Abstract
Background
A pilot study was conducted to determine whether conditioning using selective targeting of hematopoietic cells with an α-particle emitter, bismuth-213 (213Bi)-labeled anti-CD45 monoclonal antibody (MAb) is sufficient to overcome the major histocompatibility barrier in a canine model of dog leukocyte antigen-haploidentical hematopoietic cell transplantation (HCT).
Methods
Six dogs were administered 0.5 mg/kg 213Bi-labeled anti-CD45 MAb (dose 213Bi=2.26–4.9 mCi/kg) administered in 6–8 injections. For postgrafting immunosuppression all dogs received cyclosporine and mycophenolate mofetil.
Results
All dogs had initial donor engraftment, with 3 of 6 dogs having sustained engraftment to last point of follow-up. Two dogs receiving 2.26 and 3.25 mCi /kg of 213Bi rejected their grafts at day +127 and +125, respectively, while dogs receiving 213Bi doses of 3.3 mCi/kg or greater achieved high level donor chimerism.
Conclusion
The results suggest that nonmyeloablative conditioning with 213Bi labeled anti-CD45 MAb could be applicable to major histocompatibility haploidentical HCT without excessive non-hematological regimen-related toxicity.
Keywords: Bismuth-213, Anti-CD45 MAb, Haploidentical transplantation, Nonmyeloablative transplantation
INTRODUCTION
The application of systemically targeted radiation using radionuclide-labeled monoclonal antibodies (MAb) for conditioning in allogeneic hematopoietic cell transplantation (HCT) has been explored to decrease toxicity of total body γ-irradiation (TBI). Previously, β-emitting radionuclide-labeled MAbs have been evaluated in clinical trials, and have showed some efficacy (1–4). However, alpha-emitters such as bismuth-213 (213Bi) with their high linear energy transfer and short particle range may be more appropriate for targeting hematopoietic cells and therefore better suited for radioimmunotherapy as conditioning for HCT. We have previously shown that conditioning with bismuth-213 (213Bi)-labeled anti-CD45 MAb or 213Bi-labeled anti-TCRαβ MAb successfully allowed sustained engraftment in a dog leukocyte antigen (DLA)-identical littermate canine HCT model (5–8).
In recent studies, T cell-depleted or unmanipulated human leukocyte antigen (HLA)-haploidentical grafts have been applied as an alternative hematopoietic stem cell source for patients without suitable HLA-identical donors (9). The intensive conditioning used so far for haploidentical HCT resulted in a high treatment related mortality and therefore strategies for nonmyeloablative conditioning regimens are investigated (10–14). However, nonmyeloablative conditioning for HCT could increase the risk of graft rejection in an HLA-haploidentical setting. Hence, we investigated whether nonmyeloablative conditioning with 213Bi-labeled anti-CD45 MAb alone would allow a durable donor engraftment in a canine model of DLA-haploidentical HCT.
MATERIALS AND METHODS
The median age of dogs (beagles and minimongrel-beagle crossbreeds) in the study was 13 months (range, 10–16), and the median weight was 12.7 kg (range, 6.5–13.6). DLA-haploidentical littermates were selected on the basis of family typing using highly polymorphic major histocompatibility complex class I and II microsatellite markers and sequencing for DLA-DRB1 alleles.
For radiolabeling, we used the anti-CD45 MAb CA12.10C12 (IgG1) (15). 213Bi was obtained by elution from an 225Actinium generator purchased from the US Department of Energy (Oak Ridge, TN), and modification of CA12.10C12 for labeling with 213Bi was done as previously described (5).
