Abstract
Epidemiological studies suggest that estrogen therapy protects against clinical expression of chronic neurological diseases. These beneficial effects of estrogen therapy are highly modified by apolipoprotein E (apoE) through an unknown mechanism. We examined the short-term effects of estradiol replacement in ovariectomized mice on apoE expression and markers for cell proliferation, reactive gliosis, neuronal maturation, and synaptogenesis in the primary olfactory pathway of wild-type (WT) and apoE knockout (KO) mice. Three days of estradiol replacement increased apoE expression in the olfactory nerve and in the glomerular layer. Estradiol treatment also increased cell proliferation, total cell numbers, number of mature neurons in the olfactory epithelium, and reactive astrocyte numbers in the olfactory bulb (OB) in both WT and KO mice. We also found that estradiol increased glomerular synaptophysin (Syn), but the magnitude of increase was potentiated by the presence of apoE. This data suggest that apoE may be necessary to elicit the complete effect of estradiol on Syn upregualtion.
Keywords: estrogen, apoE, olfactory, glia, glial proteins, knockout mice
1. Introduction
Epidemiological studies suggest that estrogen therapy protects against clinical expression of Alzheimer’s and Parkinson’s diseases (Henderson, 2005; Paganini-Hill and Henderson, 1994; Tang et al., 1996). However, prospective studies with estrogen neither slowed the progression of dementia nor protected against cognitive decline in the Women’s Health Initiative Memory study (Espeland et al., 2004; Shumaker et al., 2003).
Previous non-human studies have demonstrated that 17β-estradiol has numerous beneficial effects on the brain, and many of these effects involve apolipoprotein E (Horsburgh et al., 2002; Nathan et al., 2004; Stone et al., 1998; Struble et al., 2007; Teter et al., 1999). ApoE is a lipid transporting protein, and it ranges in length from 279 to 310 residues, with a high degree of sequence conservation among species. The three common isoforms of apoE in humans differ by amino acids at positions 112 and 158 (Weisgraber, 1994). The most common isoform, apoE3, contains cysteine and arginine at positions 112 and 158, respectively. Both positions contain cysteine in apoE2 and arginine in apoE4. Mice have one form of apoE, which is similar to human apoE3 in its structural and functional properties, including receptor binding and lipoprotein preferences (Weisgraber, 1994).
Estradiol replacement can increase apoE in rodent models (McAsey et al., 2006; Nathan et al., 2004; Srivastava et al., 1997), and apoE has striking isoform-specific effects on the risk for several neurological diseases including Alzheimer disease (AD), Parkinson disease, and multiple sclerosis (Henderson, 2005; Paganini-Hill and Henderson, 1994; Tang et al., 1996). Cross-sectional analyses have shown an interaction between estrogen therapy and apoE genotype on learning and memory in postmenopausal women. Estrogen therapy improved performance in episodic memory only in women with apoE3, but not in women with apoE4 (Burkhardt et al., 2004). Furthermore, estrogen therapy reduced cognitive decline, but only in women who were not carriers for apoE4 allele (Yaffe et al., 2000).
ApoE allele status is also associated with olfactory dysfunction. ApoE4 carriers show a significant decline in odor threshold and odor identification, and have delays in processing of olfactory information (Bacon et al., 1998; Calhoun-Haney and Murphy, 2005; Gilbert and Murphy, 2004; Murphy et al., 1998; O’Hara et al., 1998). To our knowledge, only one study has looked at the effects of estradiol on the olfactory system repair, showing that estradiol treatment resulted in significantly faster and better recovery of olfactory discrimination performance following olfactory epithelium (OE) injury (Dhong et al., 1999). The physiological basis underlying the beneficial effects of estradiol on olfactory function is not clear. Therefore, we evaluated the effects of estradiol replacement on several parameters of olfactory system integrity and compared the effects in normal mice to those lacking apoE.
We examined the acute effects of estradiol replacement following ovariectomy on expression of apoE and markers for cell proliferation, reactive gliosis, neuronal maturation, and synaptogenesis in the primary olfactory pathway in mice as a first step towards understanding the role of estrogen in the olfactory system. The expressions of these parameters were examined in wild-type (WT) and apoE knockout (KO) mice to understand the impact of apoE deficiency on estradiol’s effect.
