Abstract
Introduction
Risk-stratification in acute heart failure syndromes (AHFS) is problematic. A recent set of recommendations describes emergency department (ED) patients with AHFS who do not fulfill high-risk criteria and may be good candidates for observation unit (OU) management. The goal of this analysis was to report on the outcomes experienced by ED patients with AHFS who do not have any of these high-risk criteria.
Methods
We performed a secondary analysis of the HEARD-IT multinational study. HEARD-IT was a multi-center study designed to test the impact of acoustic cardiography on ED decision making in patients with possible AHFS. For the purposes of the current analysis we identified a subset of HEARD-IT patients who did not fulfill any high-risk criteria based on published data. The proportion of these patients who experienced an adverse outcome was determined.
Results
The 201 subjects who fulfilled the inclusion criteria had a mean age of 64 years (SD 13), 61% were male, 34% were Caucasian and 55% were black. There were a total of 25 (12.4%) cardiac events, including 1 death due to AHFS. The majority of the cardiac events were 30-day readmissions related to AHFS (16/25, 64.0%).
Conclusion
AHFS patients at low-risk for subsequent morbidity and mortality based on recent consensus guidelines may be good candidates for early discharge after a brief period of observation in the OU or ED. Additional prospective research is needed to determine the impact of implementation of these criteria in ED patients with AHFS.
Keywords: acute heart failure, low-risk, society of chest pain center, validation
Introduction
Risk-stratification in acute heart failure syndromes (AHFS) is challenging. Over the past few decades many attempts have been made to develop prediction rules to identify subsequent risk in patients with AHFS.[1–10] Their applicability to the ED patient with AHFS has proven limited: 1) retrospective methodology has missed collection of variables that could be available to an emergency physician[1–9]; 2) frequently the only outcome considered was mortality[1–3,6,8]; 3) generally only inpatients were enrolled[1–9]; and 4) often only in-hospital events were considered [1–3,6,9]. Further, since approximately 80% of AHFS patients are admitted to the hospital, models that identify high-risk and the need for admission are arguably less useful than those recognizing low-risk and identifying a cohort eligible for safe, early discharge.[11,12] Lack of high-risk does not necessarily equal low-risk. Finding patient characteristics that identify patients at low-risk of adverse events is important if we are going to change the paradigm from nearly universal hospital admission to that of safe, early discharge.
Largely as a result of our inability to identify AHFS patients at low-risk of subsequent adverse events, the majority of ED patients with AHFS are admitted.[12] Heterogeneous etiologies and multiple comorbidities such as renal dysfunction and hyponatremia confound development of useful risk models adding to the difficulty discerning which variables are most important amongst those available. In the absence of useful decision aids for patients likely to be at low or moderate risk, a reasonable approach to avoiding the need for full hospital admission may be management in an observation unit (OU). Previous research suggests that OU management is a safe, resource conservative option for ED patients with AHFS who lack high-risk features.[13–15] A recent set of recommendations describes ED patients with AHFS who may be good candidates for OU management.[16] External validation of these criteria has not been previously performed.
The aim for this study was to report outcomes experienced by ED patients with AHFS without high-risk features to determine if their observed adverse event rate was low.