On day −3, 0.034–0.055 mg/kg non-conjugated anti-CD45 MAb was injected to prevent non-specific tissue binding of 213Bi labeled anti-CD45 MAb (16). In all dogs, a total dose of 0.5 mg/kg 213Bi labeled anti-CD45 MAb was administered in 6 to 8 injections on days −3 to −2. The 6 dogs (Table 1) received total doses ranging from 2.26 to 4.9 mCi /kg 213Bi labeled anti-CD45 MAb as a nonmyeloablative conditioning (5). Peripheral blood mononuclear cells (PBMC) were collected from DLA-haploidentical littermate donors following administration of 5 µg/kg of recombinant canine granulocyte colony stimulating factor (rc-G-CSF) administered subcutaneously (sc) twice daily from day −5 through day 0. A median of 8.9 (range, 2.2–13) × 108/kg of rc-G-CSF mobilized PBMC was intravenously infused on day 0. Postgrafting immunosuppression consisted of cyclosporine (CSP; 15 mg/kg orally twice a day on days −1 through day +100, with a taper through day +180) and mycophenolate mofetil (MMF; 10 mg/kg sc twice daily on days 0 to day +40 and then, 5 mg/kg on days +41 to +100). Donor-host cell chimerism was evaluated weekly by a polymerase chain reaction (PCR)-based assay of polymorphic (CA)n dinucleotide repeats (17). Complete peripheral blood cell counts (CBC) were measured daily starting day −4 until hematopoietic recovery and weekly thereafter. Chemistries including liver and kidney function tests were evaluated on day −3 before injection of non-conjugated MAb, days +7, +14, +21, +28 and then monthly.
Table 1.
| Recipient Dog ID |
213Bi (mCi /kg) (no. of injection) |
Transplanted MNC (×108 /kg) |
Percent donor MNC chimerism (Max-final, %) |
Duration of engraftment (weeks) |
Rejection | Cause of death* |
|---|---|---|---|---|---|---|
| G238 | 3.14 (6) | 9 | 70-12 | >35 | No | ET2 |
| G257 | 2.26 (8) | 13 | 60-0 | 18 | Yes* | ET2, GVHD |
| G310 | 3.25 (8) | 2.2 | 38-0 | 14 | Yes* | Released for adoption |
| G456 | 3.3 (6) | 7.2 | 86-72 | >10 | No | ET1- ascites, CHV |
| G481 | 3.9 (8) | 8.8 | 92-76 | >6 | No | ET1 - pneumonia, CHV |
| G485 | 4.9 (8) | 10 | 95-78 | >8 | No | ET1 - Liver failure, GVHD |
ET1 - euthanized due to poor condition. ET2 - euthanized, end of study.
Autologous marrow recovery was seen after rejection.
RESULTS
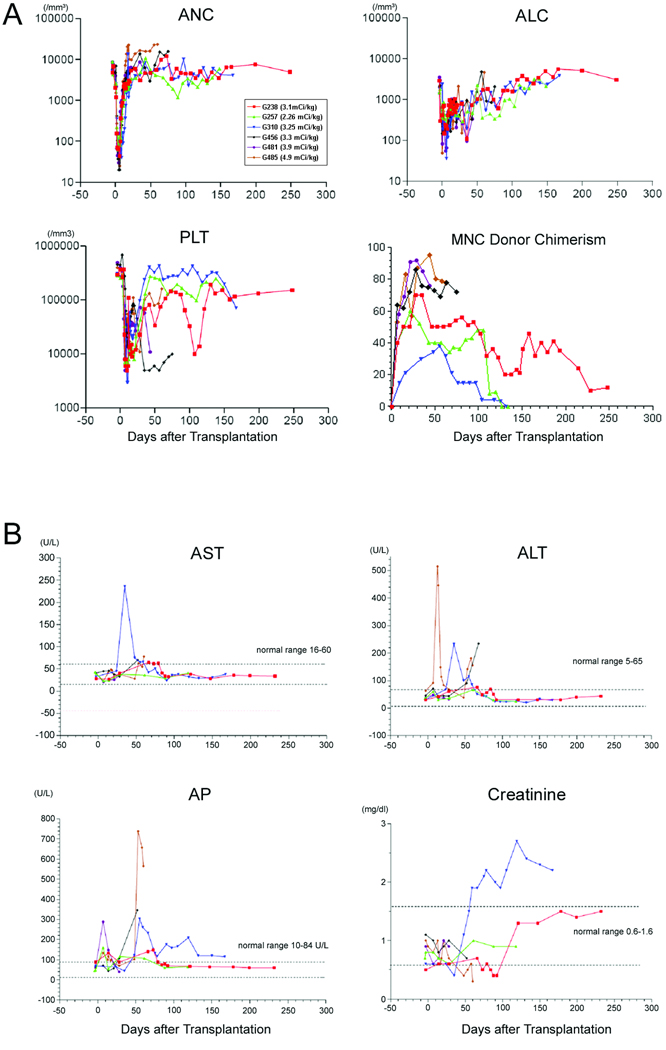
The neutrophil nadir of 20 to 62 /µL occurred on days 2 to 14 after HCT. Thrombocytopenia (<20 × 109 /L) associated with conditioning appeared between days 6 and 24 with nadirs of 3,000 to 14,000 /µL. The recoveries of neutrophil counts (>0.5 × 109 /L) were observed on days 7 to 15 (Figure 1A). All dogs achieved primary engraftment one week after HCT, with donor PBMC chimerism ranging from 15 to 58% (median, 49%). Stable engraftment was observed in the 3 of 6 dogs. Although no graft rejection occurred, the MNC chimerism of G238 receiving 3.1 mCi /kg of 213Bi declined before the end of study. Two dogs (G257 and G310) receiving 2.26 and 3.25 mCi /kg of 213Bi rejected their grafts on days +127 and +125, respectively. MMF was discontinued on day 100. G257 and G310 were still receiving CSP (6 mg/kg/day), at the time graft rejection occurred. After graft rejection, the dogs had