Abstract
Functional brain imaging has overshadowed traditional lesion studies in becoming the dominant approach to the study of brain-behavior relationships. The proponents of functional imaging studies frequently argue that this approach provides an advantage over lesion studies by observing normal brain activity in vivo without the disruptive effects of brain damage. However, the enthusiastic onslaught of brain images, frequently presented as veridical representations of mental function, has sometimes overwhelmed some basic facts about brain organization repeatedly observed over more than a century. In particular, the lateralization of speech and language to the left cerebral hemisphere in over 90% of the right-handed population does not appear to have been taken as a serious constraint in the interpretation of imaging results in studies of these functions. This paper reviews a number of areas in which standard activation assumptions yield results that are at odds with clinical experience. The activation approach will be contrasted with a performance-based analysis of functional image data, which, at least in the case of speech production, yields results in better agreement with lesion data. Functional imaging represents enormous opportunities for understanding brain-behavior relationships, but at the present level of understanding of what is being represented in such images, it is premature to adhere to a single approach based on the strong but questionable assumptions inherent in most activation studies.
Keywords: Functional imaging, Activation, Speech, Performance-based analysis, Laterality, Functional imaging methods
1. Introduction
The notion of activation is central to most current functional imaging work, and it is based on several assumptions. The first is that the experimental behavior or mental process under study will result in a significant increase in a measurable blood flow or other hemodynamic response. Such increases are assumed to be found in functionally relevant brain areas following a contrast between an experimental task and a control task or resting state. Further, activation assumes that all increases following a contrast procedure reflect neurophysiology that is directly related to the process under study either psychologically (e.g., mentally generating a verb) or motorically (e.g., pushing a button). The magnitude of the increases resulting from such contrasts is commonly assumed to be an indication of the magnitude of involvement of the areas of change in the behavior or mental process under study, at least implicitly. Because of the need to perform a contrast to demonstrate activation, several additional assumptions are made regarding the decomposition of imaging data. Decomposition assumes that specific mental processes can be isolated by contrasting images, that the data generated by such a contrast have measurement properties that support neurobehaviorally meaningful analyses, and that the significant regional increases in decomposed data are interpretable independent of the data that were eliminated by the contrast. That is to say, the activation approach characterizes functional brain systems either as independent “hot spots” (i.e., areas of significant signal increase from one condition to another) or as networks that can be defined in terms of the “hot spots” that remain following a contrast. In a real sense, the activation assumptions are invoked to bridge the gap between brain physiology and mental function. If these assumptions were true, the activation approach would provide a straightforward, even simple method for mapping the functional organization of the brain. However, the results of studies based on activation approaches are often discordant with lesion results. While it might be convenient to ignore the lesion experience in favor of activation patterns, lesions have the advantage of producing tangible, often intractable consequences that have provided the basis for functional localization in neurology. The behavior of individuals following a brain lesion may indeed reflect processes such as functional reorganization, compensation, and diaschesis to varying extents. Nonetheless, when deficits are fixed, lesion studies have been characterized as providing evidence for the necessity of specific brain areas for specific behaviors (Fellows et al., 2005). In the case of speech and language, the general features of the clinical experience have been replicated for over a century and are the reference points closest to a “gold standard” in brain-behavior research. Whereas the details regarding the specifics of language organization in the peri-sylvian area are still argued in the lesion literature, the left–right asymmetry of function has been well established. Some of the problems for functional brain imaging raised by language studies will be considered elsewhere (Van Lancker Sidtis, 2007). This paper will consider the failure of the activation approach to capture the most basic features of the neurological organization of motor speech control: the identification of relevant brain regions and the correct assignment of brain lateralization.
The lesion experience has been quite consistent at the level of gross functional anatomy. In the right-handed population, language is lateralized to the left cerebral hemisphere in over 90% of the right-handed population (e.g., Davis & Wada, 1978). Motor speech control is also strongly lateralized (Kent & Tjaden, 1997). Damage to the left inferior frontal gyrus produces problems with speech in right-handed individuals (e.g., Mohr et al., 1978; Schiff, Alexander, Naesser, & Galaburda, 1983; Tognola & Vignolo, 1980). In general, dysarthria is far more likely to occur following supratentorial lesions in the left hemisphere than the right (Urban et al., 2001). Although the lesion studies suggest that lateralization of the cerebellum for motor speech control may not be as strong as in the cerebral hemispheres, the right cerebellum is more involved in this process than the left with an estimate of the right-to-left ratio of dysarthria producing lesions in the cerebellum of six-to-one (Ackermann, Vogel, Peterson, & Poremba, 1992; Amarenco, Rosengart, DeWitt, Pessin, & Caplan, 1993; Urban et al., 2001; Urban et al., 2003). Based on the clinical experience, speech production should produce clear evidence of activation of the inferior frontal gyrus and the pyramidal motor system lateralized to the left cerebral hemisphere as well as the extrapyramidal motor system, particularly the right cerebellum. This has not been the case as average blood flow results and activation patterns have typically been bilateral (e.g., Blank, Scott, Murphy, Warburton, & Wise, 2002; Huang, Carr, & Cao, 2001; Ingham, Fox, Ingham, & Zamarripa, 2000; Murphy et al., 1997; Riecker et al., 2000; Riecker, Kassubek, Gröschel, Grodd, & Ackermann, 2006; Riecker et al., 2005; Riecker et al., 2002; Riecker, Wildgruber, Grodd, & Ackermann, 2002; Schulz, Varge, Jeffires, Ludlow, & Braun, 2005; Sidtis, Gomez, Groshong, Strother, & Rottenberg, 2006; Sidtis, Strother, Anderson, & Rottenberg, 1999; Sidtis, Strother, & Rottenberg, 2003; Urban et al., 2003; Wildgruber, Ackermann, & Grodd, 2001; Wise, Greene, Büchel, & Scott, 1999).
