline mutation was found in minisatellite sequences of children born in the heavily contaminated area of the Chernobyl nuclear accident (5).
We have shown previously that the length change mutation was induced at the mouse hypervariable minisatellite locus, Ms6hm, by irradiation of the postmeiotic spermatid stage germ cells (6). The dose-response of the induction for spermatid stage irradiation followed a linear increase up to 2 Gy and plateaued thereafter (7). Although less efficient, spermatozoa and spermatogonia stages were sensitive to radiation induction of minisatellite mutation as well. A high sensitivity of spermatid stage germ cells for the induction of minisatellite mutation was also noted when fission neutron was applied (8). Other reports have stated that only the premeiotic stage germ cells were sensitive to radiation with a doubling dose of 0.33 Gy, but not the postmeiotic stage germ cells (9, 10). Contradiction between their results and ours has to be resolved by more detailed analyses.
Our work and those by others however agree on one point in which the induced frequencies of mutation at mouse minisatellites are at least 10 to 100 times higher than those expected from the frequency of damage registered within the 3- to 4-kb region of the loci. This result raises a possibility that radiation induction of minisatellite mutation is not a targeted event, but is likely an untargeted event associated with radiation-induced genomic instability. We have previously noticed a slight elevation of the mutant frequency of the maternally derived allele of the Ms6hm locus, in addition to the paternally derived one, when male parents were irradiated at the spermatozoa stage with gamma-rays (6, 7). It is reasonable to expect an increase in the maternal allele mutation if DNA damage of irradiated sperm triggers genomic instability in zygotes, which then mutates the paternally derived allele in cis and the maternally derived allele in trans. In the present study, we have tested this possibility by analyzing the mutant frequency of the Ms6hm locus in F1 mice born to irradiated males. A statistically significant increase of the maternally derived minisatellite allele was observed in F1 born to male parents irradiated at the spermatozoa stage, proving that the expectation was indeed correct.
Materials and Methods
Selection of C3H/HeN Mice.
C57BL/6N and C3H/HeN mice were purchased from Charles River Breeding Laboratories Japan. They were housed in the animal facility and fed with commercial pellets and tap water ad libitum, under the condition of constant temperature of 23°C and a regular 12-h light/dark cycle. In this study, we analyzed the length change mutation of the C3H/HeN allele of the Ms6hm minisatellite locus. Because of the high frequency of germline mutation at the locus, a large fraction of C3H/HeN mice purchased from the supplier were heterozygous at the locus. To avoid the complication in the study of F1 mice, we first screened C3H/HeN parents by Southern analysis and used only those homozygous at the Ms6hm locus.
Irradiation and Mating of Mice.
Partial body irradiation to the testicular portion was performed on male mice of 8 to 10 weeks old. A total of 6 Gy of γ-rays was delivered at a dose rate of 0.5 Gy/min by using a 60Co source at the Research Institute for Radiation Biology and Medicine, Hiroshima University. Males were mated with females for 1 week immediately after the exposure to assess the effect of spermatozoa irradiation. For spermatogonial irradiation, matings were done 15 to 20 weeks after the irradiation.
Southern Analysis of DNA and the Minisatellite Probe.
DNA was extracted from tails of F1 mice as described previously (6). Four micrograms of DNA was digested with HaeIII and electrophoresed through 1.2% agarose gel of 20 cm in length. DNA was then transferred to a nylon membrane (Gene Screen Plus; Biotechnology Systems, NEN), and the filters were hybridized to a 32P-labeled Pc-1 probe. Pc-1 was a locus-specific clone isolated from the mouse genome (3) and was identical to the Ms6hm locus isolated by others (11). After hybridization, the filters were washed with 0.01× SSC at 65°C for 30 min and exposed to x-ray film. This stringency of hybridization enabled detection of the locus-specific Ms6hm bands, which were around 8.3 kb for the C57BL/6N allele and 3.7 kb for the C3H/HeN allele. Migration distances of the C3H/HeN allele bands of the locus were measured for the parents and offspring, and the length change mutants were scored as described below.
Scoring of Mutation at the C3H/HeN Allele of the Ms6hm Locus.
