Abstract
The ligand binding domains of the estrogen related receptors, ERRα and ERRγ were covalently immobilized onto the surface of an aminopropyl silica liquid chromatography stationary phase to create the ERRα-silica and ERRγ-silica columns and onto the surface of open tubular capillaries to create the ERRα-OT and ERRγ-OT columns. The ERR-silica and ERR-OT columns were characterized using frontal chromatographic techniques with diethylstibesterol and the binding affinities, Kd values, to the immobilized receptors were consistent with the values obtained by a radioligand binding assay. The ERRγ-silica column was also characterized using non-linear chromatographic techniques using a series of tamoxifen derivatives. The relative Kd values obtained for the derivatives were consistent with the relative ability of the compounds to inhibit the cellular proliferation of the human-derived T98G glioma cell line, expressed as IC50 values. The results indicate that the columns containing immobilized ERRα and ERRγ can be created and used to characterize the binding of compounds to the immobilized receptors and that the relative retention of compounds on these columns reflects the magnitude of their inhibitory activity.
Keywords: glioblastoma, astrocytomas, estrogen related receptor, screening, biochromatography
1. Introduction
In humans, most malignant brain tumors are gliomas and astrocytomas, which are extremely lethal with a median survival from diagnoses of 12–15 months [1]. Current clinical treatment of gliomas and astrocytomas are not effective and there are a number of active programs directed at the development of new drugs to treat these tumors. One potential class of agents for the treatment of glioblastomas and astrocytomas is selective estrogen receptor modulators (SERMs), such as tamoxifen, which has displayed antiglioma activity in both in vitro and in vivo models [4].
We have recently demonstrated that the anti-glioma effects of SERMs are due to their interactions with estrogen-related receptors ERRα and ERRγ [5]. These nuclear receptors are expressed alone or in combination in brain cancers and recent data indicate that the establishment of the ERRα and ERRγ expression in a tumor can be used to tailor the therapeutic program to the properties of that tumor. However, only a few effective ERRα and ERRγ antagonists have been indentified and these are primarily plant-derived flavanoids [6]. Thus, the objective of this project was the development of new methods to screen botanical extracts in order to identify new ERRα and ERRγ antagonists. We now report the development of immobilized ERRα and ERRγ columns for use in these screens.
We have previously demonstrated that columns which contain immobilized nuclear proteins, the estrogen receptor (ER) ligand binding domain [7] and the DNA unwinding element binding (DUE-B) protein [8], can be created, characterized and used to study ligand-protein interactions. In these studies, the ERRα and ERRγ ligand binding domains were covalently immobilized via the N-terminus onto the surface of an aminopropyl silica liquid chromatography stationary to create the ERRα-silica and ERRγ-silica columns, or on the activated surface of open tubular glass capillaries to create the ERRα-OT and ERRγ–OT columns. The results indicate that both formats can be used to study ligand-ERR interactions including the determination of binding affinities and binding sites. The results also demonstrate that the ERR-OT columns have shorter retention and wash times and, therefore, would be preferred for individual compounds characterizations, while the ERR-silica columns have significantly higher binding capacities and are the preferred format for online screening of botanical extracts.
2. EXPERIMENTAL SECTION
2.1 Materials
Tamoxifen and 4-hydroxy tamoxifen were purchased from Sigma, N-desmethyl 4-hydroxytamoxifen (Endoxifen) was purchased from Toronto research chemicals (Toronto, Canada) and diethylstilbestrol (DES) was purchased from Fisher Scientific. His-tag fusion proteins with a 4 amino acid lysine insert with the ligand binding domain of the ERR α and γ were purchased from GenScript (Piscataway, NJ). Tricorn 5/20 glass column was purchased from Amersham Bioscience (Piscataway, NJ). BSA, ammonium acetate, gluteraldehyde, glutaric acid, glycine, pyridine (99.8%), sodium azide, and Tris buffer were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). The water used in the study was prepared using a Milli-Q Water Purification System (Millipore Corporation, Bedford, MA). The aminopropyl silica (APS) stationary phase (12 µ 300 Å pores) was purchased from Regis Technologies (Morton Grove, Illinois).