Methods
Setting and Patient Population
We performed a secondary analysis of the HEart failure and Audicor technology for Rapid Diagnosis and Initial Treatment (HEARD-IT) multinational study. The primary goal of HEARD-IT was to test the impact of acoustic cardiography on ED decision making in patients with possible AHFS. This trial enrolled patients at 7 United States, 1 Swiss and 1 Taiwanese site, from March through October 2006. To be eligible for HEARD-IT patients had to be at least 40 years of age with dyspnea as a chief complaint. Only patients with dialysis-dependent renal failure, or whose dyspnea was clearly not related to acute heart failure (e.g. penetrating chest injury), were excluded. HEARD-IT was approved by the institutional review board of all participating centers. The detailed methodology has been reported elsewhere.[17]
Patients not meeting high-risk criteria have been suggested to be eligible for an OU stay according to previous recommendations.[16] High-risk criteria are: 1) systolic blood pressure < 100 mmHg; 2) electrocardiogram (ECG) changes consistent with ischemia not known to be old; 3) cardiac Troponin T (>0.1 ng/ml) or cardiac Troponin I (>0.3 ng/ml); 4) renal insufficiency (blood urea nitrogen [BUN] > 40 mg/dl or creatinine > 3 mg/dl), 5) significant hyponatremia (<135 mEq/L).[16]
Data Collection
HEARD-IT prospectively enrolled ED patients, both eventual admissions and discharges, who fulfilled inclusion and exclusion criteria. ED data were collected prospectively by study personnel, including demographics, medical history, physical examination, and ECG findings as documented by the treating emergency physician. Medications administered in the ED and prior to arrival were also recorded. Laboratory tests, chest radiography findings as documented by a board-certified radiologist, and echocardiography reports documented by a board certified cardiologist were obtained from the medical record. At the end of the ED stay, the treating physician recorded whether or not a patient had suspicion of AHFS as a component of the differential diagnosis on a standardized Case Report Form. Those patients who the treating physician suspected of having AHFS were eligible for inclusion in this secondary analysis. All patients were followed by chart review throughout their index stay to document in-hospital events. Thirty and ninety-day follow-up was obtained by telephone interview by study personnel and medical records were reviewed for all patients at the time of follow-up. Study personnel specifically inquired about whether each subject had an ED visit, a hospital admission or death at both 30-days and 90-days after their date of enrollment. The causes for the ED visit, hospitalization or death were then categorized as: 1) due to heart failure; 2) due to a cardiac cause but not heart failure (e.g. acute coronary syndrome, arrhythmia) or 3) other.
Primary Outcome
The primary outcome of interest was a combined outcome of 30-day death or readmission due to cardiac causes.
Statistical Analysis
Patients without high-risk features were described using mean and standard deviation for continuous variables and frequencies and percents for categorical variables. The proportion of patients experiencing an adverse outcome was computed, 95% confidence intervals were obtained using the score method with continuity correction. Univariable logistic regression was used to compare the odds of events among those with and without various signs and symptoms. Analyses were conducted using SPSS v16 (SPSS Inc., Chicago, IL) and Microsoft Excel (Microsoft Corporation, Redmond, WA).
Results
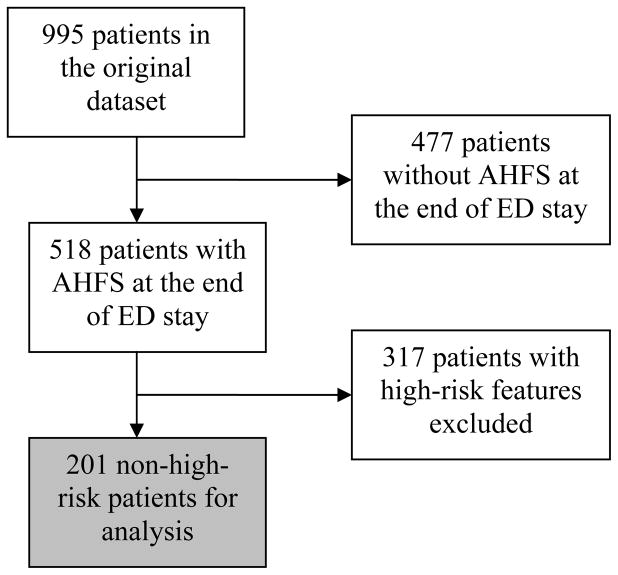
There were 995 subjects included in the original dataset. To identify a non-high-risk cohort, AHFS patients with any of the aforementioned high-risk features were excluded, resulting in 201 subjects, all of whom were successfully followed up.(Figure 1) The non-high-risk cohort had a mean age of 64 years (SD 13), 61% were male, 34% were Caucasian and 55% were black. There were 93 patients (46.3%) in this cohort that were managed in an OU or discharged directly from the ED. (Table 1)
Figure 1.
Identification of non-high-risk cohort for purposes of analysis.
Table 1.