The majority of mutation at the Ms6hm locus was of length change type, and the extent of the length changes varied which made the scoring of the mutant somewhat arbitrary. In addition, reproducibility of the experimental results became questionable when the length changes were minute. Therefore, criteria of mutation have to be made to score the length change mutation at the locus. The present study used a similar criterion of mutation as that of previous studies (6–8). Briefly, the distance between the 8.3-kb band corresponding to the C57BL/6N allele and the 3.7-kb band to the C3H/HeN allele was measured, or for most of samples, calculated from the migration of marker DNA. F1 mice were scored as mutant when the difference in the mobility from the parental C3H/HeN bands was more than 2% of the distance between 8.3 kb and 3.7 kb. This 2% shift in the mobility corresponded to an approximately 60-bp change in the length of the C3H/HeN allele and can be detected reproducibly. In addition to the 60-bp criterion, more stringent criteria of the 75-bp criterion (2.5% shift) and the 90-bp criterion (3% shift) were applied in some analyses (Table 2). All of the Southern analyses in the present experiments were repeated at least twice, and three times for some. The χ2 test was used to analyze statistical significance of the mutant frequency data.
Table 2.
Mutant frequency for spermatozoa irradiation under stringent conditions
| C3H allele | Dose, Gy | Stage | No. of mutants/No. of
F1 (%)
|
|||
|---|---|---|---|---|---|---|
| 75-bp criterion | P value | 90-bp criterion | P value | |||
| Paternally derived | 0 | 11/226 (4.9) | 5/226 (2.2) | |||
| 6 | Spermatozoa | 9/63 (14) | 0.009 | 8/63 (13) | 0.0003 | |
| Maternally derived | 0 | 11/203 (5.4) | 5/203 (2.5) | |||
| 6 | Spermatozoa | 15/117 (13) | 0.0019 | 10/117 (8.5) | 0.0013 | |
Results
Mutation Analysis at the Paternally Derived C3H/HeN Allele.
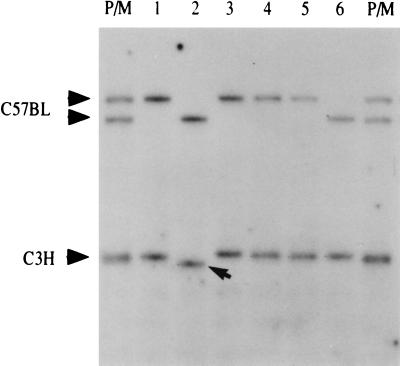
Unirradiated C3H/HeN male mice were mated with C57BL/6N females. A total of 226 mice were born to 23 C3H/HeN males mated with 35 C57BL/6N females. An example of Southern analysis of a litter of this control mating is shown in Fig. 1. The female parent to this particular litter was heterozygous at the Ms6hm locus and the alleles segregated among F1 mice. The mobility of the paternally derived C3H/HeN allele of the second F1 mouse in the litter was clearly faster than that of the father. The length of the C3H/HeN allele of the second F1 mice in Fig. 1 was found to be shorter by 93 bp than that of the male parent.
Figure 1.
A length change mutant at the paternally derived Ms6hm allele in F1 mice born to unirradiated males. An unirradiated C3H/HeN male was mated with a C57BL/6N female, and F1 mice were analyzed. Southern blotting of a litter of F1 mice is shown. P/M is the mixture of DNA from both male and female parents. A length change mutation of the paternal C3H/HeN allele of the second mouse is indicated by an arrow.
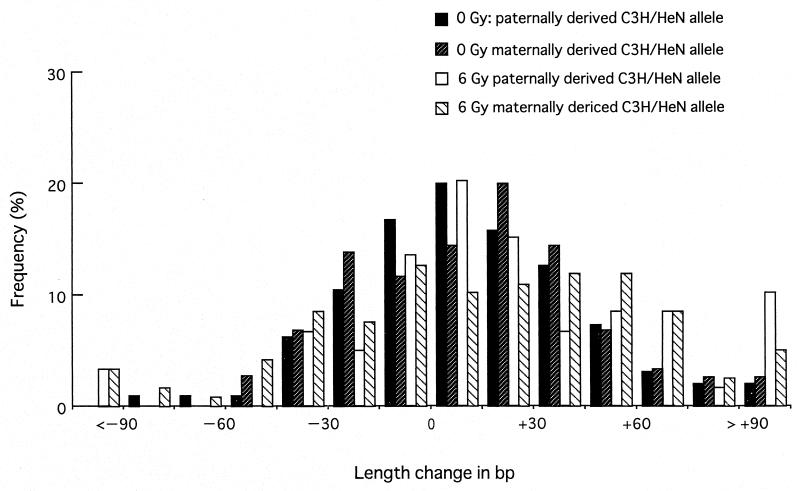
The distribution of the length change in bp is shown in Fig. 2. The pattern showed a normal distribution with a slight bias toward the gain-in-length mutation. The continuous nature of the length change mutation indicates the need for an appropriate criterion to score the mutation, especially for the alleles with small changes. The 60-bp criterion identified 19 mice as mutants, and the mutant frequency was therefore 8.4% for the C3H/HeN allele when derived from unirradiated male parents (Table 1). This value is similar to that of our previous study (7).
