Abstract
STAT3 plays important roles in cell proliferation and survival signaling and is often constitutively activated in transformed cells. In this study, we examined STAT3 activation in endothelial cells (EC) during angiogenic activation and therapeutic angiogenesis inhibition. VEGF stimulation of cultured EC induced STAT3 phosphorylation by a VEGFR2- and Src-dependent mechanism. FGF2 but not PlGF also induced EC STAT3 activation in vitro. Activated STAT3 mediated VEGF induction of EC Bcl-2 and contributed to VEGF protection of EC from apoptosis. In vivo, p-STAT3 was absent by immunohistological staining in the vascular EC of most normal mouse organs but was present in the vessels of mouse and human tumors. Tumor vascular p-STAT3 increased as tumors were induced to overexpress VEGF, indicating that VEGF is an activator of EC p-STAT3 in vivo. Tumor vascular p-STAT3 decreased during angiogenesis inhibition by antagonists of VEGF-VEGFR signaling, VEGF Trap and SU5416, indicating that VEGF contributed to the EC STAT3 activation seen in the tumors prior to treatment and that p-STAT3 may be used to monitor therapy. These studies show that p-STAT3 is a mediator and biomarker of endothelial activation that reports VEGF-VEGFR2 activity and may be useful for studying the pharmacodynamics of targeted angiogenesis inhibitors.
Keywords: endothelial cells, signal transduction, angiogenesis, antiangiogenic therapy, tumor, biomarker, STAT3, VEGF, VEGFR2, Bcl-2
Introduction
Formation of new blood vessels is necessary for sustained tumor growth.1,2 Many factors contribute to tumor neovascularization, but vascular endothelial growth factor (VEGF) is among the most critical of these factors.3,4 VEGF production is stimulated by hypoxia and is upregulated by the activity of certain oncogenes and proto-oncogenes and inactivation of certain tumor suppressor genes.5–7 These are common occurrences in cancers, making VEGF probably ubiquitously expressed in tumors. The importance of VEGF in tumor neovascularization is shown by the ability of VEGF inhibitors to retard tumor growth in preclinical and clinical therapeutic settings.8 Bevacizumab, a humanized anti-VEGF monoclonal antibody,8,9 is the first FDA-approved agent designed to inhibit angiogenesis10 and VEGF Trap, a chimeric decoy receptor, is another VEGF inhibitor in clinical trials.11
VEGF activates signaling in endothelial cells (EC) after binding cognate receptors on the cell surface. Its two best characterized receptors are the tyrosine kinases, VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2). VEGFR2 signaling activates a variety of downstream mediators in EC, including Src, Ras and members of the PI3K-AKT and Raf-MEK-ERK pathways12 and is responsible for many of the characteristic effects of VEGF on EC, including cell proliferation, survival, chemotaxis and increased vascular permeability.13–15 Antibody16 and small molecule kinase inhibitors of VEGFR2,17,18 have been shown to inhibit tumor angiogenesis and one such inhibitor, sunitinib,19 is FDA-approved for the treatment of renal cell carcinoma.
Members of the signal transducer and activator of transcription (STAT) family of latent transcription factors directly mediate signaling from the cell membrane to the nucleus. Cell stimulation by a variety of growth factors and cytokines induce STAT phosphorylation and activation by JAK, Src-family and other tyrosine kinases, resulting in their dimerization and nuclear translocation.20 STAT3 is activated by kinases with oncogenic potential and is constitutively activated in a variety of tumor types.21,22 In turn, it activates genes associated with cell proliferation and survival.23 Recent evidence has suggested that STAT3 may be involved in VEGF-induced EC signaling and activation,24,25 but the evidence has been confusing and its role in endothelial activation remains unclear. Studies presented here demonstrate that STAT3 is activated upon VEGF stimulation of EC in vitro and in vivo by a VEGFR2-dependent and Src-dependent mechanism and that STAT3 activation mediates Bcl-2 induction by VEGF. Activated STAT3 (p-STAT3) is observed in tumor endothelium and its level decreases with antiangiogenic therapeutic manipulations that inhibit VEGF-VEGFR2-induced signaling.
Results
STAT3 is activated in endothelial cells of tumors and lung but not other normal organs
A search for signaling pathways activated in angiogenic endothelium led us to stain histological sections of K1735 murine melanoma with an antibody specific for the activated, Y705 phosphorylated form of STAT3 (p-STAT3). Immunostaining for p-STAT3 by IHC (DAB chromogen) and for CD34 by IHC (SG blue chromogen) or by IF to reveal vessels and counterstaining with hematoxylin (Fig. 1A–D), approximately 22% ± 4% (Mean ± SD) of K1735 tumor vessels stained for p-STAT3 by IHC (more magnified Fig. 1B and C show vessel EC staining for p-STAT3 and D shows EC not staining for p-STAT3). Staining in the EC of these vessels was nuclear and all EC in a vessel tended to stain the same way for p-STAT3. We determined that STAT3 activation was common in tumor vasculature when we found that murine RENCA renal cell carcinomas (Fig. 1E and F) and Lewis lung carcinomas (LLC; not shown) had 13% ± 2% (Mean ± SD) and 26% ± 4% (Mean ± SD) p-STAT3 positive vessels, respectively. The nuclei of a substantial proportion of the malignant cells in these tumors also stained for p-STAT3.
Figure 1.
STAT3 is activated in endothelial cells of tumors but not in endothelial cells of most murine normal organs. Thin sections of paraffin-embedded K1735 (A–D) and RENCA (E and F) murine tumors, normal mouse organs, kidney (G and H), liver (I and J) and lung (M and N), and human colorectal cancer (O and P) were stained with anti-p-STAT3 antibody using DAB chromogen (brown) and with anti-CD34 antibody using SG blue chromogen (blue in A, B, G, H–J, O and P) or by immunofluorescence (green in C–F, M and N). Regions of 400X fields of view (A, E, G, I, M, O) outlined by red boxes are shown at higher magnification in the ensuing panels (B, F, H, J, N and P) to reveal detail. Examples of endothelial cell (EC) nuclei staining for p-STAT3 are pointed out by red arrows; examples of EC nuclei not staining for p-STAT3 in tumors are pointed out by black arrows in (D) and are abundant in normal organs (H and J) except the lung. Note that a large fraction of tumor cell nuclei stain positively for p-STAT3 (B–D and F). Normal mouse liver (K and higher magnification, L) was stained with anti-STAT3 antibody using DAB chromogen and with anti-CD34 antibody using SG blue chromogen.