We found that estradiol replacement increased apoE expression in the olfactory nerve and in the glomerular layer. Glial cells appear to be the primary producers of apoE. Estradiol treatment increased OE thickness, basal cell proliferation, the density of mature neurons in the OE, and astroglial numbers and synaptogenesis in the olfactory bulb (OB) in both WT and KO mice. We also found that estradiol increased glomerular synaptophysin (Syn), but the magnitude of increase was potentiated by the presence of apoE. This data suggest that apoE may be necessary to elicit the complete effect of estradiol on Syn upregualtion.
2. Results
2.1. ApoE
ApoE immunostaining in both the estradiol- and vehicle-treated mice was intense at the olfactory epithelial surface and in cytoplasmic processes of the sustentacular cells extending to the epithelial surface. ApoE immunoreactive processes were present between the olfactory sensory neuron (OSN) bundles in both the vehicle- and estradiol-treated groups; however, a localization difference was apparent. The immunoreactive processes in the estradiol-treated mice terminated on faintly stained globular structures above the unstained basal lamina. We previously showed that these globular structures expressed GBC-1, a marker for globose basal cells (Nathan et al., 2007). In contrast, in the absence of estradiol, apoE immunostaining was concentrated on oblong cells that were arranged on a plane parallel to the unstained basal lamina. The morphology and location of these oblong cells resemble horizontal basal cells, but this conclusion warrants further studies (Holbrook et al., 1995).
The endothelial cells of blood vessels and cells surrounding the olfactory nerve in the lamina propria were intensely stained for apoE in both vehicle- and estradiol-treated groups. ApoE immunostaining was present throughout the core of the nerve bundles in estradiol-treated mice. In contrast, only very faint apoE staining was observed in the vehicle-treated mice. In essence, estradiol treatment slightly increased apoE expression in the olfactory nerve.
Estradiol treatment intensified apoE staining in the olfactory nerve and glomerular layers in the OB. In the olfactory nerve of estradiol-treated mice, the large fascicles were consistently stained, and were demarcated from each other by densely stained cellular processes. Glomeruli were less intensely stained than the olfactory nerve and were clearly outlined by immunostained cells in the septae surrounding the glomeruli as previously described (Nathan et al., 2001; Struble et al., 1999). In the vehicle-treated mice apoE immunostaining was weak throughout the OB.
2.2. GFAP
Astrocytes are the primary producers of apoE in the CNS (Pitas, 1987). Irrespective of the apoE genotype, the density of glial fibrillary acidic protein (GFAP) immunoreactive processes was increased by estradiol treatment compared to vehicle. Quantification of the GFAP process density revealed that estradiol treatment significantly (F1,8=24.46; p<0.001) increased process density in both the WT and KO mice. Neither genotype (F1,8=1.39. NS) or the interaction between genotype and treatment (F1,8=0.049; NS) reached significance. Estradiol replacement appeared to directly affect the expression of immunoreactive GFAP irrespective of the presence of apoE.
2.3. OE thickness
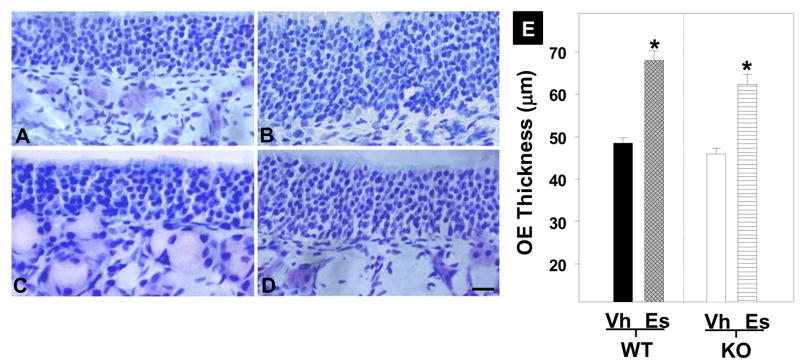
Measures of OE thickness showed that estradiol replacement resulted in a thicker (28%) OE compared to vehicle treated groups regardless of apoE genotype (F1,8=10.17; p<0.02). Neither genotype (F1,8=0.62; NS) nor the interaction between genotype and treatment (F1,8=0.17; NS) reached significance. ApoE presence or absence did not modulate the estradiol effect on thickness.