2.2 Immobilization of ERR α or ERR γ via N-terminus
2.2.1. Immobilization of ERRα or ERRγ on Silica Stationary phase
The ERRs were immobilized based on a previously published protocol [9]. Briefly, 50 mg of APS was added to 10 ml of pyridine [10 mM, pH 6.0] in a 15 ml conical plastic tube and the mixture was rotated for 15 min, centrifuged at 1500 × g for 10 min, and the supernatant was discarded. The APS was suspended in 10 ml of 5% gluteraldehyde, rotated for 3 h and then centrifuged. The supernatant was discarded and the activated APS was washed three times with 10 ml portions of pyridine [10 mM, pH 6.0], a suspension of 50 µg of ERRα or γ protein in 300 µl of pyridine [10 mM, pH 6.0] was added and the mixture left for 24 h at 4°C. After the mixture had warmed to room temperature, 5 ml of glutaric acid [1M, pH 8.0] was added and the resulting mixture rotated for 30 min at 200 rpm in an orbital shaker, centrifuged at 1500 × g for 10 min and the supernatant discarded. The ERR α or γ -silica was rinsed three times with 5 ml portions of Tris-HCl buffer [10 mM, pH 7.4] containing 150 mM NaCl, 0.1 % (w/v) BSA, 1mM EDTA, 0.1% sodium azide. The suspension containing the ERR α or γ -silica was packed into a Tricorn 5/20 glass column (50 × 5 mm I.D.) and the column washed with Tris-HCl buffer [10 mM, pH 7.4] for 2 h at a flow rate 0.2 ml/min at 25 °C.
2.2.2. Immobilization of ERRα or ERRγ on the surface of open tubular capillaries
The ERRα-OT and ERRγ-OT were synthesized as described above with slight modifications. Briefly, the open tubular capillary (25 cm × 100 µm i.d.) was primed with 1 M NaOH, rinsed with water, and dried at 95 °C for 1 h. A 10% aqueous solution of aminopropyltrimethoxysilane was passed through the capillary for 5 min followed by a 30 min incubation at 95 °C and then repeated. After 18 h, a 1% gluteraldehyde aqueous solution was passed through the capillary for 1h followed by a solution of ERRα or ERRγ (50 µg in 2 ml ammonium acetate buffer [10mM, pH 7.4]) which was recycled through the capillary for 60 min. Both tips of the capillary were submerged into the solution for 18 h at 4°C. After which ammonium acetate buffer [10mM, pH 7.4] was passed through for 5 min followed by a 1 M solution of glutaric acid for 1 h.
2.3 Chromatographic system, frontal and non-linear chromatographic studies
The chromatographic system consisted of a series 1100 liquid chromatography/mass selective detector (LC/MSD) (Agilent Technologies, Palo Alto, CA) equipped with a vacuum de-gasser, a binary pump, an autosampler, a mass selective detector supplied with atmospheric pressure ionization electrospray (API-ES). The chromatographic system was interfaced to a 250 MHz Kayak XA computer (Hewlett-Packard, Palo Alto, CA) using ChemStation software (Rev B.10.00, Hewlett-Packard). The mobile phase was composed of ammonium acetate [10 mM, pH 7.4] and the experiments were carried out at a flow rate of 0.5 ml/min at ambient temperature. The parameters on the MSD were set at 10 lpm for the drying gas flow, 350 °C for the drying gas temperature, 54 psig for nebulizer pressure, and 70 V for the fragmentor. DES, 4-hydroxytamoxifen, endoxifen and tamoxifen were monitored using single ion monitoring (M-1) at m/z values 267.2 and (M+1) at m/z values of 388.1, 374.2 and 372.3 respectively.
2.3.1 Frontal chromatography studies
Serial concentrations of DES [0.05, 0.1, 0.25, 0.5, 1 µM] were prepared in ammonium acetate [10 mM, pH 7.4]: methanol (94:6, v/v). The observed retention volumes were used to calculate binding affinities (Kd values) of the studied ERRγ inhibitors using a previously described approach [10]. The data were analyzed by nonlinear regression with the sigmoidal response curve using Prism 4 software (Graph Pad Software Inc., San Diego, CA).