In contrast, p-STAT3 immunostaining was not seen in the vessels of most normal mouse organs examined (<2% of vessels in kidney, liver, spleen, mammary gland, small intestines and large intestines) (kidney shown in Fig. 1G and H and liver shown in and J). STAT3 was present in EC of normal mouse organs (liver shown in Fig. 1K and L), indicating that the absence of p-STAT3 was not due to absence of the parent protein. An exception among normal mouse organs was the lung, where pulmonary EC stained for nuclear p-STAT3 (Fig. 1M and N). Nuclear p-STAT3 was also found in the EC of human cancers. In 12 human colorectal carcinomas, we found a mean of 20% (range 11%–27%) of tumor vessels immunostaining for p-STAT3 (Fig. 1O and P).
VEGF activation of STAT3 in endothelial cells is VEGFR2- and Src-dependent
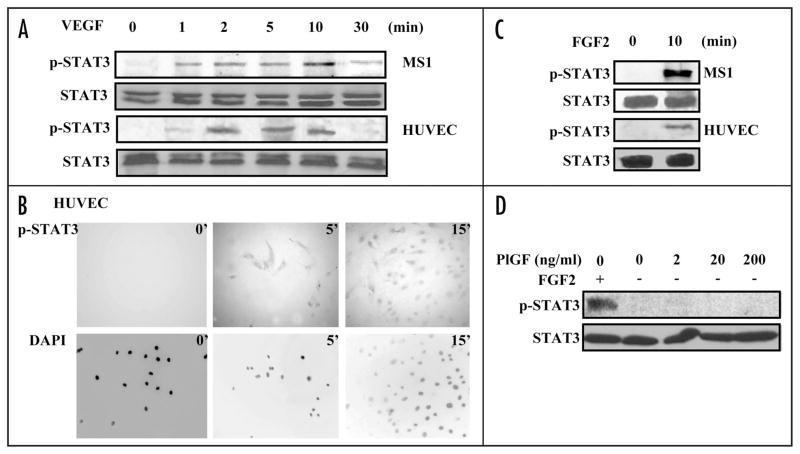
To understand the in vivo association of p-STAT3 with tumor endothelium, we studied STAT3 activation in EC following VEGF stimulation in vitro. STAT3 but not p-STAT3 was detected in Western blots of lysates of human umbilical vein endothelial cells (HUVEC) and MS1 endothelial cells (immortalized tumor microvascular endothelial cells)30 cultured in media containing .5% fetal calf serum. Addition of 10 ng/ml VEGF-A 165 amino acid isoform (VEGF) rapidly induced STAT3 activation in these cells without a change in STAT3 levels (Fig. 2A). Immunostaining of these cells confirmed the rapid induction of p-STAT3 in EC by VEGF and showed, in addition, its translocation to nuclei (Fig. 2B and Suppl. Fig. 1). STAT3 could be activated in EC by growth factors other than VEGF, as shown by the ability of fibroblast growth factor 2 (FGF2) to induce p-STAT3 (Fig. 2C). However, placenta growth factor (PlGF), which is a ligand for VEGFR1, failed to activate STAT3 in EC (Fig. 2D).
Figure 2.
VEGF and FGF2 activate STAT3 in endothelial cells. HUVEC and MS1 endothelial cells were cultured in medium containing 0.5% serum for 24 hours and then cultured in medium containing 10 ng/ml VEGF (A and B) or 25 ng/ml FGF2 (C) for different durations, or 2–200 ng/ml PlGF (D) in HUVEC cell for 10 minutes. Western blots of cell lysates were probed for p-STAT3, stripped and reprobed for STAT3 (A, C and D). HUVEC cells were fixed and stained using anti-p-STAT3 antibody. DAPI counterstaining of nuclei is shown (B). All experiments were performed twice with similar results.
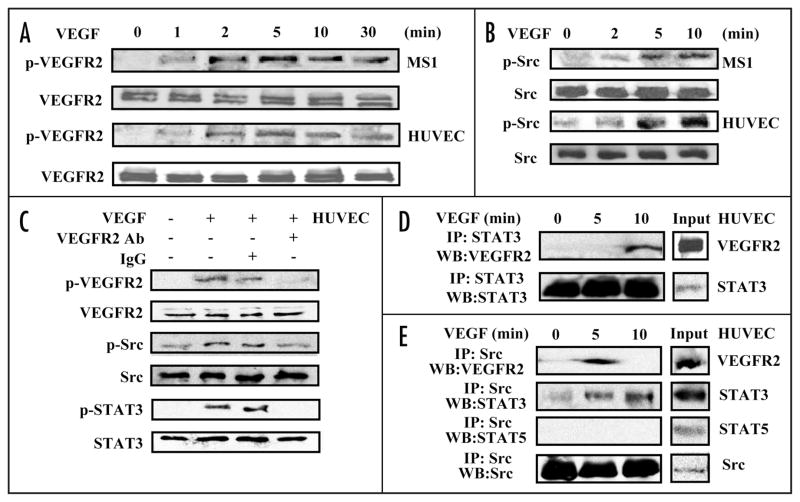
We examined VEGFR2, which mediates many of VEGF’s effects on EC, as a potential mediator of p-STAT3 induction by VEGF. As expected, VEGF treatment of HUVEC and MS1 cells resulted in VEGFR2 phosphorylation (Fig. 3A). VEGF treatment also induced phosphorylation of Src, although low-level Src activation could be seen at baseline (Fig. 3B). Pretreatment of HUVEC with an anti-human VEGFR2 antibody previously shown to inhibit receptor activation31 prevented VEGF activation of VEGFR2, Src and STAT3, suggesting that VEGFR2 mediated VEGF induction of STAT3 activation (Fig. 3C). Next, we performed co-immunoprecipitation studies to examine whether these kinases become physically associated with STAT3 following VEGF stimulation. Immunoprecipitation of STAT3 followed by blotting for VEGFR2 revealed that these two proteins were physically associated in HUVEC lysates following VEGF stimulation (Fig. 3D). Src immunoprecipitation followed by blotting for VEGFR2 revealed that these two were also associated after VEGF stimulation. STAT3, but not STAT5, also co-immunoprecipitated with Src, although this association was detectable at low levels before VEGF treatment and became more pronounced following treatment (Fig. 3E). Similar results were obtained in co-immunoprecipitation studies performed on MS1 cell lysates following VEGF treatment (Suppl. Fig. 2).