2.4. BrdU
Estrogen treatment marginally increased BrdU staining in the OE of WT and KO mice, although this did not reach standard levels of significance (F1,8=5.036; p<0.055) in both genotypes. No other contrast approached significance.
2.5. OMP
Olfactory marker protein (OMP) is a marker for mature OSN in the OE. We found that WT mice had about 20% more OMP labeled cells in the OE than the KO mice (F1,8=9.93; p<0.014). Estradiol treatment increased (almost doubled) the density of OMP cells in the OE as compared to vehicle (F1,8=71.46; p<0.001) regardless of genotype. We found no interaction between treatment and genotype (F1,8=0.65; NS) indicating that the effects of estradiol and genotype on OMP expression were independent. Estradiol treatment increased OMP staining in the glomerular layer of both genotypes as compared to their respective vehicle group. As observed in the OE, OMP staining was more intense in the estradiol-treated WT than in the estradiol treated KO mice.
2.6. Syn
Syn staining of glomeruli presented a complex picture with a significant interaction between genotype and estradiol replacement (F1,8=84.17; p<0.001). Syn staining was more intense in the vehicle-treated WT mice than in the vehicle-treated KO mice. Estradiol treatment increased Syn immunoreactivity by about 19% in the KO mice to reach the level of the vehicle-treated WT group. Estradiol replacement in WT mice increased Syn immunoreactivity by 30%. In essence, the absence of apoE in KO mice was associated with less Syn and an attenuated, but still significant increase in response to estradiol replacement.
3. Discussion
Two major differences in apoE staining pattern were observed in the OE of estradiol- versus vehicle-treated groups. First, apoE staining in the olfactory fascicles of estradiol-treated mice was diffuse, but consistently present. In contrast, in the vehicle-treated group, apoE expression in the olfactory nerve was undetectable. Second, in the estradiol-treated group, apoE staining above the basal lamina was weak and was punctuate around globular structures. In a previous study we showed that these apoE-expressing punctae were the end feet of sustentacular cells on globose basal cells (Nathan et al., 2007). In contrast, apoE was concentrated in horizontal and flat cellular structures in the basal cell zone in the vehicle-treated group. The distribution and morphology of these cells appears to be horizontal basal cells (Holbrook et al., 1995).
We also found that estradiol replacement following ovariectomy increased apoE in the olfactory nerve and in the glomerular layer of the OB. The distribution and morphology of the cells contributing to this increased apoE production appear to be ensheathing glial cells in the olfactory nerve and astroglial cells surrounding the glomeruli (Nathan et al., 2007). Estradiol induced increase in apoE has been observed in various neural and non-neuronal tissues (Nathan et al., 2004; Srivastava et al., 2001; Srivastava et al., 1996; Srivastava et al., 1997; Stone et al., 1997; Wang et al., 2006).
Estradiol replacement was associated with substantial increase in GFAP process density in the glomerular layer of WT mice. How estradiol increases GFAP process density is unclear; however, previous studies have reported this phenomenon (Lewis et al., 2008; Ritz and Hausmann, 2008). The presence of apoE is not critical for this process since estradiol replacement in KO mice also resulted in a significant increase in GFAP process density. An alternative explanation for the increase in process density is that estradiol simply increased the expression and or the detection of GFAP, without affecting process growth. A previous study has shown that estradiol affects the amount and or the distribution of GFAP in the astrocyte, and there by increases GFAP staining intensity (Garcia-Segura et al., 1989).
Estradiol replacement was associated with increased epithelial thickness by about 28%, and this effect was independent of apoE presence. The thickness increase could indicate an increase in cell division. Several previous studies have shown that estradiol can increase BrdU incorporation and cell counts (Barha et al., 2009; Pawluski et al., 2009). Estradiol can indirectly increase basal cell proliferation by increasing signaling factors promoting basal cell proliferation (Beites et al., 2005). The precise mechanism whereby estradiol increases epithelial thickness is unclear; however, a partial explanation may be obtained from the OMP data noted below.