Figure 2.
Distribution of the length change of the C3H/HeN allele of the Ms6hm locus in F1 mice. Distribution of the length change in bp is shown for four groups of F1 mice: F1 mice from matings of control C3H/HeN males with C57BL/6N females (filled bar), control C57BL/6N males with C3H/HeN females (forward slash bar), 6-Gy irradiated C3H/HeN male with C57BL/6N females (open bar), and 6-Gy irradiated C57BL/6N males with C3H/HeN females (back slash bar). In the abscissa, <−90 and >+90 include all of the samples, with the decrease and the increase respectively in length of more than 90 bp.
Table 1.
Mutant frequency at the C3H/HeN allele of the Ms6hm locus in F1 mice
| Male
|
Female
|
F1 No. | C3H allele mutant*
|
|||||
|---|---|---|---|---|---|---|---|---|
| Strain | No. | Dose, Gy | Stage | Strain | No. | No. (%) | P value | |
| C3H | 23 | 0 | C57BL | 35 | 226 | 19 (8.4) | ||
| 10 | 6 | Spermatozoa | 14 | 63 | 14 (22) | 0.0022 | ||
| 7 | 6 | Spermatogonia | 10 | 69 | 13 (19) | 0.014 | ||
| C57BL | 27 | 0 | C3H | 28 | 203 | 20 (9.8) | ||
| 21 | 6 | Spermatozoa | 28 | 117 | 23 (20) | 0.013 | ||
| 6 | 6 | Spermatogonia | 11 | 61 | 5 (8.2) | 0.698 | ||
Mutants of this table were scored by the 60-bp criterion.
Male C3H/HeN mice were then irradiated at the testicular portion of the body with 6 Gy of 60Co γ-rays and immediately mated with C57BL/6N females to assess the effect of irradiation to spermatozoa. A total of 63 F1 mice were born to 10 males mated with 14 females. Analyses with the 60-bp criterion identified 14 mutant mice at the paternally inherited C3H/HeN allele. Therefore, the mutant frequency was 22% for the C3H/HeN allele derived from male parents irradiated at the spermatozoa stage (Table 1). This mutant frequency was significantly higher than that of the unirradiated group (P = 0.002 by χ2 test).
Irradiated males were kept for 15 weeks and then mated with C57BL/6N females to assess the effect of spermatogonia irradiation. A total of 69 F1 mice were obtained from 7 males mated with 10 females. Thirteen F1 mice were found to carry the C3H/HeN allele differing in length more than 60 bp, giving 19% mutant frequency for the spermatogonial irradiation (Table 1). This increase was statistically significant (P = 0.014 by χ2 test).
Mutation at the Maternally Derived C3H/HeN Allele.
Analyses of the maternally derived C3H/HeN allele were made on 203 F1 mice born from 28 C3H/HeN females mated with 27 unirradiated C57BL/6N males. The distribution of the length change of the maternal C3H/HeN allele in this group is as shown in Fig. 2. The pattern did not differ significantly from that of the paternally derived C3H/HeN allele of the control unirradiated group. The number of mutants identified with the 60-bp criterion was 20, giving the mutant frequency of 9.8%, which was similar to the value of the paternally derived C3H/HeN allele (Table 1). Therefore, the mutability of the C3H/HeN allele of the Ms6hm locus did not differ by the parental origin.