Breakdown of 30-day events based on patient disposition from the ED. Variables are listed as number and percentages. Events are deaths and 30-day readmissions related to cardiac causes.
| No event (N=176) | Event (N=25) | Total (N=201) | |
|---|---|---|---|
| Discharge home | 55 (31.3) | 3 (12.0) | 58 (28.9) |
| Observation | 32 (18.2) | 3 (12.0) | 35 (17.4) 36 (17.9) |
| Admit (floor) | 33 (18.8) | 3 (12.0) | |
| Admit (monitored) | 51 (29.0) | 15 (60.0) | 66 (32.8) |
| Admit (ICU) | 5 (2.8) | 1 (4.0) | 6 (3.0) |
Characteristics of those with and without adverse events
There were 518 cases with AHFS in their differential diagnosis. Of these, 317 were considered high-risk based on the OU criteria. The event rate in these high-risk AHFS patients was 14.8%, of which 5.7% (18/317) were death, and the remainder was due to cardiac or AHFS readmissions. In the non-high-risk cohort of 201 patients, there were a total of 25 (12.4%) cardiac events, including 1 (0.5%) death due to AHFS. The majority of the cardiac events were 30-day readmissions related to AHFS (16/25, 64.0%). Of the 106 patients who were managed in an OU or discharged directly home there were 6 events (7%). The 6 events were comprised of 2 admissions for AHFS and 4 cardiac (non-AHFS) admissions. There were no deaths among patients discharged or admitted to an OU.
Patients with a prior MI or pacemaker and those with cardiomyopathy due to alcohol or cocaine use had a greater odds of experiencing an adverse event than those without these findings.(Table 2) Elevated BNP and serum sodium level also increased the odds of an event, while an elevated temperature was associated with decreased odds of an event. Vital signs and a number of parameters described in the inpatient population as being important predictors of outcomes (e.g. hemoglobin) were not helpful in identifying patients at lower-risk of subsequent adverse outcomes.(Table 2).
Table 2.
Demographics, medical history, symptoms, physical exam variables and test results in the overall non-high-risk cohort stratified by 30-day adverse event. Continuous variables are described using means and standard deviations, categorical variables are described using frequencies and percentages. *For continuous data, odds ratios represent the change in odds for a unit increase in the value of the variable or, in the case of BNP, for a 100 unit increase. The number of cases with each variable measured is also shown.
| No event | Event | OR | 95%CI | P-Value | |
|---|---|---|---|---|---|
| Age at enrollment | 64 (13) | 60 (14) | 0.98 | (0.95 – 1.01) | 0.175 |
| Male | 105 (59.7) | 18 (72.0) | 1.74 | (0.69 – 4.38) | 0.240 |
| Vital signs | |||||
| Heart Rate | 89 (19) | 92 (27) | 1.01 | (0.99 – 1.03) | 0.385 |
| Temperature (F) | 97.8 (0.9) | 97.3 (1.0) | 0.61 | (0.38 – 0.98) | 0.040 |
| Systolic BP | 149 (26) | 156 (35) | 1.01 | (0.99 – 1.02) | 0.219 |
| Diastolic BP | 84 (19) | 87 (24) | 1.01 | (0.99 – 1.03) | 0.429 |