autologous marrow recovery (Figure 1A). In G310, transient moderate elevation of hepatic enzymes was observed without other signs of liver dysfunction. Elevations of serum creatinine also appeared after day +55. Renal toxicity caused by CSP and concurrent dehydration might have contributed to renal dysfunction in this dog. However, after discontinuation of CSP at day 164 after graft rejection, the serum creatinine level did not normalize. G310 was released for adoption after rejection of graft. G257 was euthanized at the end of study. The pathological examination at necropsy of G257 showed that lymphoid cells scattered throughout the triad region of the liver, as well as in the lobular and interstitial regions, and occasional foci had infiltration of small bile ducts by the lymphocyte, suggesting minimal degree of acute graft versus host disease (GVHD). G257 clinically showed only a mild elevation of a hepatic enzyme, alkaline phosphatase (AP) (Figure 1B). GVHD occurred in two dogs (G257 and G485), which received the two highest doses of rc-G-CSF mobilized PBMC (Table 1). Acute GVHD of liver was clinically suspected in G485 because the dog had jaundice, reduced body weight, extensive ascites and significant elevation of hepatic enzymes (maximum total bilirubin 1.1 mg/dl, alanine aminotransferase 515 and AP 738 U/L) (Figure 1B). The pathological examination at necropsy confirmed minimal evidence of bile duct abnormalities, which were consistent with grade I acute GVHD. On completion of study, G238 was euthanized and pathological examination after an extensive autopsy showed no abnormalities and no evidence of GVHD. G456 was euthanized on day +77 because of poor condition due to systemic canine herpes virus (CHV) infection with elevation of hepatic enzyme and ascites. G481 was also euthanized because of pneumonia associated with CHV infection on day +45.
Figure 1.
A) Peripheral blood absolute neutrophil (ANC), lymphocyte (ALC), platelet counts (PLT) and mononuclear cell (MNC) donor chimerism of dogs which underwent nonmyeloablative dog lymphocyte antigen-haploidentical hematopoietic cell transplantation. ANC, ALC and PLT of peripheral blood in the 6 dogs which received total doses of 2.26–4.9 mCi/kg 213Bi labeled anti-CD45 monoclonal antibody as nonmyeloablative conditioning. B) Liver enzymes and creatinine levels in the dogs treated with nonmyeloablative dog lymphocyte antigen-haploidentical hematopoietic cell transplantation (HCT). Values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP) and creatinine in the 6 dogs which received nonmyeloablative dog lymphocyte antigen-haploidentical HCT.
DISCUSSION
The data presented demonstrates that the use of 213Bi-labeled anti-CD45 MAb, targeting an ubiquitous antigen for hematopoietic cells, can be sufficient to allow sustained engraftment in haploidentical HCT with minimal non-hematopoietic toxicity. In addition, CD45 might be an excellent target because it is ubiquitously expressed on not only nonmalignant hematopoietic cells but also malignant cells such as lymphoma and leukemia. Therefore the use of anti-CD45 MAb might have a potential for therapeutic effects.