Figure 3.
VEGF induces endothelial cell VEGFR2, Src and STAT3 activation and intermolecular association. HUVEC and MS1 endothelial cells were cultured in medium containing 0.5% serum for 24 hours and then cultured in medium containing 10 ng/ml VEGF for different durations. Western blots of the lysates were probed with antibody specific for phospho-VEGFR2, stripped and reprobed for VEGFR2 (A), and probed with antibody specific for phospho-Src, stripped and reprobed for Src (B). HUVEC cells were pretreated with anti-human VEGFR2 inhibitory antibody (1 μg/ml) or IgG (1 μg/ml) for one hour and were stimulated with 10 ng/ml VEGF for 10 min. Western blots of the lysates were probed with antibody specific for phospho-VEGFR2, phospho-Src, phospho-STAT3, stripped and reprobed for VEGFR2, Src and STAT3 (C). HUVEC lysates were immunoprecipitated with anti-STAT3 antibody and Western blots of the precipitate were probed with antibody to VEGFR2, stripped and reprobed with antibody to STAT3 (D). HUVEC lysates were immunoprecipitated with anti-Src antibody and Western blots of the precipitate were probed sequentially with antibodies to VEGFR2, STAT3, STAT5 and Src (E). All experiments were performed twice with similar results.
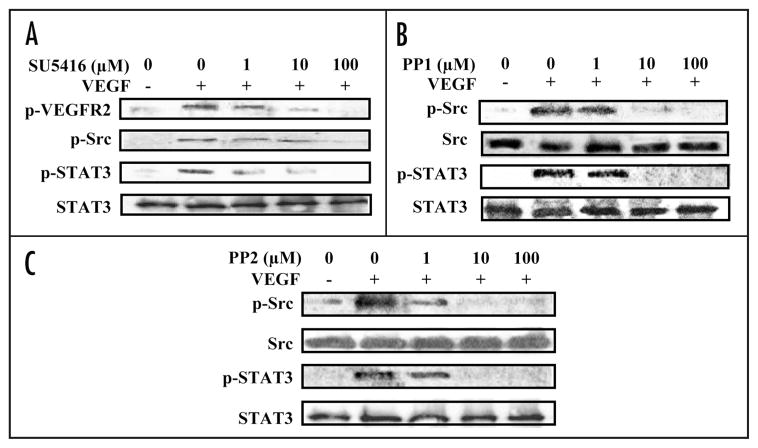
The association of STAT3 with VEGFR2 and with Src following VEGF treatment led us to use inhibitors to test the functional relationship between these kinases and STAT3 activation. As expected, exposure of HUVEC to the small molecule VEGFR2 kinase inhibitor, SU5416,32 prevented VEGF induction of VEGFR2 phosphorylation in a dose-dependent manner. SU5416 treatment also inhibited VEGF induction of Src and STAT3 phosphorylation (Fig. 4A). Treatment with Src inhibitor PP1 or PP2,33 inhibited Src phosphorylation due to VEGF stimulation and also inhibited STAT3 phosphorylation (Fig. 4B and C). The pattern of inhibition by this panel of agents indicated that VEGF induction of EC STAT3 phosphorylation is dependent on VEGFR2 and Src.
Figure 4.
VEGFR2 or Src inhibition abrogates STAT3 activation by VEGF. HUVEC were cultured in medium containing 0.5% serum for 24 hours and exposed to different concentrations of SU5416, PP1 or PP2 inhibitor for one hour. VEGF (10 ng/mg) was added, and cell lysates were prepared 10 minutes later. Western blots of lysates from cells treated with SU5416, a VEGFR2 inhibitor, were probed with antibodies to p-VEGFR2, p-Src and p-STAT3; the last blot was stripped and reprobed with antibody to STAT3 (A). Blots of lysates from cells treated with c-Src inhibitors PP1 (B) or PP2 (C) were probed with antibodies to p-Src and p-STAT3; these blots were stripped and reprobed with antibodies to Src and STAT3, respectively. All experiments were performed twice with similar results.