2.3.2 Non-linear chromatography studies (NLC)
The binding kinetics of 4-hydroxytamoxifen, endoxifen and tamoxifen were determined by NLC were carried out on the ERRγ-silica column as previously described [11]. The mobile phase was composed of ammonium acetate [10 mM, pH 7.4]:methanol (80:20, v/v) delivered at a flow rate of 0.5 ml/min at room temperature. The studies utilized 20 µl injections of 4-hydroxytamoxifen [1.0, 2.5, 5.0, 10.0, and 20.0 µM], endoxifen [2.5, 5.0, 10.0, and 20.0 µM] and tamoxifen [5.0, 10.0, 20.0 and 40.0 µM]. The mathematical approach was the Impulse Input Solution [12] and the chromatographic data were analyzed using PeakFit v4.12 for Windows Software (SPSS Inc., Chicago, IL, USA) following a previously reported protocol [13].
2.4. MTS Proliferation
The CellTiter 96® Aqueous cell proliferation assay was performed according to the manufacturer’s protocol (Promega, Madison, WI) with slight modifications [5]. Briefly, T98G cells were seeded in 96-well plates at a density of 1750 cells/well and cultured in complete phenol red-free medium for 24 h. Test compounds dissolved in DMSO and mixed with culture medium were added to the cells in the following concentrations: tamoxifen [0.1, 1.0, 2.0, 3.0, 5.0, 7.0, 10 and 20 µM]; 4-hydroxytamoxifen [0.625, 1.25, 2.5, 3.75, 5, 7.5, 10 and 15 µM]; endoxifen [0.625, 1.25, 2.5, 3.75, 5, 7.5, 10 and 15 µM]. Control cultures were treated with DMSO. After 48 h, 20µl of 0.5 mg/ml MTS solution was added to each well, and the cultures were further incubated for 30 min. The absorbance was measured at 490nm with a microplate reader (Thermo Scientific, USA). Change in growth rate was calculated as follows: [Abs490nm of treated cells/Abs490nm of control cells]. Three wells were used for each treatment, and the experiments were repeated three times. IC50s were calculated using the Graph Pad Prism Software (La Jolla, CA).
3. RESULTS AND DISCUSSION
3.1 Characterization of the ERRα–OT and ERRγ–OT columns
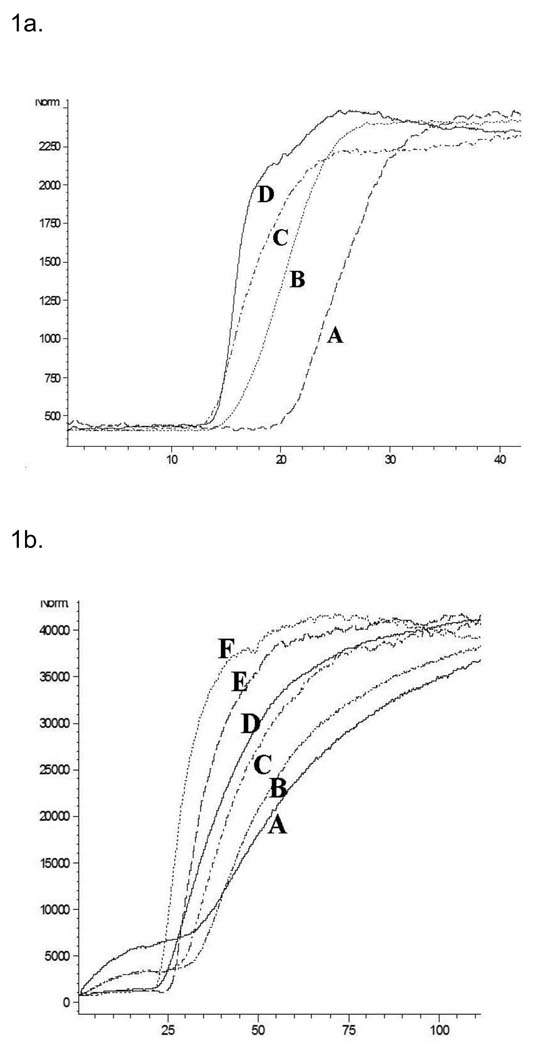