In marrow transplantation, the engraftment rate largely depends on the degree of histocompatibility disparity between donors and recipients. Specifically, conditioning with 920 cGy TBI allowed sustained engraftment in 95% of DLA-identical littermates HCT, while it resulted in 50% of DLA-identical unrelated marrow recipients and in a mere 8% of DLA-non-identical related or unrelated marrow recipients. A dose of 1800 cGy TBI was required to establish DLA-non-identical related or unrelated marrow grafts (18,19). If the marrow graft is replaced by rc-G-CSF mobilized PBMCs, the TBI dose needed for sustained engraftment could be decreased to 920 cGy in DLA-non-identical settings (20). In our study, rc-G-CSF mobilized PBMCs were used as grafts in all the dogs. A dose of 3.3 to 4.9 mCi/kg of 213Bi labeled anti-CD45 MAb, which is approximately equivalent to low-dose 200 cGy TBI (5), could successfully allow durable donor engraftment. We speculated that the use of radionuclide immunotherapy targeting a specific ubiquitous antigen of hematopoietic cells, CD45, might be very effective in reducing requisite radioactivity level necessary for engraftment. We previously reported that difference in requisite TBI dose might be partly derived from differences in radiosensitivity between host immune cells, T-cells and the large granular lymphocytes with natural killer (NK) activity which causes graft rejection across different histocompatibility barriers (19). T-cells that play a critical role on graft rejection, by responding to minor histocompatibility antigens in the DLA-identical HCT, are more radiosensitive than large granular lymphocytes with NK function in dogs. In addition, NK-cells are known to contribute to initial graft rejection in mice models (21,22). Indeed, our previous study showed that the donor chimerism levels in dog conditioned with 213Bi-labeled anti-TCRαβ MAb (CA15.9D95) were lower than those observed in dogs conditioned with 213Bi labeled anti-CD45 MAb (6), suggesting 213Bi-labeled anti-CD45 MAb is more effective in killing residual cells such as NK-cells. Radioimmunotherapy using alpha-emitter-labeled anti-CD45 MAb may have the unique ability to overcome the radioresistance of NK cells. However, the short-half life and high cost of Bi-213 might be obstacles for application to humans, because multiple preparations and injections will be required to obtain adequate doses of clinical material. For that reason, we have initiated studies using another alpha-emitter, astatine-211, which has a half life of 7.2 hours. Preliminary data in mice suggest that it may be more effective (23).
In this investigation, 3.3 to 4.9 mCi/kg of 213Bi labeled anti-CD45 MAb successfully allowed donor engraftment in the DLA-haploidentical setting without significant non-hematological regimen related toxicity. These pilot data demonstrated that TBI in nonmyeloablative conditioning for HCT could be completely replaced by an α-particle emitting radionuclide, including in the MHC-mismatched setting.
Acknowledgements
The authors would like to thank George Sale, M.D. for performing the pathology studies, Michele Spector, D.V.M. for providing veterinary care along with the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center. We also would like to thank Stacy Zellmer and Patrice Stroup for DLA-typing and the technicians of the hematology department for analyzing peripheral blood counts. The authors are indebted to Amgen for the gift of canine G-CSF. We are also grateful for the assistance of Helen Crawford, Bonnie Larson, and Sue Carbonneau in manuscript preparation.
Grant support: This study was supported by the National Institutes of Health HL36444 and CA118940. H.N. was funded by the Graduate School of Medicine, Osaka City University, Osaka, Japan. F.R.K. was supported by a grant from FAPESP/Brazil and an award from the Oncology Research Faculty Development Program from the Department of Health and Human Services, NIH, Bethesda, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Hirohisa Nakamae drafted and revised the manuscript, analyzed and interpreted data.
- Fabio R Kerbauy designed and conducted the study, drafted and revised manuscript, and analyzed and interpreted data.
- D. Scott Wilbur conceived, designed and conducted the study, contributed vital new conjugates, drafted and revised manuscript, and supervised interpretation of data.