STAT3 mediates VEGF induction of Bcl-2 and pro-survival effects in EC
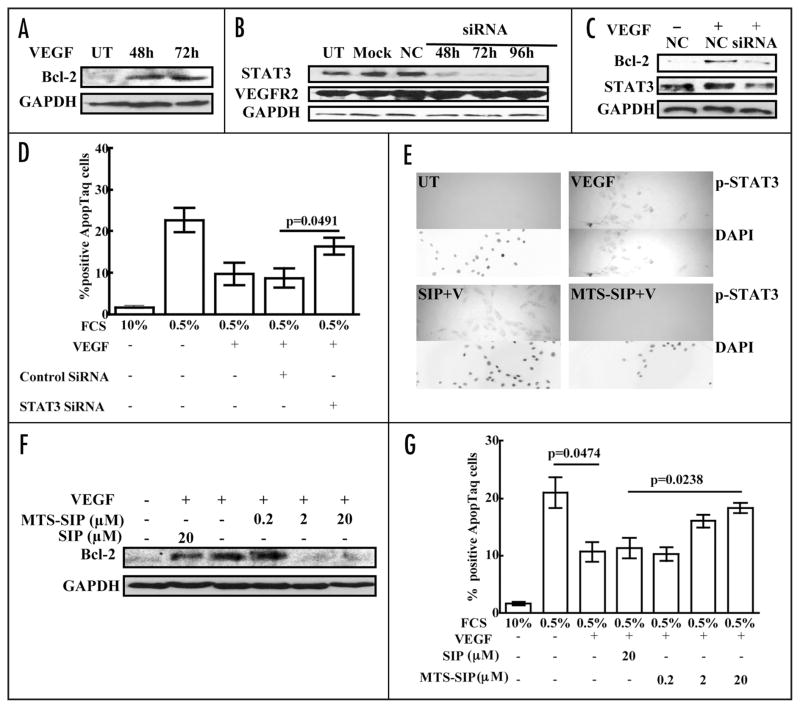
The activation of STAT3 by VEGF suggested it had a role in mediating VEGF effect in EC. VEGF was previously shown to induce Bcl-2 in EC34 and STAT3 is known to regulate Bcl-2 expression in other cell types.35,36 These observations prompted us to examine induction of Bcl-2 as a potential role for STAT3 during EC stimulation by VEGF (Fig. 5A). Transfection of STAT3 siRNA specifically reduced STAT3 levels in HUVEC (Fig. 5B) and attenuated VEGF induction of Bcl-2 in these cells (Fig. 5C). This effect was specific, as control siRNA (NC) had no effect on STAT3 levels and did not inhibit Bcl-2 induction by VEGF. The STAT3-dependence of VEGF induction of Bcl-2 and the demonstrated importance of Bcl-2 for VEGF protection from EC death37 suggested that STAT3 siRNA treatment might have an effect on HUVEC survival. To examine this, we placed HUVEC in low-serum medium. HUVEC cultured in medium with 10% FCS had 1% TUNEL-positive cells, whereas those cultured in medium with 0.5% FCS had 23% TUNEL-positive cells. The presence of 100 ng/ml VEGF in 0.5% FCS medium reduced HUVEC death to 10% TUNEL-positive cells (Fig. 5D), showing that VEGF partially prevented apoptosis due to serum withdrawal. HUVEC transfected with STAT3 siRNA and placed in 0.5% FCS medium containing 100 ng/ml VEGF had 16% TUNEL-positive cells, while cells transfected with control siRNA had 9% TUNEL-positive cells. These results show that STAT3 inhibition significantly impaired VEGF promotion of EC survival.
Figure 5.
VEGF induces Bcl-2 and protects endothelial cells from death by a STAT3-dependent mechanism. HUVEC were placed in serum-free medium overnight, stimulated with medium containing 0.5% serum + VEGF (100 ng/ml) for 48 or 72 hours. Blots of the lysates were probed with antibody to Bcl-2, stripped and reprobed with antibody to GAPDH (A). HUVEC were transfected with STAT3 siRNA (siRNA; 100 nM) for 48, 72 or 96 hours, transfected with negative control siRNA (NC; 100 nM) for 96 hours, mock transfected (Mock) or untransfected (UT). Blots were probed sequentially with antibodies to STAT3, VEGFR2 and GAPDH (B). HUVEC were transfected with STAT3 siRNA (siRNA; 100 nM) or negative control siRNA (NC; 100 nM) for 72 hours. The transfected cells were cultured in serum-free medium overnight and then placed in medium containing 0.5% serum + VEGF (100 ng/ml). Cell death was assayed after 24 hours and cell lysates were prepared after 48 hours. Blots were probed with antibodies to Bcl-2 and STAT3, stripped and reprobed with antibody to GAPDH (C). Cell death was assayed by TUNEL staining (D). This experiment was performed a total of three times with similar results. HUVEC were treated with 20 μM p-STAT3 inhibitory peptide linked to a membrane translocating sequence (MTS-SIP) or with unlinked SIP at 20 μM in serum-free medium for 16 hours. They were then stimulated with VEGF (100 ng/ml) for 10 minutes. Cells were fixed and stained using anti-p-STAT3 antibody. DAPI counterstaining of nuclei is shown (E). HUVEC were treated with 0.2, 2 or 20 μM p-STAT3 MTS-SIP or with unlinked SIP at 20 μM in serum-free medium for 16 hours. They were then cultured in medium containing 0.5% serum + VEGF (100 ng/ml) and the peptides. Cell death was assayed after 24 hours and cell lysates were prepared after 48 hours. Blots were probed with antibodies to Bcl-2, stripped and reprobed with antibody to GAPDH (F). Cell death was assayed by TUNEL staining (G). All Western blot experiments were performed twice and the other experiments were performed a total of three times with similar results.
Although the siRNA results supported a role for activated STAT3 in VEGF induction of Bcl-2 and prosurvival effects, reduction of EC STAT3 levels by siRNA may have had adventitious effects, so we examined the effect of STAT3 activation by another approach. We used a p-STAT3 inhibitory peptide (SIP) linked to a membrane translocation peptide (MTS).38 HUVEC treatment with MTS-SIP inhibited p-STAT3 induction by VEGF (Fig. 5E), which showed that this peptide inhibited STAT3 activation.39 Treatment with MTS-SIP inhibited VEGF induction of Bcl-2 (Fig. 5F) and attenuated VEGF prosurvival effects on serum-deprived HUVEC (Fig. 5G). Treatment with SIP not linked to MTS, which enters cells poorly, did not inhibit VEGF induction of EC p-STAT3 or Bcl-2 and did not attenuate VEGF promotion of HUVEC survival (Fig. 5E–G). Together, these results demonstrated that STAT3 activation helps mediate VEGF induction of Bcl-2 and promotion of survival in EC.