The ERRα–OT and ERRγ–OT columns were characterized using frontal chromatographic techniques using DES as the marker ligand. On both columns, the DES chromatographic traces contained a relatively flat initial portion representing nonspecific and specific binding of the marker to the stationary phase, followed by a vertical breakthrough representing saturation and then a plateau, c.f. Fig. 1a. Increasing mobile phase concentrations of DES produced a corresponding decrease in the breakthrough volume, Fig. 1a, and the data was used to calculate the affinity, expressed as Kd values, using previously described techniques [14]. The chromatographically determined Kd values for the binding of DES to the ERRα and ERRγ were 929 and 237 nM respectively, Table 1. The Kd value obtained for DES on the ERRγ was similar to previously reported value of 870 nM [15] and the relative order, i.e. Kd (ERRγ) < Kd (ERRα), is consistent with the previously reported IC50 values of 700 nM (ERRγ) [15] and 10 µM (ERRα) [15]. The chromatographic data was also used to calculate the amount of active sites (Bmax) on the OT columns which were 106 pmoles on the ERRγ-OT column and 600 pmoles on the ERRα-OT column.
Figure 1.
A. The effect of increasing concentration of diethylstilbesterol on its chromatographic retention on the ERRγ-OT column from right to left (0.05, 0.1, 0.25 and 0.5 µM). B. The effect of increasing concentration of diethylstilbesterol on its chromatographic retention on the ERRγ- column from right to left (0.05, 0.1, 0.25, 0.5, 1, 2 µM).
Table 1.
Calculated binding affinities (Kd values) for diethylstilbesterol on the ERRα-OT and the ERRγ-OT columns determine using frontal chromatographic techniques.
| Kd Obs | IC50* | |
|---|---|---|
| ERRα | 929 nM | 10 µM |
| ERRγ | 237 nM | 700 nM |
Reported EC50 values for DES (Coward et al., 2001).
3.2 Characterization of the ERRγ- and ERRα-silica columns
Frontal chromatographic studies were used to determine the Kd for DES on the ERRγ-silica column, Fig 1.b. The calculated Kd value, 251 nM, was the same as the Ki value, 237 nM, obtained on the ERRγ-OT column indicating that the DES-protein interactions were similar on both columns. However, the calculated Bmax value was 4 nmoles, a 40-fold increase relative to the ERRγ-OT column. The ERRα-silica column was characterized using the ERRα agonist biochanin A, and the Kd value, 64 nM, was consistent with the previously reported Kd of 45 nM [5]. It is interesting to note that unlike the ERRγ-silica column, the immobilization of ERRα on the silica support did not significantly increase the calculated Bmax value, relative to ERRα-OT column, 700 pmol and 600 pmol, respectively.