The OMP phenotype represents a “mature” OSN that has made contact with the post-synaptic cells of the OB (Graziadei et al., 1980; Sydor et al., 1986). Hence, an estradiol replacement-induced increase in the number of OMP+ OSN in the OE of both the WT and KO mice suggests that estradiol replacement either maintained the adult population in response to loss of ovarian hormones or estradiol facilitated OSN maturation. Estradiol could also have facilitated OSN maturation by promoting synaptogenesis with the neurons in the OB. Alternatively, estradiol induced increase in cell division could have contributed to an increase in the OMP+ cell counts in the OE. However, this possibility is unlikely as previous studies have shown that it takes at least 7 days for a newly generated cell in the OE to transform into a mature OSN phenotype, and therefore, three days of estradiol is probably not adequate (Graziadei and Graziadei, 1979). Hence, changes in BrdU may not be significant at this stage after OVX.
We also found that the OMP cell density in OE and intensity of OMP staining in the OB was greater in the WT than the KO suggesting an effect of apoE on OE maintenance. One possible explanation for this difference is that cell genesis rates in KO and WT are comparable but, as we have shown axonal growth and synaptogenesis following a lesion of the OE are delayed in KO mice (Nathan et al., 2005; Nwosu et al., 2008). Hence, increased OMP cell density in WT OE, regardless of estradiol replacement indicates that apoE facilitates maturation of OSN to phenotypically adult cells.
An incomplete or aberrant status of synaptogenesis in the KO compared to the WT is supported by Syn density. Overall, estradiol was associated with increased Syn but the baseline level and magnitude of increase was suppressed by the absence of apoE. A decrease in Syn staining has been shown in the hippocampus, neocortex, and OB of apoE KO mice (Masliah et al., 1995; Nwosu et al., 2008). Our data parallel these previous studies and present a possible mechanism to explain this. Specifically, normal regeneration may be slightly delayed in apoE KO mice and this result in fewer synapses. Estradiol replacement following ovariectomy can increase several synaptic proteins, including Syn perhaps by stimulation of synaptic maturation. ApoE may be necessary to elicit the complete effect of estradiol on Syn upregualtion.
Our data may be explained by hypothesizing two independent factors simultaneously affecting the OE and OB, estradiol and apoE. Estradiol deprivation in ovariectomized mice may lead to degeneration of the OE owing to the loss of trophic actions of estrogen. This hypothesis is based on the following observations. First, degeneration of the OE in the vehicle-replaced mice would explain the thinner OE as compared to estradiol-replaced mice. Second, reduced OMP immunoreactivity in the OE of vehicle-replaced mice could represent death of mature OSN in the OE. Third, decreased OMP staining in the glomerular layer of the vehicle-replaced mice may be due to loss of mature axons of OSN in the glomeruli. Finally, the distribution of apoE, from globose cells in the estradiol treated mice to horizontal cells in the vehicle treated mice, may also represent OE degeneration. Horizontal cells are multipotent precursor cells, as they are capable of producing the full complement of cell types in the adult OE (Carter et al., 2004). Increased expression of apoE in the horizontal cells of the vehicle-replaced mice may facilitate lipid recycling from degenerating OSN to support rapid proliferation of horizontal cells, and their subsequent differentiation to other OE cell types (Nathan et al., 2005).
OB changes in apoE immunoreactivity could represent a direct effect of estradiol on the production of apoE as we have previously reported for the OB (McAsey et al., 2006; Struble et al., 2003). The increase in apoE is associated with an increase in GFAP+ cells in the estradiol treated mice. Since astrocytes are the chief producer of apoE in the nervous system, it is possible that estradiol increased apoE production by increasing astrocyte numbers. Alternatively, estradiol’s effect on increasing apoE and GFAP are unrelated. Increase in GFAP could represent either a direct effect of estradiol on GFAP expression/detection. Our data suggest either of these scenarios is reasonable.
Finally, decreased OMP and Syn in apoE KO mice may represent fewer axons with adult phenotypes in the KO than the WT. Lower OMP levels could also represent slower synaptogenesis in the apoE KO mice combined with the effects of degenerating OSNs. Hence, we would expect an interaction between genotype and conversion of remaining OSN axons to the adult phenotype expressing OMP. To further clarify these issues of degeneration following ovariectomy, a long-term study needs to be performed to determine if the OE has reached asymptote. Interventions after asymptote would further clarify the role of estradiol and the integrity of the OE following ovariectomy.