| Resp Rate | 22 (5) | 23 (5) | 1.03 | (0.96 – 1.11) | 0.386 |
| Pulse Ox | 95 (4) | 95 (7) | 0.97 | (0.89 – 1.05) | 0.455 |
| Labs | |||||
| BNP$ | 709 (845) | 1260 (917) | 1.06 | (1.02 – 1.11) | 0.008 |
| Sodium | 140 (3) | 141 (4) | 1.15 | (1.01 – 1.30) | 0.030 |
| Glucose | 124 (60) | 131 (49) | 1.00 | (0.99 – 1.01) | 0.584 |
| BUN | 19.4 (7.9) | 21.0 (8.3) | 1.02 | (0.97 – 1.08) | 0.356 |
| Creatinine | 1.1 (0.4) | 1.2 (0.3) | 1.54 | (0.59 – 3.99) | 0.379 |
| Hemoglobin | 12.5 (2.2) | 11.9 (3.3) | 0.91 | (0.76 – 1.08) | 0.263 |
| X-ray findings | |||||
| Pneumonia | 8 (4.9) | 0 (0.0) | ND | ||
| Cephalization | 17 (10.4) | 3 (12.0) | 1.17 | (0.32 – 4.33) | 0.813 |
| Pleural Effusion | 31 (19.0) | 8 (32.0) | 2.00 | (0.79 – 5.06) | 0.142 |
| Interstitial Edema | 50 (30.7) | 11 (44.0) | 1.78 | (0.75 – 4.18) | 0.189 |
| Symptoms | |||||
| Wheezing | 38 (22.9) | 5 (20.8) | 0.89 | (0.31 – 2.53) | 0.822 |
| Rales | 76 (45.2) | 16 (64.0) | 2.15 | (0.90 – 5.14) | 0.085 |
| Rhonchi | 20 (12.3) | 3 (13.0) | 1.07 | (0.29 – 3.94) | 0.916 |
| S3 | 11 (7.6) | 3 (13.6) | 1.91 | (0.49 – 7.47) | 0.353 |
| Elevated JVP | 35 (22.9) | 6 (28.6) | 1.35 | (0.49 – 3.74) | 0.565 |
| Edema | 97 (57.4) | 13 (54.2) | 0.88 | (0.37 – 2.07) | 0.765 |
| HF Etiology | |||||
| Alcohol | 1 (0.9) | 2 (11.1) | 13.38 | (1.15 – 156.13) | 0.039 |
| Cocaine | 1 (0.9) | 3 (16.7) | 21.40 | (2.09 – 219.24) | 0.010 |
| Hypertensive | 11 (10.2) | 3 (16.7) | 1.76 | (0.44 – 7.06) | 0.423 |
| Ischemic | 16 (14.8) | 3 (16.7) | 1.15 | (0.30 – 4.43) | 0.839 |
| Valvular | 7 (6.5) | 1 (5.6) | 0.85 | (0.10 – 7.34) | 0.882 |
| Other/Unknown | 76 (70.4) | 12 (66.7) | 0.84 | (0.29 – 2.44) | 0.751 |
| Medical History | |||||
| Chronic Renal | 21 (12.4) | 3 (12.5) | 1.01 | (0.28 – 3.67) | 0.992 |
| HF | 110 (63.6) | 21 (84.0) | 3.01 | (0.99 – 9.15) | 0.053 |
| COPD | 40 (23.7) | 9 (37.5) | 1.94 | (0.79 – 4.76) | 0.150 |
| DM | 70 (40.9) | 11 (44.0) | 1.13 | (0.49 – 2.64) | 0.771 |
| Hyperlipidemia | 59 (34.9) | 11 (44.0) | 1.46 | (0.63 – 3.43) | 0.379 |
| Hypertension | 141 (81.0) | 18 (72.0) | 0.60 | (0.23 – 1.56) | 0.296 |
| MI | 35 (20.3) | 10 (41.7) | 2.80 | (1.15 – 6.82) | 0.024 |
| Pulmonary Embolism | 7 (4.1) | 2 (8.3) | 2.12 | (0.41 – 10.84) | 0.368 |
| CABG | 30 (17.4) | 8 (32.0) | 2.23 | (0.88 – 5.63) | 0.091 |
| PCI/Stent | 23 (13.6) | 4 (16.7) | 1.27 | (0.40 – 4.05) | 0.687 |
| Stroke | 32 (18.5) | 1 (4.2) | 0.19 | (0.02 – 1.47) | 0.112 |
| Pacemaker, ICD, CRT | 26 (15.1) | 10 (41.7) | 4.01 | (1.61 – 9.99) | 0.003 |
| Prosthetic Valve | 7 (4.1) | 1 (4.2) | 1.02 | (0.12 – 8.66) | 0.987 |
BP=blood pressure; BNP=b-type natriuretic peptide; BUN=blood urea nitrogen; JVP=jugular venous pressure; HF= heart failure;COPD=chronic obstructive pulmonary disease; DM= diabetes mellitus; MI=myocardial infarction; CABG= coronary artery bypass graft; PCI= percutaneous coronary intervention; ICD=implantable cardioverter/defibrillator; CRT= chronic resynchronization therapy; ND-not done.