p-STAT3 is induced by VEGF and reports VEGF-VEGFR2 signaling invivo
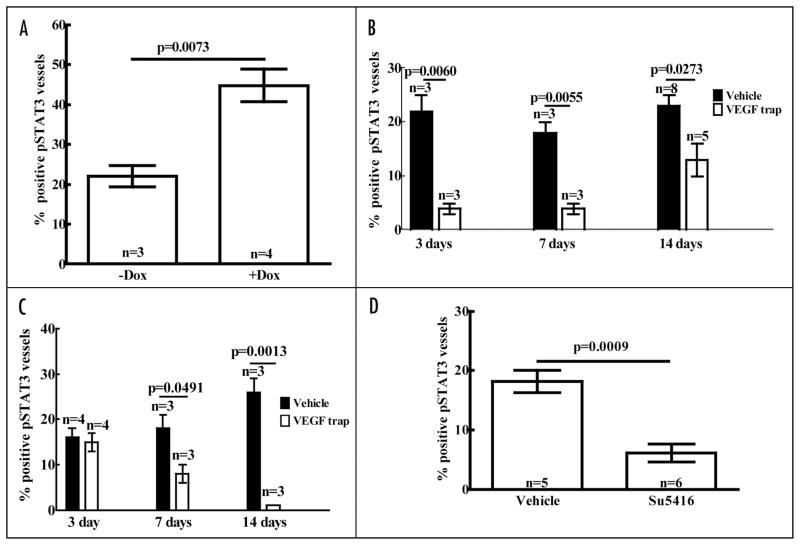
Published studies on effects of VEGF on STAT3 activation in cultured EC report varying results, some of which may be attributable to differences in the EC studied.24,25 To determine whether our in vitro studies accurately portrayed events in vivo, we sought confirmation of VEGF activation of STAT3 in tumor endothelium. We used K1735.VI4 tumors, which were generated from K1735 tumors cells genetically engineered to express murine VEGF in the presence of doxycycline (Dox). Two days after Dox was added to the drinking water of mice bearing K1735. VI4 tumors, +Dox tumors had 45-fold more VEGF in their lysates measured by ELISA than −Dox tumors. p-STAT3 was present in 22% of vessels in −Dox tumors, similar to the frequency seen in wild-type K1735 tumors, whereas it was present in 45% of vessels in +Dox tumors (Fig. 6A), showing that VEGF induced EC STAT3 activation in vivo.
Figure 6.
Tumor endothelial cell p-STAT3 is induced by VEGF and decreased by inhibitors of VEGF and VEGFR2. Tumors were raised in syngeneic mice by subcutaneous inoculation of cultured tumor cells. When tumors reached 5 mm in diameter, hosts were treated with the agents described and the tumors harvested at various times after start of treatment. Vascular p-STAT3 expression was examined immunohistochemically by dual staining with anti-p-STAT3 antibody (DAB chromogen, brown chromogen) and anti-CD34 antibody (SG blue, blue chromogen). C3H/HeN mice bearing K1735.VI4 tumors (engineered to express murine VEGF165 under doxycycline, Dox, induction) were given Dox (2 mg/ml) in their drinking water or not for two days (number of tumors analyzed for each group indicated within histogram) (A). Mice bearing K1735 tumors were treated with control Fc or VEGF Trap (25 mg/kg given subcutaneously twice a week) and their tumors excised 3, 7 or 14 days after start of treatment (B). BALB/c mice bearing RENCA tumors were treated with control Fc or VEGF Trap and their tumors analyzed as described (C). Mice bearing K1735 tumors were treated with vehicle or SU5416 (20 mg/kg daily) (D) and their tumors analyzed after seven days of treatment. Differences were analyzed by Student’s test and p value was shown.
STAT3 activation seen in tumor endothelium presumably results from EC stimulation by angiogenic factors in the tumor microenvironment. VEGF is present in these tumors and may contribute to the level of STAT3 activation seen. We treated tumor-bearing mice with inhibitors of VEGF and VEGFR2 to determine the effect of treatment on EC p-STAT3. Treatment with VEGF Trap significantly inhibited growth of both K1735 tumors (71% and 70% growth inhibition at day 7 and 14, respectively, compared to Fc-treated control tumors) and RENCA tumors (35% and 50% growth inhibition at day 7 and 14, respectively), suggesting that VEGF contributed to angiogenesis in both tumor types. Immunostaining of K1735 and RENCA tumors revealed a marked decrease in vessel staining for p-STAT3 in VEGF Trap-treated tumors compared to Fc-treated tumors. A decrease in the percentage of K1735 vessels staining for p-STAT3 was evident by day 3 of therapy and persisted to the end of therapy on day 14 (Fig. 6B; 82%, 78% and 43% decreases at days 3, 7 and 14, sequentially, compared to tumors treated with control Fc injection). A decrease in the percentage of RENCA vessels staining for p-STAT3 was evident by day 7 of therapy and became more pronounced at the end of therapy on day 14 (Fig. 6C; 56% and 96% decreases at days 7 and 14, sequentially). These results indicated that VEGF was responsible for a significant portion of EC p-STAT3 in these tumors. To examine the relationship between VEGF endothelial activation and STAT3 activation using another inhibitor, we studied K1735 tumors treated with SU5416.32 Treatment significantly reduced tumor vessels staining for p-STAT3 (Fig. 6D; 67% decrease at day 7), suggesting that signaling through VEGFR2 was responsible for much of the p-STAT3 in the EC of these tumors.
Discussion
The studies presented show that VEGF activation of VEGFR2 in cultured EC rapidly induces the molecular association of VEGFR2, Src and STAT3 and results in STAT3 phosphorylation by a VEGFR2- and Src-dependent mechanism. Immunocytochemical staining indicates that p-STAT3 localizes largely to nuclei and, accordingly, is positioned to affect EC gene expression. Others have examined EC STAT3 activation following in vitro VEGF stimulation but have reported variable and often inconsistent results.24,25 Thus, even though our studies showed VEGF induced STAT3 phosphorylation and nuclear localization in both MS1 cells and HUVEC, it was important to examine events in vivo to determine the significance and relevance of the in vitro observations. Immunohistological studies showed that p-STAT3 is generally absent in the quiescent microvessels of most normal mouse organs, with the lung being a notable exception. In contrast, p-STAT3 is present in the nuclei of a significant fraction of microvascular EC in three kinds of murine tumors, indicating that STAT3 is activated in angiogenic tumor EC. While these observations provided no indication of the factors that might be activating STAT3 in EC in vivo, the increase seen following induction of VEGF overexpression in K1735 tumors showed that VEGF can activate EC p-STAT3 in vivo and the marked decrease seen following treatment with agents that inhibit VEGF or VEGFR2 showed that VEGF is a primary activator of endothelial STAT3 in the tumors studied. Together, these data show that STAT3 is a mediator of VEGF-VEGFR2 signaling in angiogenic tumor endothelium.