4. Materials and Methods
4.1. Animals
Breeding pairs of WT C57BL/6 strain and homozygous apoE KO mice were purchased from the Jackson Laboratories, Bar Harbor, ME. ApoE genotype of the litters were verified by PCR and confirmed by immunoblotting using anti-apoE as described below. Four months old mice were used in this study.
4.2. Ovariectomy
WT and KO littermate mice, four months of age at the start of this study, were used. Mice were ovariectomized (OVX) by a dorsal, bilateral approach under ketamine (100 mg/kg) and xylazine (50 mg/kg) (Sigma, St. Louis, MO) anesthesia and sterile operating conditions. The animals recuperated for five days prior to estradiol/vehicle pellet placement. In previous studies we have shown that at five days post-OVX, estradiol levels were undetectable in mice (Cheng et al., 2007; McAsey et al., 2006). The animals were randomly assigned to either replacement with a pellet containing estradiol (0.36 mg, 60 day release, Innovative Research of America, Sarasota, FL) or a pellet containing only the binder (vehicle). Estradiol- or vehicle-pellets were placed subcutaneously using a trochar at the mid-scapular level. Preliminary results from our laboratory, and published results from several laboratories, have demonstrated that the estradiol pellets maintain 17 β-estradiol at a constant proestrus level for at least 60 days (Cheng et al., 2007; Katovich and O’Meara, 1987; McAsey et al., 2006; Rosenblum et al., 1985).
4.3. Tissue preparation
Mice were sacrificed three days following implantation of estradiol- or vehicle-pellets. Mice received an intraperitoneal injection of BrdU (50 mg/kg) (Sigma Aldrich, St. Louis, MO) 12 hours prior to sacrifice. For fluorescence immunohistochemistry, mice were anesthetized as described above and transcardially perfused with cold saline (0.9% NaCl), followed by 4% paraformaldehyde in 0.1M PBS. Olfactory turbinates were removed and cryoprotected overnight in 30% sucrose in 0.1 M PBS. After cryoprotection, the turbinates were frozen with dry ice and sections were cut on a cryostat at 18 μm, and air dried for 2 h at room temperature.
4.4. CV Staining
Sections were rinsed in distilled water for 10 minutes and placed in the oven for 2 hours at 37°C. The sections were then defatted with xylene for 30 minutes. The sections were hydrated in a series of ethanol (100%, 95%, and 70%) for 10 minutes each. Sections were rinsed in water and stained in cresyl violet acetate solution (Sigma, St. Louis, MO) for 4 minutes. Sections were rinsed in water and in a series of ethanol (70, 95, and 100%). Following incubation in xylene for 30 minutes, sections were coverslipped using permount (Fisher Scientific, Fair Lawn, NJ).
4.5. Immunohistochemical Analysis
Sections on slides were rinsed in 0.1 M PBS, and permeabilized with 0.2 % Triton X-100 (Sigma, St. Louis, MO) in PBS for 30 minutes at room temperature. The slides were rinsed once with PBS and treated with 70, 90, 100, 90, and 70% ethanol for two minutes each (Jang et al., 2003). Non-specific immunoreactivity was attenuated by incubation in 2.25% gelatin in 0.1 M PBS for 1 h, followed by overnight incubation with primary antisera solution at 4 °C (see Table 1 for source and concentration used). The sections were washed three times in PBS, and incubated for 1 hour at room temperature with secondary antibody solution as listed in Table 1. The sections were washed three times in PBS, mounted in Vectashield (Vector labs, Burlingame, CA). For apoE staining, KO mice were processed in parallel with WT mice. Specificity was determined by incubation with normal serum in place of the primary antisera which resulted in no staining.
Table 1.