Limitations
While our data show a low adverse event rate among those patients eligible for observation, the exact event time relative to ED presentation was not known. An event that occurs 3 days after ED or hospital discharge is likely more related to acute management decisions than that which occurs 29 days after discharge. Further, while patients hospitalized were included in the non-high-risk cohort, the impact of the possible protective (or damaging) effect of hospitalization is difficult to extrapolate. Had these patients been managed in an OU or discharged directly from the ED their adverse events may have been different. These criteria are meant to be used for guidance along with clinician gestalt, and not in place of it. Before they can be implemented unilaterally in clinical practice they need to be tested in a prospective fashion.
While AHFS was considered by the treating ED physician to be a possibility in all subjects, the actual role of AHFS in the acute presentation is unknown. Patients may have had more than one etiology for dyspnea. While this is reflective of clinical practice and makes our findings generalizable to subjects where “heart failure is considered as an etiology at the end of the ED stay” it may not have been the sole cause of dyspnea. Finally, while HEARD-IT attempted to enroll the undifferentiated dyspneic patient who may have AHFS, only those patients who were able to give consent could be enrolled.
This could have introduced selection bias, resulting in much higher-risk patients being excluded from participation. Finally, multiple univariable comparisons were made to determine whether any of the clinical characteristics were associated with adverse events. This analysis was meant to be exploratory. The large number of comparisons increases the likelihood that there would be a finding of significance based on chance alone.
Discussion
Our results from this prospective, ED-based study suggest that patients who do not fulfill the previous high-risk criteria are at low-risk for subsequent mortality. Our adverse event rate is similar to, or lower than that observed in other heart failure studies, including those that studied patients in the OU.[15,18] The difference in the overall event rate between the high-risk and non-high-risk cohorts was not significantly different. However, there was only one (0.5%) death in the non-high-risk cohort compared to 18 (5.7%) in the high-risk group.
The majority of recent prognostic studies in patients with AHFS continue to try to identify variables that place patients at high-risk of subsequent events. How does a marker of high-risk impact ED decision making when the default decision is admission in the vast majority of patients? We are in need of prospective studies that identify risk-profiles associated with a low-risk of subsequent morbidity and mortality. The potential impact of this line of research should not be underestimated. Identifying an additional 10% of patients who could be safely discharged home after a period of observation in the ED or OU would result in significant cost savings. This is the focus of two ongoing studies supported by the National Heart, Lung and Blood Institute.[19] The two studies aim to answer the following two questions: 1) Does this ED patient with AHFS need to be admitted, and if so to what level of care? 2) In this ED patient who is admitted to the hospital, what is the earliest time point they can be safely discharged home?
Readily available data such as medical comorbidities, and either elevated BNP or serum sodium levels may help identify patients who are at increased risk for recidivism in this non-high-risk cohort. While this may not impact the decision to admit or discharge, it may impact discharge planning to prevent an unscheduled visit. Unlike previous studies, our data were not based on retrospectively identified patients whose hospital discharge diagnosis may not have been reflective of their acute ED presentation. Only patients whom the treating ED physician considered to have AHFS were included in this study. However, in order to be enrolled in HEARD-IT patients were required to give informed consent, thus limiting the proportion of patients presenting in-extremis that could be enrolled. This may have partially explained the unexpected small difference we observed in overall event rates between the high-risk and non-high-risk groups. Moreover, given the limitations of confounding related to hospitalization, a prospective study of disposition decision making guided by these OU recommendations is needed before we can definitively determine whether they provide a useful addition to clinical gestalt.
Heart failure is characterized as a chronic underlying disease process interspersed with acute episodes of clinical worsening, often manifested as signs and symptoms of congestion. This cyclical nature is typical of other chronic diseases such as diabetes or chronic obstructive pulmonary disease where temporary clinical worsening is the rule rather than the exception. It is expected that a subset of AHFS patients who are discharged from the ED, OU or hospital will be readmitted within 30-days. Consistent with recently published data, the vast majority of the observed events in this analysis were due to readmission related to AHFS or cardiac causes.[20] Patient decision making has a significant impact on readmission. Non-adherence to medication and diet, previous AHFS admissions, and poor social support have been related to AHFS readmissions.[21,22]Interventions aimed at education, dietary and medication assessment, discharge planning and close outpatient follow-up reduce AHFS readmissions.[23] Thus, it is not surprising that we found an association between subjects with poor health behavior, such as alcohol and cocaine use, and an increased risk for readmission.