Src is known to be activated following VEGFR2 engagement by VEGF40 and Src phosphorylation of STAT3 has been described in other cell types.23 Thus, Src-mediated STAT3 activation in EC follows a pathway established in other cell types for involving STAT3 signaling during cellular activation. In tumor cells ectopically expressing VEGFR2, VEGF has been shown to activate STAT3,41 but the mediator(s) downstream of VEGFR2 was(were) not identified. Src involvement in EC STAT3 activation suggests that other factors that stimulate EC and recruit Src in the process may also activate STAT3. FGF2 is one such factor and was shown to induce STAT3 activation. Interestingly, PlGF did not induce STAT3 activation, indicating that not all EC activators involve STAT3 signaling.
STAT3 can be activated by cytokines (e.g., IL-6), growth factors (e.g., EGF, TGFα and HGF) and oncoproteins (e.g., Src) in various cell types. In these cells, its phosphorylation by Janus (JAK), receptor tyrosine or Src-family kinases has been shown to promote cell proliferation and survival and/or contribute to cell transformation.23 STAT3 acts by modulating expression of genes that regulate the cell cycle, apoptosis, epithelial-mesenchymal transition (EMT) and cell invasion. The pleiotropic effects of STAT3 activation suggest that it probably impacts numerous processes and events in VEGF-stimulated EC. Using a candidate approach based on the known relationship between VEGF and Bcl-2,34 and cell survival,37 we identified a role for STAT3 in activated ECs. Inhibition of STAT3 activity by siRNA and an inhibitory phosphopeptide showed that VEGF induction of EC Bcl-2 and enhancement of EC survival are mediated, at least in part, by STAT3 activation. STAT3 promotion of EC survival may go beyond just Bcl-2 induction, because STAT3 has also been shown to activate expression of the VEGF gene in EC24 and also in other cell types.42 EC production of VEGF may initiate an autocrine mechanism for cell survival as well as help sustain other EC effects of VEGF. EC STAT3 is activated by angiogenic factors other than VEGF (e.g., FGF2) and the induction of VEGF expression by STAT3 provides a potential mechanism for these other factors to enlist VEGF participation in their activities and effects.43 Such a mechanism may help explain why inhibitors of VEGF and VEGFR2 interfere with in vitro and in vivo angiogenesis induced by FGF2.44
STAT3 anti-apoptotic activity is demonstrable in EC in vitro, but its effects during VEGF-induced angiogenesis in vivo is less clear. Mice with conditional endothelial STAT3 knockout are born at the expected Mendelian ratio and develop normally,45 signifying that developmental angiogenesis, a VEGF-dependent process, can proceed without EC STAT3. VEGF signaling through other pathways, such as PI3K-AKT46 or Raf,47 may provide redundant signals and compensate for the absence of endothelial STAT3 during development. The endothelium is abnormal in the absence of STAT3 function, however, as evidenced by the observations that EC STAT3 knockout mice exhibit an exaggerated inflammatory response and lethal susceptibility to lipopolysaccharide (LPS) challenge,45 increased susceptibility to hyperoxia-induced lung EC injury48 and enhanced post-ischemia myocardial injury.49 How STAT3 deficiency impacts tumor angiogenesis, which is often VEGF-driven, is currently unclear, as tumor studies in EC knockout mice have not been published to date.
The presence of p-STAT3 in tumor endothelium distinguishes it from the quiescent endothelium of most normal mouse organs and reflects its activated state. Factors other than VEGF can activate EC STAT3 and stimulate tumor angiogenesis, which makes it difficult to know which factors might be responsible for STAT3 activation in tumor endothelium without additional information. In the case of colorectal carcinoma, the therapeutic efficacy of the VEGF inhibitor, bevacizumab,10 provides supplementary information and suggests that at least a portion of the activated STAT3 seen in human colon carcinoma vessels may be due to VEGF. The fact that VEGF has been shown or is suspected to be an inducer of angiogenesis in many tumor types suggests that it probably contributes to STAT3 activation in the endothelium of many tumors. This was proven in the case of K1735 and RENCA mouse tumors by p-STAT3 downregulation following therapeutic interventions targeting VEGF and VEGFR2. These results also suggest that p-STAT3, if present in tumor endothelium prior to therapy, may be useful for monitoring therapeutic VEGF-VEGFR2 inhibition. We undertook these studies in a search for histological reporters of endothelial activation associated with angiogenesis that provide pathway information usable for investigating the pharmacodynamics of targeted antiangiogenic agents in preclinical and clinical settings. Based on the studies reported herein, endothelial p-STAT3 appears suitable for this purpose.
In summary, a combination of in vitro and in vivo studies establishes the participation and contribution of STAT3 activation during VEGF endothelial activation. EC STAT3 activation distinguishes quiescent and angiogenic mouse endothelium and can be a reporter of VEGF activity in tumors. Levels of EC p-STAT3 change with therapeutic modulation of VEGF-VEGFR2 signaling, making it potentially useful for monitoring the effect of this class of angiogenesis inhibitors. As part of a biomarker panel reporting on the activity of EC signaling pathways and cell fate decisions, p-STAT3 can help delineate the pharmacodynamics of antiangiogenic agents in vivo.