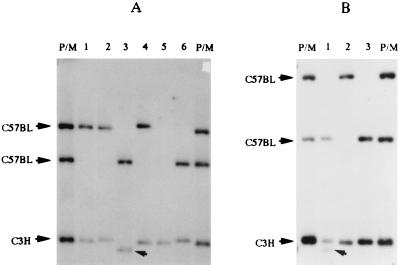
Male C57BL/6N mice were irradiated with 6 Gy to the testicular portion and mated immediately with C3H/HeN females. A total of 117 F1 mice were born to 21 such males mated with 28 C3H/HeN females. The distribution of the mobility shift of the maternally derived C3H/HeN allele is as shown in Fig. 2, and the pattern exhibited a wider distribution of the allele length, especially toward larger sizes. The 60-bp criterion identified 23 length change mutations, which gave the mutant frequency of 20% (Table 1). This increase in the mutant frequency for the maternally derived C3H/HeN allele was statistically significant (P = 0.013 by χ2 test). Some of the Southern analyses are shown in Fig. 3. The maternally derived C3H/HeN allele of the third F1 mouse of the litter lost approximately 220 bp (Fig. 3A). The male parent of Fig. 3A was heterozygous at the C57BL/6N allele. It is interesting to note that this paternal allele suffered a length change mutation in the 4th F1 mouse and deletion mutation in the 5th F1 mouse in the litter. Another example of length change mutation is shown in Fig. 3B, in which the first F1 mouse of the litter was mosaic at the maternally derived C3H/HeN allele, differing in length by 150 bp.
Figure 3.
Length change mutations at the maternally derived Ms6hm locus in F1 mice born to spermatozoa irradiated males. F1 mice born to immediate mating of 6-Gy irradiated C57BL/6N males to C3H/HeN females were analyzed. P/M is the mixture of DNA from both male and female parents. (A) Examples of length change mutations. Note the shift in the band mobility of the C3H/HeN allele in the third mouse. (B) An example of mosaic mutant. A litter of F1 mice is shown. Note an extra C3H/HeN band in the first mouse.
Male C57BL/6N mice irradiated at the testicular region of the body were kept for 15 weeks and mated with C3H/HeN female mice to test the effect of spermatogonial irradiation. Mating 6 such males with 11 females produced 61 F1 mice, of which 5 were identified as mutants (Table 1), giving the mutant frequency of 8.2%, which did not differ significantly from that of the control. Thus, parental irradiation at the spermatogonia stage did not affect the mutant frequency of the maternally derived unirradiated C3H/HeN allele in F1 mice.
Mutant Frequencies Under More Stringent Conditions.
The degree of length change to be scored as mutant allele determines the frequency of mutation of minisatellite analysis. Although one can obtain more mutants, the 30-bp criterion corresponding to the 1% mobility shift suffers a reliability problem. The 60-bp criterion used in the present study yielded reproducible results. We have applied more stringent criteria of the 75-bp and 90-bp difference for the analysis of F1 mice born to irradiated spermatozoa (Table 2). The mutant frequencies of the paternally derived C3H/HeN allele were 4.9% by the 75-bp criterion and 2.2% by the 90-bp criterion in F1 mice born to unirradiated males. The frequencies increased to statistically significant values of 14% by the 75-bp criterion (2.5% criterion, P = 0.009 by χ2 test) and 13% by the 90-bp criterion (3% criterion, P = 0.0003 by χ2 test) for F1 born to irradiated males. Similarly, application of the criteria for analysis of the maternally derived C3H/HeN allele yielded mutant frequencies of 5.4% by the 75-bp criterion and 2.5% by the 90-bp criterion for F1 born to unirradiated males. Mutant frequencies of the maternally derived allele were elevated to 13% by the 75-bp criterion (P = 0.0019 by c2 test) and 8.5% by the 90-bp criterion (P = 0.0013 by χ2 test) in F1 born to irradiated spermatozoa. The decreases in the P values under more stringent criteria were likely due to the presence of mutant alleles, with distinctively large differences in the length among F1 born to irradiated males.