The timing of events relative to ED and OU discharge and their relationship to the index stay is perhaps the most compelling issue that needs to be rigorously studied in future efforts. Those events that occur soon thereafter discharge (i.e. 5 days) likely have an association with acute therapeutic and disposition decisions. Events occurring 25–30 days, or later, after OU or ED discharge are less likely related to the prior acute presentation. We would suggest that these readmissions are not unexpected, and two brief periods of OU management may be more cost-effective at reducing total hospital days in the hospital when compared to one prolonged inpatient admission.[13,15]
There are several possible limitations to consider when interpreting our results. While our data show a low adverse event rate among those patients eligible for observation, the exact event time relative to ED presentation was not known. An event that occurs 3 days after ED or hospital discharge is likely more related to acute management decisions than that which occurs 29 days after discharge. Further, while patients hospitalized were included in the non-high-risk cohort, the impact of the possible protective (or damaging) effect of hospitalization is difficult to extrapolate. Had these patients been managed in an OU or discharged directly from the ED their adverse events may have been different. These criteria are meant to be used for guidance along with clinician gestalt, and not in place of it. Before they can be implemented unilaterally in clinical practice they need to be tested in a prospective fashion.
While AHFS was considered by the treating ED physician to be a possibility in all subjects, the actual role of AHFS in the acute presentation is unknown. Patients may have had more than one etiology for dyspnea. While this is reflective of clinical practice and makes our findings generalizable to subjects where “heart failure is considered as an etiology at the end of the ED stay” it may not have been the sole cause of dyspnea. Finally, while HEARD-IT attempted to enroll the undifferentiated dyspneic patient who may have AHFS, only those patients who were able to give consent could be enrolled. This could have introduced selection bias, resulting in much higher-risk patients being excluded from participation. Finally, multiple univariable comparisons were made to determine whether any of the clinical characteristics were associated with adverse events. This analysis is exploratory in nature and should be tempered by the large number of comparisons, which increases the chance of a type I error.
Conclusion
Those patients without high-risk features during their ED evaluation are at low-risk for subsequent morbidity and mortality and may be good candidates for early discharge after a brief period of observation in the OU or ED. Whether subsequent re-admissions could have been prevented by taking different action at the time of the presentation is unknown; future studies should attempt to relate adverse events to the visit so that decision making can occur relative to modifiable outcomes.
Acknowledgments
The authors would like to acknowledge Ms. Sarah Brown for her assistance in manuscript preparation.
Supported in part by 1 K23 HL085387-01A2 and 1 1R01HL088459
Inovise Medical, Inc. provided study support for patient enrollment and data management. Analysis and manuscript production was performed independently by the investigators. Three of the authors, Drs. Frank Peacock, Deborah Diercks and Alan Maisel serve as members of the Inovise Scientific Advisory Board. I have previously received honoraria from Inovise Medical, Inc.
Contributor Information
Sean P. Collins, University of Cincinnati, Department of Emergency Medicine.
Christopher J. Lindsell, University of Cincinnati, Department of Emergency Medicine.
Allen J. Naftilan, Vanderbilt University, Division of Cardiology.
W. Frank Peacock, Cleveland Clinic Foundation, Department of Emergency Medicine.
Deborah Diercks, Department of Emergency Medicine, University of California-Davis.
Brian Hiestand, Department of Emergency Medicine, The Ohio State University.
Alan Maisel, Division of Cardiology, San Diego VA Medical Center.
Alan B. Storrow, Vice Chair for Research and Academic Affairs, Vanderbilt University, Department of Emergency Medicine.