Materials and Methods
Reagents
Recombinant human and mouse VEGF, recombinant human and mouse FGF2, recombinant human PlGF and anti-human inhibitory VEGFR2 antibody were purchased from R&D Systems (Minneapolis, MN). SU5416 was purchased from Sigma-Aldrich (St. Louis, MO). Selective inhibitors of Src tyrosine kinase, PP1 and PP2, were purchased from Biomol (Plymouth Meeting, PA) and Calbiochem (San Diego, CA), respectively. VEGF Trap (aflibercept) was obtained from Regeneron Pharmaceuticals (Tarrytown, NY). SIP with or without MTS was purchased from Calbiochem (San Diego, CA). ApopTag kit was obtained from Chemicon (Temecula, CA).
Rat anti-mouse CD34 was purchased from Abcam (Cambridge, MA), mouse anti-human CD31 was obtained from Dako (Carpinteria, CA), rabbit monoclonal anti-p-STAT3 (Tyr 705), rabbit anti-STAT3, rabbit anti-STAT5, rabbit monoclonal anti-p-VEGFR2 (Tyr 1175), rabbit monoclonal anti-VEGFR2, rabbit anti-p-Src (Tyr 416), rabbit anti-Src, rabbit anti-Src, rabbit anti-GAPDH antibodies were obtained from Cell Signaling (Beverly, MA). Rabbit polyclonal anti-Bcl-2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Biotinylated anti-rabbit and rat immunoglobulin secondary antibodies, ABC reagent, 3, 3-diamin-obenzidine26 and SG blue chromogen were obtained from Vector Laboratories (Burlingame, CA).
Cell culture
MS1 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in a DMEM, supplemented with 25 mM NaHCO3, 10% fetal bovine serum (FBS), penicillin/streptomycin. HUVECs were maintained in EBM-2 complete endothelial growth medium (Clonetics, Walkersville, MD) according to the instructions of the supplier.
Immunohistochemistry and immunocytochemistry
Five-μm paraffin-embedded tissue sections were subjected to antigen retrieval in citric acid solution and primary antibody to analyte was applied overnight. After washing, slides were process for immunohistochemistry (IHC) by incubating with biotinylated secondary antibodies and ABC reagent and immune complexes were detected with DAB. The same sections were subsequently stained for vessels using anti-CD34 antibody by IHC using SG blue as chromogen or by immunofluorescence (IF) using a fluorescent-labeled secondary antibody. p-STAT3 was quantitatively detected only by IHC, whereas CD34 was detected equally well by IHC and by IF. Immunostainable p-STAT3 was observed to decrease significantly if slides were stained more than a month after sectioning. Accordingly, all slides for p-STAT3 analysis were stained within two weeks of sectioning. A vessel was counted positive for p-STAT3 when at least one EC within the vessel stained for nuclear p-STAT3.
For cultured MS1 or HUVEC cells, cells were fixed in 4% paraformaldehyde and immunocytochemistry was performed as detailed previously.27 Dying cells were identified by TUNEL staining using the ApopTag followed by DAPI counterstain.28
Western blotting and immunoprecipitation
For Western blotting, 30 to 60 μg cultured cells lysate were boiled for five minutes in Laemmli’s sample buffer and subjected to an 8–10% SDS-PAGE. Gels were transferred to nitrocellulose and probed with different primary antibodies overnight at 4°C. After washing, blots were incubated with the corresponding horseradish-peroxidase (HRP)-conjugated IgG (1:2000) (Amersham, Arlington Heights, IL) and developed for enhanced chemiluminescence (Amersham, Arlington Heights, IL). Blots were subsequently stripped and reprobed.
For immunoprecipitation, cells were lysed in cold RIPA buffer. Lysates were then cleared by centrifugation and then incubated overnight with primary antibody and an additional three hours with protein-A sephrose beads (Invitrogen, Carlsbad, CA). Beads were then washed five times with cold RIPA buffer, boiled in sample buffer, separated on 10% SDS-PAGE, transferred to a nitrocellulose membrane and probed with the appropriate detection antibody for determining endogenous protein-protein association.
siRNA
HUVECs were seeded at a density of 80,000/ml in complete EBM-2 in a six well plate. Cells were transfected with STAT3 siRNA (SMARTpool) or negative control (NC) siRNA using siIMPORTER transfection reagent according to manufacturer’s instruction (Millipore, Billerica, MA). Protein expression was verified by Western blot analysis as indicated above.
Tumor studies
K1735, RENCA and LLC tumors were generated in female C3H/HeN, BALB/c and C57BL/6 mice, respectively, as previously reported.29 Tumor-bearing mice were treated with (a) VEGF Trap (25 mg/kg) or control Fc injected subcutaneously twice a week or (b) SU5416 (20 mg/kg) or vehicle injected intraperitoneally daily. Mice were euthanized according to guidelines established by the Institutional Animal Care and Use Committee.
To generate K1735 tumor cells with doxycycline (Dox)-inducible expression of VEGF, K1735 cells were stably transfected with pEF2-rTtA (neo) to obtain clones expressing the reverse tetracycline transactivator. One clone of transfectants was subsequently stably transfected with TRE-VEGF (hygro) to obtain K1735.VI4 cells, which overexpress murine VEGF164 in the presence of Dox. For induction in vivo, Dox (2 mg/ml) was added to the drinking water of mice bearing K1735.VI4 tumors, tumors were excised two days later and VEGF levels in tumor lysates were assayed by ELISA (R&D, Minneapolis, MN). For histological studies, tumors were processed as previously reported.29
Statistics
Unless specifically mentioned, all values were expressed as Mean ± SEM. Student’s t test was used to determine statistical significance of differences between two groups (GraphPad Prism, GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank Dr. Jeff Tsai and Dr. Steve Huang for helpful discussion and advice, Dr. Hui Wang for providing mouse lung specimens and Sosina Makonnen for performing VEGF ELISA assays. We acknowledge the Center for Molecular Studies of Liver and Digestive Diseases Morphology Core (P30 DK50306) for use of its facilities and services.