- Wolfgang Bethge designed, conducted the study, revised manuscript and supervised interpretation of data.
- Donald K. Hamlin conceived, designed and conducted the study, contributed vital new conjugates, and supervised interpretation of data.
- Erlinda B. Santos designed and conducted the study, drafted and revised manuscript, and analyzed and interpreted data.
- Rainer Storb conceived, designed, revised manuscript and supervised interpretation of data.
- Brenda M. Sandmaier conceived, designed, conducted the study, drafted and revised manuscript, and supervised interpretation of data.
References
- 1.Matthews DC, Appelbaum FR, Eary JF, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–1131. [PubMed] [Google Scholar]
- 2.Matthews DC, Martin PJ, Nourigat C, Appelbaum FR, Fisher DR, Bernstein ID. Marrow ablative and immunosuppressive effects of 131 I-anti-CD45 antibody in congenic and H2-mismatched murine transplant models. Blood. 1999;93:737–745. [PubMed] [Google Scholar]
- 3.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of 131I-Anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–1247. [PubMed] [Google Scholar]
- 4.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100:318–326. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 6.Bethge WA, Wilbur DS, Storb R, et al. Selective T-cell ablation with bismuth-213-labeled anti-TCRab as nonmyeloablative conditioning for allogeneic canine marrow transplantation. Blood. 2003;101:5068–5075. doi: 10.1182/blood-2002-12-3867. [DOI] [PubMed] [Google Scholar]
- 7.Bethge WA, Wilbur DS, Storb R, et al. Radioimmunotherapy with Bismuth-213 as conditioning for nonmyeloablative allogeneic hematopoietic cell transplantation in dogs: a dose deescalation study. Transplantation. 2004;78:352–359. doi: 10.1097/01.tp.0000128853.62545.b2. [DOI] [PubMed] [Google Scholar]
- 8.Bethge WA, Sandmaier BM. Targeted cancer therapy using radiolabeled monoclonal antibodies. Technology in Cancer Research and Treatment. 2005;4:393–405. doi: 10.1177/153303460500400407. [DOI] [PubMed] [Google Scholar]
- 9.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 10.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 11.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 12.Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa H, Ikegame K, Kaida K, et al. Unmanipulated HLA 2–3 antigen-mismatched (haploidentical) bone marrow transplantation using only pharmacological GVHD prophylaxis. Exp Hematol. 2008;36:1–8. doi: 10.1016/j.exphem.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caniatti M, Roccabianca P, Scanziani E, Paltrinieri S, Moore PF. Canine lymphoma: immunocytochemical analysis of fine-needle aspiration biopsy. Veterinary Pathology. 1996;33:204–212. doi: 10.1177/030098589603300210. [DOI] [PubMed] [Google Scholar]
- 16.Bianco JA, Sandmaier B, Brown P, et al. Specific marrow localization of an 131I-labeled anti-myeloid antibody in normal dogs: Effects of a "cold" antibody pretreatment dose on marrow localization. Exp Hematol. 1989;17:929–934. [PubMed] [Google Scholar]
- 17.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 18.Storb R, Deeg HJ. Failure of allogeneic canine marrow grafts after total body irradiation: Allogeneic "resistance" vs transfusion induced sensitization. Transplantation. 1986;42:571–580. doi: 10.1097/00007890-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Raff RF, Sandmaier BM, Graham T, Loughran TP, Jr, Pettinger M, Storb R. "Resistance" to DLA-nonidentical canine unrelated marrow grafts is unrestricted by the major histocompatibility complex. Exp Hematol. 1994;22:893–897. [PubMed] [Google Scholar]
- 20.Sandmaier BM, Storb R, Santos EB, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–3513. [PubMed] [Google Scholar]
- 21.Warner JF, Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982;300:31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- 22.Warner JF, Dennert G. Bone marrow graft rejection as a function of antibody-directed natural killer cells. J Exp Med. 1985;161:563–576. doi: 10.1084/jem.161.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamae H, Wilbur DS, Hamlin DK, et al. Biodistribution, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the a-emitting radionuclides bismuth-213 or astatine-211. Cancer Res. 2009;69:2408–2415. doi: 10.1158/0008-5472.CAN-08-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]