Discussion
In our previous studies, mouse germ cells were found to be most sensitive to radiation induction of minisatellite mutation when irradiated at the spermatid stage, but the induction of minisatellite mutation by spermatozoa and spermatogonial irradiation was only suggestive (6, 7). The present study clearly demonstrated that, although less efficient, spermatozoa and spermatogonia stages are sensitive to radiation induction of length change mutation of the Ms6hm minisatellite locus. This pattern of sensitivity to mutation induction is quite similar to those found for the specific locus test, in which the sensitivity was highest for the spermatid stage, followed by the spermatogonia and the spermatozoa stages (12). Thus, our data demonstrate that irradiation of postmeiotic stage germ cells leads to minisatellite mutation in F1 mice. In addition, the present study demonstrates that spermatozoa irradiation leads to destabilization of unirradiated maternal allele of the Ms6hm minisatellite locus. In contrast to our findings, others have reported that the postmeiotic spermatid stage was refractory to radiation induction of minisatellite mutation .
At present, there is no explanation for the discrepancy between our results and the results in refs. 9 and 10, except for the difference in the criteria of minisatellite mutation and the mouse strains used. The presence of variant sequence motifs within the repeat enables exact analysis of length changes and the pattern of rearrangements for human minisatellites (13). However, the mouse Ms6hm locus is composed entirely of simple repeat of GGGCA (3), making the length change measurements only approximate. Therefore, we scored the mutants for the 3.7-kb C3H/HeN allele when F1 mice carried the minisatellite band differing in their length from the parent by more than 60 bp. A statistically significant increase in the mutant frequency was observed for the paternally derived allele of the Ms6hm locus. In addition, more stringent criteria of 75- and 90-bp differences also yielded statistically significant increases in the mutant frequencies for the paternal allele of the locus for irradiation at the postmeiotic spermatozoa stage as well as the spermatogonia stage. The criterion of the length change mutation in other studies has not been fully described, but may differ from ours (9, 10). The strain of mouse used in our study is the F1 between C3H/HeN and C57BL/6N, and that used by others is CBA/H. It has been reported that some strains of mice for example, BALB/c are highly susceptible to radiation induction of chromosome instability in somatic tissues (14, 15). This hypersensitivity was found to be associated with the decreased activity of DNA-dependent protein kinase (DNA-PK) (16). Detailed comparison may be necessary for radiation induction of minisatellite mutation between C3H/HeN × C57BL/6N F1 and CBA/H strains.
The induced frequencies for the recessive visible mutation in mice were in the order of 10−5/Gy/locus (17, 18). Molecular analyses of mutants revealed large deletions of the marker gene, characteristic to the direct involvement of radiation damage in mutation induction (12, 19). In the present study on the mouse hypervariable minisatellite locus, we have observed an increase of the mutant frequency by 10%, or 10−1, in F1 mice born to 6-Gy irradiated spermatozoa. The amount of DNA damage expected in the oocytes fertilized with 6-Gy irradiated haploid sperm is equivalent to that of 3-Gy irradiated oocytes. It has been estimated that around 30 double strand breaks are induced in diploid cells exposed to 1 Gy of X-rays (20). The number of all types of DNA damage is 100 times that of double strand breaks. A simple calculation can estimate the chance of the locus of around 3 kb carrying any of this damage to be in the order of 10−3 to 10−2 for the dose of 3 Gy. Therefore, one has to assume an indirect mechanism of induction of length change mutation at the Ms6hm locus in F1 mice born to irradiated males (7–10). Examples of indirectly induced untargeted mutation by radiation are found in several other systems. The increase in the frequencies of reversion at the mouse pink-eyed unstable allele was around 10%/Gy (21) and that at the Drosophila white-ivory allele was around 2%–3%/Gy (22, 23). These reversions are due to recombination of tandem duplications, and recombination/gene conversion events seem to be quite sensitive to radiation.
Radiation induction of genomic instability has been suggested by recent studies (24). Delayed chromosome mutation can occur in descendants of irradiated cells after many cell divisions in vitro and in vivo (25, 26). Delayed gene mutation was shown to be predominantly of spontaneous type with point mutation and small deletions, suggesting indirect involvement of radiation in the process (24). The present study demonstrated that minisatellite mutation is another outcome of radiation-induced genomic instability.