List of primary and secondary antibodies used in this study
| Antibodies | Host | Source | Dilution |
|---|---|---|---|
| ApoE | Goat | Calbiochem, San Diego, CA | 1:1000 |
| OMP | Goat | Wako, Richmond, VA | 1:500 |
| Syn | Rabbit | Cell Marque, Rocklin, CA | 1:500 |
| BrdU | Rat | Accurate, Westbury, NY | 1:500 |
| GFAP | Mouse | Accurate, Westbury, NY | 1:500 |
| FITC-anti goat | Donkey | Jackson, West Grove, PA | 1:500 |
| Cy3-anti goat | Donkey | Jackson, West Grove, PA | 1:500 |
| Cy3-anti rat | Goat | Jackson, West Grove, PA | 1:500 |
| Alexa-anti rabbit | Donkey | Invitrogen, Eugene, OR | 1:200 |
Stained sections were examined using an Olympus BX-50 microscope. Images were captured using a Pixera Digital Camera (Pixera, Los Gatos, CA) and saved as high resolution TIF files. Figures from images were assembled using Photoshop (Adobe, San Jose, CA). Image analysis was performed using Scion Image software (Scion Image, Frederick, MD).
Morphological thickness of the OE was determined from image calibration of a stage micrometer in Scion Image. Thickness was repeatedly measured from the horizontal basal cell layer to the head of the sustentacular cells. The number of OMP+ in ten 100 μm segments of OE and BrdU+ cells in 1 mm of OE was counted utilizing Scion Image. GFAP+ processes were counted by placing a 5×5 grid box over the digital image, and astrocyte processes were marked with a dot if they crossed the grid (Struble et al., 2006). Syn quantification was performed as described (Nwosu et al., 2008).
4.6. Statistical Analysis
All quantification procedures were performed using three mice per genotype (WT, KO) and three mice per treatment (estrogen, vehicle). A total of 10 measurements were taken from each animal. The data in individual experiments were presented as mean ± standard error and statistical analysis (ANOVA, Repeated Measures ANOVA) was performed using SYSTAT. A blinded procedure was employed in all experiments so the experimenter was unaware of the genotype (WT versus KO) and treatment (estradiol versus vehicle) received by the animals.
Fig. 1.

Effects of estradiol on apoE expression in adult mouse olfactory epithelium. ApoE immunoreactivity in ovariectomized WT mice implanted for 3 days with either a vehicle (A) or estradiol pellet (B). Sus, sustentacular cells, OSN, olfactory sensory neuron, BC, basal cell zone, BV, blood vessel, OF olfactory fascicle. Arrow indicates basal lamina. ApoE immunoreactivity in vehicle- and estradiol-treated mice was intense in the perikarya of the sustentacular cells and was faint around the OSN. Intense apoE staining above the basal lamina was present in oblong cells (arrow heads) in the vehicle group, and in punctas surrounding globose cells in the estradiol treated group (asterisks). Endothelial cells of the blood vessels in both treatment groups were strongly stained. ApoE immunostaining was present throughout the core of the olfactory fascicle in estradiol treated mice, and was very faint in the vehicle treated mice. Scale bars = 10 μm.
Fig. 2.
ApoE immunostaining in the olfactory nerve and glomerular layer of the OB of ovariectomized WT mice implanted with either vehicle (A) or estradiol (B) pellet. ApoE immunostaining was weak throughout the OB in the vehicle-treated mice. In the estradiol- treated mice, dense apoE immunoreactivity was observed in cellular processes (arrows) in the olfactory nerve fascicles and in cells surrounding the glomeruli (arrow heads). Scale bars = 10 μm.
Fig. 3.
GFAP immunoreactivity in the glomerular layer of the OB. Estradiol treatment increased GFAP immunoreactive processes in WT (B) and KO (D) mice as compared to vehicle treated WT (A) and KO (C) mice. Scale bars = 10 μm. (E) Quantification of GFAP immunoreactive process density in vehicle (Vh) and estradiol (Es) treated WT and KO mice (Mean and SE). Estrogen treatment significantly increased GFAP process density (*).
Fig. 4.
Cresyl violet stained sections of OE from WT (A, B) and KO (C, D) mice implanted with an estradiol (B, D) or a vehicle (A, C) pellet. Irrespective of the genotype, estradiol treatment increased OE thickness. Scale bars = 10 μm. (E) Quantification of OE thickness in WT and KO mice treated with estradiol (Es) or vehicle (Vh) (Mean and SE). Estrogen treatment significantly increased OE thickness (*).