This work was supported by grants from the National Institutes of Health (RO1 CA99519 and RO1 CA77851 to W.M.F.L. and R21CA111874 to Peter J. O’Dwyer).
Abbreviations
- DAB
3, 3-diaminobenzidine
- Dox
doxycycline
- EC
endothelial cells
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transition
- FBS
fetal bovine serum
- FGF2
fibroblast growth factor 2
- HGF
hepatocyte growth factor
- HIF-α
hypoxia inducible factor α
- HRP
horseradish-peroxidase
- HUVEC
human umbilical vein endothelial cells
- IHC
immunohistochemistry
- IF
immunofluorescence
- LLC
Lewis lung carcinomas
- MMP-2
matrix metalloproteinase-2
- MMP-9
matrix metalloproteinase-9
- MTS
membrane translocation peptide
- PlGF
placenta growth factor
- SFK
Src family kinase
- SIP
p-STAT3 inhibitory peptide
- STAT
signal transducer and activator of transcription
- TGFα
transforming growth factor-α
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Note
Supplementary materials can be found at: www.landesbioscience.com/supplement/ChenCBT7-12-Sup.pdf
References
- 1.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–64. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 4.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605–10. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–301. [PubMed] [Google Scholar]
- 6.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–80. [PubMed] [Google Scholar]
- 7.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 8.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: A VEGF blocker with potent anti-tumor effects. Proc Natl Acad Sci USA. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tallquist MD, Soriano P, Klinghoffer RA. Growth factor signaling pathways in vascular development. Oncogene. 1999;18:7917–32. doi: 10.1038/sj.onc.1203216. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe Y, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor inhibits anchorage-disruption-induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp Cell Res. 1997;233:340–9. doi: 10.1006/excr.1997.3583. [DOI] [PubMed] [Google Scholar]
- 15.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–8. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 16.Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–18. [PubMed] [Google Scholar]
- 17.Beebe JS, Jani JP, Knauth E, Goodwin P, Higdon C, Rossi AM, Emerson E, Finkelstein M, Floyd E, Harriman S, Atherton J, Hillerman S, Soderstrom C, Kou K, Gant T, Noe MC, Foster B, Rastinejad F, Marx MA, Schaeffer T, Whalen PM, Roberts WG. Pharmacological characterization of CP-547, 632, a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for cancer therapy. Cancer Res. 2003;63:7301–9. [PubMed] [Google Scholar]
- 18.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–21. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 19.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Approval summary: Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 20.Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 21.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–8. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–3. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: Modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MB, Caldwell RB. VEGF differentially activates STAT3 in microvascular endothelial cells. Faseb J. 2003;17:1562–4. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 25.Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, Detmar M, Hashimoto K. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026–31. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- 26.Larrea E, Aldabe R, Molano E, Fernandez-Rodriguez CM, Ametzazurra A, Civeira MP, Prieto J. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection: In vivo and in vitro studies. Gut. 2006;55:1188–96. doi: 10.1136/gut.2005.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabaglo L, Ormerod MG, Dowsett M. Measurement of proliferation marker Ki67 in breast tumour FNAs using laser scanning cytometry in comparison to conventional immunocytochemistry. Cytometry B Clin Cytom. 2003;56:55–61. doi: 10.1002/cyto.b.10055. [DOI] [PubMed] [Google Scholar]
- 28.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy DA, Makonnen S, Lassoued W, Feldman MD, Carter C, Lee WM. Inhibition of tumor endothelial ERK activation, angiogenesis and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–85. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–6. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Yu Y, Duh EJ. Vascular endothelial growth factor upregulates expression of ADAMTS1 in endothelial cells through protein kinase C signaling. Invest Ophthalmol Vis Sci. 2006;47:4059–66. doi: 10.1167/iovs.05-1528. [DOI] [PubMed] [Google Scholar]
- 32.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 33.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 34.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–6. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 35.Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, Henze C, Loffler D, Koczan D, Thiesen HJ, Burger R, Gramatzki M, Horn F. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood. 2004;103:242–51. doi: 10.1182/blood-2003-04-1048. [DOI] [PubMed] [Google Scholar]
- 36.Piva R, Pellegrino E, Mattioli M, Agnelli L, Lombardi L, Boccalatte F, Costa G, Ruggeri BA, Cheng M, Chiarle R, Palestro G, Neri A, Inghirami G. Functional validation of the ana-plastic lymphoma kinase signature identifies CEBPB and BCL2A1 as critical target genes. J Clin Invest. 2006;116:3171–82. doi: 10.1172/JCI29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinci MC, Visentin B, Cusinato F, Nardelli GB, Trevisi L, Luciani S. Effect of vascular endothelial growth factor and epidermal growth factor on iatrogenic apoptosis in human endothelial cells. Biochem Pharmacol. 2004;67:277–84. doi: 10.1016/j.bcp.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation and cell transformation. J Biol Chem. 2001;276:45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 39.Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: A nonsteroidal approach to contraception. Proc Natl Acad Sci USA. 2005;102:8585–90. doi: 10.1073/pnas.0502343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem. 2004;279:39175–85. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Chen H, Yel F, Wang F, Xie X. VEGF induces phosphorylation of STAT3 through binding VEGFR2 in ovarian carcinoma cells in vitro. Eur J Gynaecol Oncol. 2006;27:363–9. [PubMed] [Google Scholar]
- 42.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity upregulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 43.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tille JC, Wood J, Mandriota SJ, Schnell C, Ferrari S, Mestan J, Zhu Z, Witte L, Pepper MS. Vascular endothelial growth factor (VEGF) receptor-2 antagonists inhibit VEGF- and basic fibroblast growth factor-induced angiogenesis in vivo and in vitro. J Pharmacol Exp Ther. 2001;299:1073–85. [PubMed] [Google Scholar]
- 45.Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, Fu XY. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med. 2003;198:1517–25. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–81. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 47.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–6. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. Faseb J. 2006;20:2156–8. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol. 2007;293:2101–8. doi: 10.1152/ajpheart.00125.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.