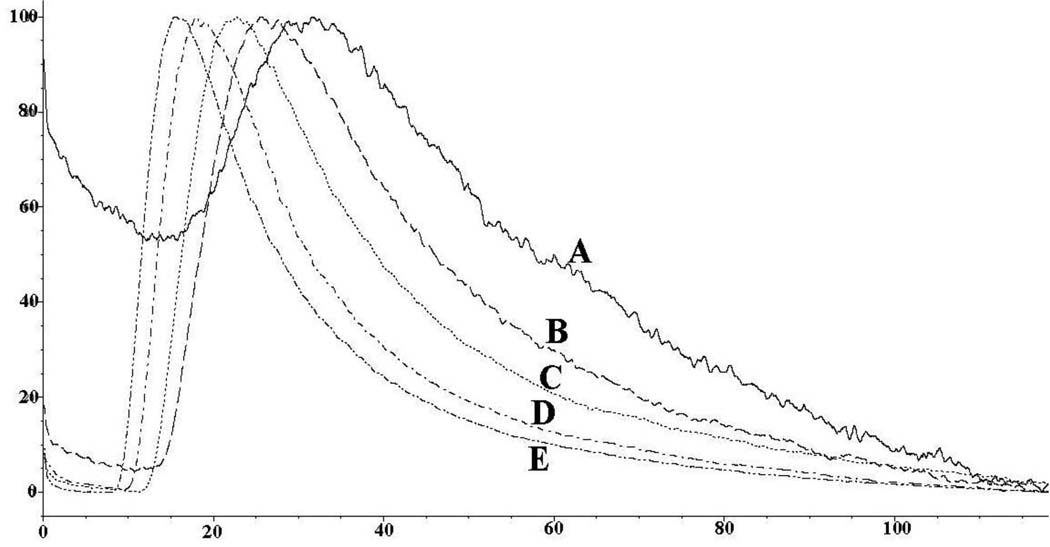
Based upon the increased capacity of the ERRγ-silica column, this format was further characterized for use in online screening using NLC and 4-hydroxytamoxifen, endoxifen and tamoxifen. The injection of increasing concentrations of these ligands resulted in chromatographic traces containing asymmetric peaks, which are indicative of the NLC process [11], Fig. 2. The calculated Kds were averaged over the area where a3 versus concentration were linear. Analysis of the data showed that NLC parameters can be obtained from peak profiles produced by injections of 20 µl of these solutions in concentrations ≤ 5 µM. Using this approach, the calculated Kd values of 4-hydroxytamoxifen, endoxifen and tamoxifen were 16.3 nM, 43.2 nM and 1.5 µM respectively, Table 2, which were consistent with previously reported Ki values of 75 nM for 4-hydroxytamoxifen and 870 nM for tamoxifen [15]. Tamoxifen, endoxifen and 4-hydroxytamoxifen were not specifically retained on the ERRα column (data not shown), which is consistent with the observation that these compounds have no affinity for the ERRα[15].
Figure 2.
Effects of increasing concentration of 4-hydroxtamoxifen from 1 µM to 20 µM on the ERRγ-column using non-linear chromatographic techniques.
Table 2.
Calculated binding affinities (Kd values) of the ERRγ antagonists, 4-hydroxytamoxifen, endoxifen and tamoxifen determined using non-linear chromatographic techniques on the ERRγ column and the corresponding dose-dependent effect on cellular proliferation (IC50 values) determined using a 48 hour MTS proliferation assay carried out using the human-derived T98G glioma cell line.
| Kd values ERRγ Column |
IC50 values T98G cell line |
|
|---|---|---|
| 4-Hydroxytamoxifen | 16.3 nM | 14.5 uM |
| Endoxifen | 43.2 nM | 3.4 uM |
| Tamoxifen | 1510 nM | 118 uM. |
3.3. Relationship between chromatographically determined Kd values and inhibition of cellular proliferation IC50 values
The chromatographically determined Kd values for 4-hydroxytamoxifen, endoxifen and tamoxfien were compared to the effect of these compounds on the proliferation of the human-derived T98G glioblastoma cell line. Previous studies have established that T98G cells expressed ERRγ and that cellular proliferation was affected by ERRγ agonists and antagonists [5]. Treatment of T98G cells with 4-hydroxytamoxifen, endoxifen and tamoxifen for 48 hours decreased cell proliferation in a dose-dependant manner and the calculated IC50 values were 3.4 µM (endoxifen), 14.5 µM (4-hydroxytamoxifen) and 118 µM (tamoxifen), Table 2. The results indicate that the chromatographically determined Kd values did not accurately predict the 3-fold difference in IC50 values between endoxifen and 4-hydroxytamoxifen, but did correctly reflect the 10-fold differences in activity between these compounds and tamoxifen. Thus, the data indicate that the ERRγ column can be used as an initial online screen for the isolation of compounds that bind to the ERRγ.