References
- 1.Formiga F, Chivite D, Manito N, Casas S, Riera A, Pujol R. Predictors of in-hospital mortality present at admission among patients hospitalised because of decompensated heart failure. Cardiology. 2007;108:73–78. doi: 10.1159/000095885. [DOI] [PubMed] [Google Scholar]
- 2.Rohde LE, Goldraich L, Polanczyk CA, Borges AP, Biolo A, Rabelo E, Beck-Da-Silva L, Clausell N. A simple clinically based predictive rule for heart failure in-hospital mortality. J Card Fail. 2006;12:587–593. doi: 10.1016/j.cardfail.2006.06.475. [DOI] [PubMed] [Google Scholar]
- 3.Barsheshet A, Garty M, Grossman E, Sandach A, Lewis BS, Gottlieb S, Shotan A, Behar S, Caspi A, Schwartz R, Tenenbaum A, Leor J. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006;166:1613–1619. doi: 10.1001/archinte.166.15.1613. [DOI] [PubMed] [Google Scholar]
- 4.Auble TE, Hsieh M, Gardner W, Cooper GF, Stone RA, McCausland JB, Yealy DM. A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med. 2005;12:514–521. doi: 10.1197/j.aem.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh M, Auble TE, Yealy DM. Validation of the acute heart failure index. Ann Emerg Med. 2008;51:37–44. doi: 10.1016/j.annemergmed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O’Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 9.Chin MH, Goldman L. Correlates of major complications or death in patients admitted to the hospital with congestive heart failure. Arch Intern Med. 1996;156:1814–1820. [PubMed] [Google Scholar]
- 10.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 11.Smith WR, Poses RM, McClish DK, Huber EC, Clemo FL, Alexander D, Schmitt BP. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest. 2002;121:1610–1617. doi: 10.1378/chest.121.5.1610. [DOI] [PubMed] [Google Scholar]
- 12.Graff L, Orledge J, Radford MJ, Wang Y, Petrillo M, Maag R. Correlation of the agency for health care policy and research congestive heart failure admission guideline with mortality: Peer review organization voluntary hospital association initiative to decrease events (provide) for congestive heart failure. Ann Emerg Med. 1999;34:429–437. doi: 10.1016/s0196-0644(99)80043-2. [DOI] [PubMed] [Google Scholar]
- 13.Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: Preliminary analysis of safety and cost. Congest Heart Fail. 2005;11:68–72. doi: 10.1111/j.1527-5299.2005.03844.x. [DOI] [PubMed] [Google Scholar]
- 14.Peacock WFt, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19:209–232. doi: 10.1016/s0733-8627(05)70176-0. [DOI] [PubMed] [Google Scholar]
- 15.Peacock WFt, Young J, Collins S, Diercks D, Emerman C. Heart failure observation units: Optimizing care. Ann Emerg Med. 2006;47:22–33. doi: 10.1016/j.annemergmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Peacock WF, Fonarow GC, Ander DS, Maisel A, Hollander JE, Januzzi JL, Jr, Yancy CW, Collins SP, Gheorghiade M, Weintraub NL, Storrow AB, Pang PS, Abraham WT, Hiestand B, Kirk JD, Filippatos G, Gheorghiade M, Pang PS, Levy P, Amsterdam EA. Society of chest pain centers recommendations for the evaluation and management of the observation stay acute heart failure patient: A report from the society of chest pain centers acute heart failure committee. Crit Pathw Cardiol. 2008;7:83–86. doi: 10.1097/01.hpc.0000317706.54479.a4. [DOI] [PubMed] [Google Scholar]
- 17.Collins SP, Peacock WF, Lindsell CJ, Clopton P, Diercks DB, Hiestand B, Hogan C, Kontos MC, Mueller C, Nowak R, Chen WJ, Huang CH, Abraham WT, Amsterdam E, Breidthardt T, Daniels L, Hasan A, Hudson M, McCord J, Naz T, Wagoner LE, Maisel A. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Ann Emerg Med. 2009 doi: 10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Storrow AB, Collins S, Lindsell CJ, Disalvo T, Han J, Weintraub NL. Improving heart failure risk stratification in the ed: Stratify 1r01hl088459-01; treatment endpoints in acute decompensated heart failure 1k23hl085387-01a2. Vanderbilt University and University of Cincinnati, NHLBI; 2007. [Google Scholar]
- 20.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 21.Vinson JM, Rich MW, Sperry JC, Shah AS, McNamara T. Early readmission of elderly patients with congestive heart failure. J Am Geriatr Soc. 1990;38:1290–1295. doi: 10.1111/j.1532-5415.1990.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, Crombie P, Vaccarino V. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–89. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 23.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]