Human minisatellite sequences are well analyzed, and their germline mutations involve complex intraallelic rearrangements. In contrast, minisatellite mutation in human somatic cells is the result of simple intraallelic exchanges occurring rather infrequently (27). At present, the mechanism of minisatellite mutation in mice is not known, but is likely to involve a similar process of recombination/gene conversion event. Premeiotic exposure to radiation is supposed to increase recombination during meiosis, which leads to the increases in minisatellite mutation of the irradiated allele. In addition, our present study revealed that spermatozoa irradiation induced minisatellite mutation of the paternally derived allele in F1 mice. Chromatin of mature spermatozoa is tightly packed and biochemically inactive. Therefore, mutation by spermatozoa irradiation must have occurred after fertilization. In this respect, minisatellite mutation observed in our present study is likely due to somatic mutation rather than germline mutation. In the study of the mouse Ms6hm locus, Kelly et al. reported that somatic mutation at this locus is rather frequent, especially for the first few divisions of mouse development, and suggested that it is difficult to distinguish the somatic event from the germline event (11).
Double strand breaks are known to activate the process of homologous recombination (28). The activated recombination complex is known to operate in trans and induction of recombination in the unirradiated genome was reported in yeast after fusion with irradiated haploid cells (29). DNA replication in fertilized oocytes takes place separately in male and female pronuclei. It is reasonable to assume that DNA damage brought in by irradiated sperm induces genomic instability in the oocytes and triggers recombinogenic activity that operates in cis on the genome of the male pronucleus and in trans on that of the female pronucleus. The maternal allele mutation for spermatozoa irradiation is consistent with the hypothesis that DNA damage introduced by sperm induces recombinogenic activity in oocytes and subsequent early stages of mouse development. In our previous study, we observed an increase in the mutant frequency from 12% for the control to 15% for those born to 3-Gy irradiated spermatozoa (7). In the present study, the increase was from 9.8% to 20% when the dose to spermatozoa was increased to 6 Gy. Therefore, it is tempting to speculate that the increase in the recombinogenic activity is linearly related to the dose of radiation and the number of DNA damage.
DNA double strand breaks are well known to trigger cellular responses of G1/S arrest and G2/M arrest in somatic cells (30). Interestingly, oocytes fertilized by irradiated spermatozoa seem to lack these important damage responses (T. Shimura and O.N., unpublished observations). In addition, mouse embryonal carcinoma cells, a model of early stage embryonal stem cells, are shown to lack G1/S arrest (31, 32). Lack of cell cycle checkpoints may facilitate the induction of genomic instability during early stage mouse development by forcing the cells to divide in the presence of damage. The F1 mouse mosaic at the maternally derived allele suggests that the recombinogenic activity may be elevated at least for several cell divisions during early embryogenesis (Fig. 3B). Similar mosaic mutation at the maternal allele was also reported previously in F1 born to irradiated spermatids (6). The elevated level of recombination is likely to survive even further, and a recent report on transgenerational destabilization of minisatellite sequences demonstrates that the activity persists through development of germ cells in F1 mice (33).
Minisatellite mutation is not restricted in germ cells and in somatic cells of early developmental stages. X-ray-induced transformation of C3H/10T1/2 cells was found to be associated with minisatellite mutations (34). We have previously shown that mouse sarcomas carrying the amplified c-myc gene exhibit instability of the Ms6hm locus (35). The molecular mechanism of maintenance of minisatellite stability may be suppressed in mouse zygote by DNA damage of irradiated sperm. Recent studies (36–38) have shown that ATM and Mre11 are the key regulators of radio responses and recombination repair of mammalian cells. Homologous recombination and nonhomologous end-joining compete with each other for repair of double strand breaks (28). Fibroblast lines established from severe combined immunodeficient (SCID) mice were found to undergo extensive mutation of the Ms6hm locus (39). It is likely that the maternal allele mutation of the Ms6hm locus in F1 of irradiated spermatozoa is induced as part of DNA damage responses of mouse zygotes.
Transgenerational destabilization of genome was first reported for the induction of the lethal mutation in germ cells of the subsequent generations of mustard gas-treated Drosophila melanogaster (40). Transgenerational carcinogenesis and teratogenesis might also be the manifestation of the genomic instability in somatic cells of F1 mice born to irradiated parents (41, 42). These pioneering works have revealed a potential hazard of parental irradiation to the subsequent generations. Our present study and those by others (33) indicate that this destabilization of genome includes elevated levels of recombination, which induces length change mutation of mouse minisatellites. The trans-acting nature of the recombinogenic activity demonstrates that the risk of radiation is not restricted to the irradiated allele, but may extend to undamaged allele inherited from the unirradiated parent in the F1 generation. To our knowledge, no other studies have reported on the previously unnoticed hazard to the maternally derived genome by DNA damage of the paternal genome.