4. Conclusions
The data from this study indicate that ERRα and ERRγ have been successfully immobilized onto the silica stationary phase, as well as the surface of the open tubular capillaries, creating the ERR-silica and ERR-OT columns. The data also demonstrate that the binding affinities calculated by frontal displacement chromatography correlated with IC50 values obtained using cellular uptake studies, suggesting that this method can be used in place of current cellular uptake studies, which are time consuming and laborious. In addition, this technique can be used for a preliminary screen for drug candidates using the differences of their binding affinities.
Acknowledgements
The research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wrensch M, Rice T, Miike R, McMillan A, Lamborn KR, Aldape K, Prados MD. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro. Oncol. 2006;8:12–26. doi: 10.1215/S1522851705000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. Changing paradigms--an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003;9:149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hui AM, Zhang W, Chen W, Xi D, Purow B, Friedman GC, Fine HA. Agents with selective estrogen receptor (ER) modulator activity induce apoptosis in vitro and in vivo in ER-negative glioma cells. Cancer Res. 2004;64:9115–9123. doi: 10.1158/0008-5472.CAN-04-2740. [DOI] [PubMed] [Google Scholar]
- 5.Gandhari MK, Frazier CR, Hartenstein JS, Cloix JF, Bernier M, Wainer IW. Identification and characterization of estrogen receptor-related receptor alpha and gamma in human glioma and astrocytoma cells. Mol Cell Endocrinol. 2010;315:314–318. doi: 10.1016/j.mce.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 2003;1(2003):981–991. [PubMed] [Google Scholar]
- 7.Moaddel R, Lu L, Baynham M, Wainer IW. Immobilized receptor- and transporter-based liquid chromatographic phases for on-line pharmacological and biochemical studies: a mini-review. J. Chromatogr. B. 2002;768:41–53. doi: 10.1016/s0378-4347(01)00484-4. [DOI] [PubMed] [Google Scholar]
- 8.Moaddel R, Price GB, Juteau JM, Leffak M, Wainer IW. The synthesis and initial characterization of an immobilized DNA unwinding element binding (DUE-B) protein chromatographic stationary phase. J. Chromatogr. B. 2005;820:197–203. doi: 10.1016/j.jchromb.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Marszall M, Moaddel R, Jozwiak K, Bernier M, Wainer IW. Initial synthesis and characterization of an immobilized heat shock protein 90 column for online determination of binding affinities. Anal. Biochem. 2008;373:313–321. doi: 10.1016/j.ab.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura T, Perry J, Anzai N, Pritchard J, Moaddel R. Development and Characterization of immobilized human organic anion transporter based liquid chromatographic stationary phase: hOAT1 and hOAT2. J. Chromatogr. B. 2007;859:267–271. doi: 10.1016/j.jchromb.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jozwiak K, Haginaka J, Moaddel R, Wainer IW. Displacement and nonlinear chromatographic techniques in the investigation of interaction of noncompetitive inhibitors with an immobilized alpha3beta4 nicotinic acetylcholine receptor liquid chromatographic stationary phase. Anal.Chem. 2002;74:4618–4624. doi: 10.1021/ac0202029. [DOI] [PubMed] [Google Scholar]
- 12.Wade D, Yang CS, Metral CJ, Roman JM, Hrabie JA, Riggs CW, Anjo T, Keefer LK, Mico BA. Deuterium isotope effect on denitrosation and demethylation of N-nitrosodimethylamine by rat liver microsomes. Cancer Res. 1987;47:3373–3377. [PubMed] [Google Scholar]
- 13.Jozwiak K, Ravichandran S, Collins JR, Wainer IW. Interaction of noncompetitive inhibitors with an immobilized alpha3beta4 nicotinic acetylcholine receptor investigated by affinity chromatography, quantitative-structure activity relationship analysis, and molecular docking. J. Med. Chem. 2004;47:4008–4021. doi: 10.1021/jm0400707. [DOI] [PubMed] [Google Scholar]
- 14.Moaddel R, Wainer IW. The preparation and development of cellular membrane affinity chromatography columns. Nat. Protocols. 2009;4:197–205. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coward P, Lee D, Hull VM, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor γ. Proc.Natl.Acad.Sci. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]