Fig. 5.
BrdU staining in the basal cell zone of OE. Estradiol treatment increased BrdU immunoreactive cells in WT (B) and KO (D) mice as compared to vehicle treated WT (A) and KO (C) mice. Scale bars = 10 μm. (E) Quantification of BrdU+ cells in vehicle (Vh) and estradiol (Es) treated WT and KO mice (Mean and SE).
Fig. 6.
OMP stained sections of OE from WT (A, B) and KO (C, D) mice implanted with an estradiol (B, D) or a vehicle (A, C) pellet. Irrespective of the genotype, estradiol treatment increased OMP+ cells; however, estradiol-treated WT mice had about 20% more OMP labeled cells than the estradiol-treated KO mice. Scale bars = 10 μm. (E) Quantification of OMP+ cells in WT and KO mice treated with estradiol (Es) or vehicle (Vh) (Mean and SE). Estrogen treatment significantly increased OMP+ cells (*).
Fig. 7.
OMP staining in the glomerular layer of the OB in WT (A, B) and KO (C, D) mice implanted with an estradiol (B, D) or a vehicle (A, C) pellet. Estradiol increased OMP staining in both the genotypes, but the effect was more pronounced in the estradiol-treated WT as compared to estradiol-treated KO. Scale bars = 10 μm.
Fig. 8.
Syn staining in the glomerular layer of the OB. Estradiol treatment increased Syn staining in WT (B) and KO (D) mice as compared to vehicle treated WT (A) and KO (C) mice. However, the magnitude of increase was significantly greater in the WT than in the KO. Scale bars = 10 μm. (E) Densitometric quantification of Syn density in vehicle (Vh) and estradiol (Es) treated WT and KO mice. Estrogen treatment significantly increased Syn density (*).
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorder (DC 003889), Illinois Department of Public Health grant, and Eastern Illinois University CFR grants.
Abbreviations
- apoE
apolipoprotein E
- OB
olfactory bulb
- OE
olfactory epithelium
- OSN
olfactory sensory neuron
- GBC
globose basal cells
- Sus
Sustentacular cells
- OMP
olfactory marker protein
- GFAP
glial fibrillary acidic protein
- Syn
synaptophysin
- BrdU
bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–31. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–66. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–16. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, Stuckey BG, Clarnette R, Nolan D, Hewson-Bower B, Martins RN. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis. 2004;6:221–8. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C. Apolipoprotein epsilon4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn. 2005;58:178–82. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–83. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, McAsey ME, Li M, Randall S, Cady C, Nathan BP, Struble RG. Estradiol replacement increases the low-density lipoprotein receptor related protein (LRP) in the mouse brain. Neurosci Lett. 2007;417:50–4. doi: 10.1016/j.neulet.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Dhong HJ, Chung SK, Doty RL. Estrogen protects against 3-methylindole-induced olfactory loss. Brain Res. 1999;824:312–5. doi: 10.1016/s0006-8993(99)01241-x. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Torres-Aleman I, Naftolin F. Astrocytic shape and glial fibrillary acidic protein immunoreactivity are modified by estradiol in primary rat hypothalamic cultures. Brain Res Dev Brain Res. 1989;47:298–302. doi: 10.1016/0165-3806(89)90186-7. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–94. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Stanley RS, Graziadei PP. The olfactory marker protein in the olfactory system of the mouse during development. Neuroscience. 1980;5:1239–52. doi: 10.1016/0306-4522(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–9. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–46. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab. 2002;22:1189–95. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–40. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katovich MJ, O’Meara J. Effect of chronic estrogen on the skin temperature response to naloxone in morphine-dependent rats. Can J Physiol Pharmacol. 1987;65:563–7. doi: 10.1139/y87-095. [DOI] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–22. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, Struble RG. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197:197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–50. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Nisar R, Randall S, Short J, Sherrow M, Wong GK, Struble RG. Apolipoprotein E is upregulated in olfactory bulb glia following peripheral receptor lesion in mice. Exp Neurol. 