Acknowledgments
We thank Dr. S. Abrahamson, Dr. Christian Streffer, and the late Dr. J. Neel for critical reading of the manuscript. We also thank Ms. Aiko Kinomura for matings of mice and preparation of DNA samples. This work was conducted at the Research Institute for Radiation Biology and Medicine, Hiroshima University, where O.N. worked until March 1997. This work was supported by a grant-in-aid from the Ministry of Health and Welfare of Japan, and a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031439298.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031439298
References
- 1.Jeffreys A J. Clin Sci. 1997;93:383–390. doi: 10.1042/cs0930383. [DOI] [PubMed] [Google Scholar]
- 2.Jeffreys A J, Royle N J, Wilson V, Wong Z. Nature (London) 1988;332:278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- 3.Mitani K, Takahashi Y, Kominami R. J Biol Chem. 1990;265:15203–15210. [PubMed] [Google Scholar]
- 4.Dubrova Y E, Jeffreys A J, Malashenko A M. Nat Genet. 1993;5:92–94. doi: 10.1038/ng0993-92. [DOI] [PubMed] [Google Scholar]
- 5.Dubrova Y, Nesterov V N, Krochinsky N G, Ostapenko V A, Neumann R, Neil D L, Jeffreys A J. Nature (London) 1996;380:683–686. doi: 10.1038/380683a0. [DOI] [PubMed] [Google Scholar]
- 6.Sadamoto S, Suzuki S, Kamiya K, Kominami R, Dohi K, Niwa O. Int J Radiat Biol. 1994;65:549–557. doi: 10.1080/09553009414550641. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y-J, Wang Z, Sadamoto S, Ninomiya Y, Kotomura N, Kamiya K, Dohi K, Kominami R, Niwa O. Int J Radiat Biol. 1995;68:177–183. doi: 10.1080/09553009514551081. [DOI] [PubMed] [Google Scholar]
- 8.Niwa O, Fan Y, Numoto M, Kamiya K, Kominami R. J Radiat Res. 1996;37:217–224. doi: 10.1269/jrr.37.217. [DOI] [PubMed] [Google Scholar]
- 9.Dubrova Y E, Plumb M, Brown J, Fennelly J, Bois P, Goodhead D, Jeffreys A J. Proc Natl Acad Sci USA. 1998;95:6251–6255. doi: 10.1073/pnas.95.11.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubrova Y E, Plumb M, Brown J, Jeffreys A J. Int J Radiat Biol. 1998;74:689–696. doi: 10.1080/095530098140952. [DOI] [PubMed] [Google Scholar]
- 11.Kelly R, Bulfield G, Collick A, Gibbs M, Jeffreys A J. Genomics. 1989;5:844–856. doi: 10.1016/0888-7543(89)90126-2. [DOI] [PubMed] [Google Scholar]
- 12.Russell W L, Bangham J W, Russell L B. Genetics. 1998;148:1567–1578. doi: 10.1093/genetics/148.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamaki K, Huang X L, Mizutani M, Yamamoto T, Katsumata R, Uchihi R, Katsumata Y, Jeffreys A J. J Forensic Sci. 1999;44:863–867. [PubMed] [Google Scholar]
- 14.Ullrich R L, Ponnaiya B. Int J Radiat Biol. 1998;74:747–754. doi: 10.1080/095530098141023. [DOI] [PubMed] [Google Scholar]
- 15.Ullrich R L, Davis C M. Radiat Res. 1999;152:170–173. [PubMed] [Google Scholar]
- 16.Okayasu R, Suetomi K, Yu Y, Silver A, Bedford J S, Cox R, Ullrich R L. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- 17.Russel W L, Kelly E M. Proc Natl Acad Sci USA. 1982;79:542–544. doi: 10.1073/pnas.79.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neel J, Lewis S E. Annu Rev Genet. 1990;24:327–362. doi: 10.1146/annurev.ge.24.120190.001551. [DOI] [PubMed] [Google Scholar]
- 19.Rinchik E M, Stoye J P, Frankel W N, Coffin J, Kwon B S, Russell L B. Mutat Res. 1993;286:199–207. doi: 10.1016/0027-5107(93)90184-h. [DOI] [PubMed] [Google Scholar]