2001;172:128–36. doi: 10.1006/exnr.2001.7762. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145:3065–73. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Nisar R, Short J, Randall S, Grissom E, Griffin G, Switzer PV, Struble RG. Delayed olfactory nerve regeneration in ApoE-deficient mice. Brain Res. 2005;1041:87–94. doi: 10.1016/j.brainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Nannapaneni S, Gairhe S, Nwosu I, Struble RG. The distribution of apolipoprotein E in mouse olfactory epithelium. Brain Res. 2007;1137:78–83. doi: 10.1016/j.brainres.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu I, Gairhe S, Struble RG, Nathan BP. Impact of apoE deficiency during synaptic remodeling in the mouse olfactory bulb. Neurosci Lett. 2008;441:282–5. doi: 10.1016/j.neulet.2008.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara R, Yesavage JA, Kraemer HC, Mauricio M, Friedman LF, Murphy GM., Jr The APOE epsilon4 allele is associated with decline on delayed recall performance in community-dwelling older adults. J Am Geriatr Soc. 1998;46:1493–8. doi: 10.1111/j.1532-5415.1998.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140:256–61. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–57. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E- containing lipoproteins. Biochim Biophys Acta. 1987;917:48–61. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–88. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, el-Sabban F, Allen AD, Nelson GH, Bhatnagar AS, Choi SC. Effects of estradiol on platelet aggregation in cerebral microvessels of mice. Stroke. 1985;16:980–4. doi: 10.1161/01.str.16.6.980. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Chowdhury PR, Averna M, Srivastava RA. Estrogen increases hepatic lipase levels in inbred strains of mice: a possible mechanism for estrogen-dependent lowering of high density lipoprotein. Mol Cell Biochem. 2001;220:87–93. doi: 10.1023/a:1010845032399. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Bhasin N, Srivastava N. Apolipoprotein E gene expression in various tissues of mouse and regulation by estrogen. Biochem Mol Biol Int. 1996;38:91–101. [PubMed] [Google Scholar]
- Srivastava RA, Srivastava N, Averna M, Lin RC, Korach KS, Lubahn DB, Schonfeld G. Estrogen up-regulates apolipoprotein E (ApoE) gene expression by increasing ApoE mRNA in the translating pool via the estrogen receptor alpha-mediated pathway. J Biol Chem. 1997;272:33360–6. doi: 10.1074/jbc.272.52.33360. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143:313–8. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. J Neurosci. 1998;18:3180–5. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble RG, Short J, Ghobrial M, Nathan BP. Apolipoprotein E immunoreactivity in human and mouse olfactory bulb. Neurosci Lett. 1999;267:137–40. doi: 10.1016/s0304-3940(99)00352-3. [DOI] [PubMed] [Google Scholar]
- Struble RG, Rosario ER, Kircher ML, Ludwig SM, McAdamis PJ, Watabe K, McAsey ME, Cady C, Nathan BP. Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17beta estradiol. Exp Neurol. 2003;183:638–44. doi: 10.1016/s0014-4886(03)00215-2. [DOI] [PubMed] [Google Scholar]
- Struble RG, Afridi S, Beckman-Randall S, Li M, Cady C, Nathan B, McAsey ME. Neocortical and hippocampal glial fibrillary acidic protein immunoreactivity shows region-specific variation during the mouse estrous cycle. Neuroendocrinology. 2006;83:325–35. doi: 10.1159/000095340. [DOI] [PubMed] [Google Scholar]
- Struble RG, Nathan BP, Cady C, Cheng X, McAsey M. Estradiol regulation of astroglia and apolipoprotein E: an important role in neuronal regeneration. Exp Gerontol. 2007;42:54–63. doi: 10.1016/j.exger.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Sydor W, Teitelbaum Z, Blacher R, Sun S, Benz W, Margolis FL. Amino acid sequence of a unique neuronal protein: rat olfactory marker protein. Arch Biochem Biophys. 1986;249:351–62. doi: 10.1016/0003-9861(86)90011-1. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Teter B, Harris-White ME, Frautschy SA, Cole GM. Role of apolipoprotein E and estrogen in mossy fiber sprouting in hippocampal slice cultures. Neuroscience. 1999;91:1009–16. doi: 10.1016/s0306-4522(98)00630-7. [DOI] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci U S A. 2006;103:16983–8. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–54. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]