- 20.Lobrich M, Cooper P K, Rydberg B. Int J Radiat Biol. 1996;70:493–503. doi: 10.1080/095530096144680. [DOI] [PubMed] [Google Scholar]
- 21.Schiestl R H, Khogali F, Carls N. Science. 1994;266:1573–1576. doi: 10.1126/science.7985029. [DOI] [PubMed] [Google Scholar]
- 22.Green M M, Todo T, Ryo H, Fujikawa K. Proc Natl Acad Sci USA. 1986;83:6667–6671. doi: 10.1073/pnas.83.18.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshikawa I M, Hoshi M, Ikenaga M. Mutat Res. 1996;357:35–42. doi: 10.1016/0027-5107(96)00076-0. [DOI] [PubMed] [Google Scholar]
- 24.Little J B. Int J Radiat Biol. 1998;6:663–671. doi: 10.1080/095530098140925. [DOI] [PubMed] [Google Scholar]
- 25.Pampfer S, Streffer C. Int J Radiat Biol. 1989;55:85–92. doi: 10.1080/09553008914550091. [DOI] [PubMed] [Google Scholar]
- 26.Kadhim M A, MacDonald D A, Goodhead D T, Lorimore S A, Marsden S J, Wright E G. Nature (London) 1992;355:738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- 27.Jeffreys A J, Barber R, Bois P, Buard J, Dubrova Y E, Grant G, Hollies C R, May C A, Neumann R, Panayi M, Ritchie A E, Shone A C, Signer E, Stead J D, Tamaki K. Electrophoresis. 1999;20:1665–1675. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1665::AID-ELPS1665>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Harber J E. Trends Genet. 2000;16:259–264. doi: 10.1016/s0168-9525(00)02022-9. [DOI] [PubMed] [Google Scholar]
- 29.Fabre F, Roman H. Proc Natl Acad Sci USA. 1977;74:1667–1671. doi: 10.1073/pnas.74.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartwell L H, Kastan M B. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 31.Malashicheva A B, Kislyakova T V, Aksenov N D, Osipov K A, Pospelov V A. Oncogene. 2000;19:3858–3865. doi: 10.1038/sj.onc.1203736. [DOI] [PubMed] [Google Scholar]
- 32.Taga M, Shiraishi K, Shimura T, Uematsu N, Kato T, Nishimune Y, Aizawa S, Oshimura M, Niwa O. J Radiat Res. 2000;41:227–241. doi: 10.1269/jrr.41.227. [DOI] [PubMed] [Google Scholar]
- 33.Dubrova Y, Plumb M, Gutierrez B, Boulton E, Jeffreys A J. Nature (London) 2000;405:37. doi: 10.1038/35011135. [DOI] [PubMed] [Google Scholar]
- 34.Paquette B, Little J B. Cancer Res. 1992;52:5788–5793. [PubMed] [Google Scholar]
- 35.Niwa O, Kamiya K, Furihata C, Ninomiya Y, Kotomura N, Wang Z, Numoto M, Kominami R. Cancer Res. 1995;55:5670–5676. [PubMed] [Google Scholar]
- 36.Lim D S, Kim S T, Xu B, Maser R S, Lin J, Petrini J H, Kastan M B. Nature (London) 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- 37.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrini J H. Am J Hum Genet. 1999;64:1264–1269. doi: 10.1086/302391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai H, Nakagama H, Komatsu K, Shiraishi T, Fukuda H, Sugimura T, Nagao M. Proc Natl Acad Sci USA. 1997;94:10817–10820. doi: 10.1073/pnas.94.20.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auerbach C. Mutation Research. London: Chapman and Hall; 1976. pp. 270–276. [Google Scholar]
- 41.Nomura T. Nature (London) 1982;296:575–577. doi: 10.1038/296575a0. [DOI] [PubMed] [Google Scholar]
- 42.Pils S, Muller W U, Streffer C. Mutat Res. 1999;429:85–92. doi: 10.1016/s0027-5107(99)00101-3. [DOI] [PubMed] [Google Scholar]