It has been suggested that right hemisphere activation in speech and language tasks represent normal function. However, this claim appears to be based solely on the presence of bilateral activation rather than on clinical evidence. Although functional imaging data on reorganization following focal damage is limited, it appears that recovery of language after stroke is most successful when traditional, left-lateralized language areas, or adjacent areas, rather than homotopic areas in the right hemisphere are involved (Weiller, 1998). In degenerative cerebellar disease, Broca's area, but not its right-side homologue, shows what appears to be compensatory increases in blood flow during speech (Sidtis & Gomez, 2003). Mapping functional reorganization during recovery presents a complex problem in which activity in homologous brain regions may change, but may have no relationship with the eventual extent of recovery (Small, Hlustik, Noll, Genovese, & Solodkin, 2002). At present, the claim that there is normally bilateral functional involvement in lateralized tasks is just that. In the case of speech production, the opposite has been argued with data that suggest that bilateral activation that is greater than normal is associated with stuttering (Braun et al., 1997; De Nil, Kroll, Kapur, & Houle, 2000; Fox, Ingham, Zamarripa, Xiong, & Lancaster, 2000).
2. Mapping task contrasts
If the activation approach is suited to the study of motor speech control, the relatively simple behavior of repeating syllables should be decomposable into components related to the rapid changes in articulatory structures and the phonation associated with vowel production. In a PET study that required normal, right-handed speakers to repeat the syllables /pa, ta, ka/, perform an articulatory task (rapid silent lip closures as if producing the syllable /pa/), and a phonation task (sustaining the vowel sound /ah/), the ability to identify articulatory and phonatory components in functional images by task subtraction was examined (Sidtis et al., 1999). Subtraction of the phonation image from the syllable repetition image did not produce results that resembled the articulation image, nor did the subtraction of the articulation image from the syllable repetition image resemble the phonation image. This result was repeated both when the tasks were performed during different scanning sessions and at the same scanning session. Task subtractions failed to produce activations in known speech areas and introduced activations in anomalous areas. Simple resting state subtractions for each task were more like the patterns expected from lesion data but there were several surprises with this contrast as well. One of the most striking aspects of these data was the bilaterality of the blood flow patterns in all conditions. A second feature was the higher levels of normalized blood flow in many areas during the constituent articulatory and phonatory tasks compared to the more complex syllable task. Whereas it was clear that task contrasts were not successful in identifying the functional anatomy of motor speech control documented in lesion studies, it was equally clear that while known speech areas could be identified in resting state contrasts, they were not properly lateralized nor did the areas traditionally associated with speech represent the highest levels of activity. The difficulties with image subtraction and the selection of an appropriate “baseline” for task contrasts have been recognized by many investigators (Friston et al., 1996; Jennings, McIntosh, Kapur, Tulving, & Houle, 1997; Newman, Tweig, & Carpenter, 2001; Sidtis et al., 1999, 2003, Sidtis, Strother, & Rottenberg, 2004; Stark & Squire, 2001). This should not be surprising. In spite of periodic reappearances of subtractive logic in behavioral studies, approaches to decomposing mental events based on subtraction studies using reaction times were recognized as flawed as early as 1905 (Ach, 1905; Watt, 1905; described in Boring, 1929; Leeper, 1951; Miller, 1951; Woodworth, 1938). The problem was and is that Donders's (1869) insertion assumption, which holds that a task is made more complex by the insertion of an additive measurable process, is incorrect. Not only do components of complex tasks interact, but attempts at decomposing behavioral measures do not yield unique solutions (Van Zandt & Ratcliff, 1995). This particular problem with the activation approach has plagued attempts at decomposing independent measures of behavior for over a century. Functional imaging has been acquiring data with 21st century technology but often interpreting it with 19th century concepts (Sidtis, 2000). New methods for analyzing functional image data that do not require activation measures are emerging (e.g., Hansen, 2007), but have not yet achieved widespread application.
3. Mapping resting state contrasts
The use of a resting condition is intended to control for all effects during scanning other than performance, whether covert or overt, of the experimental task. Any scanning procedure, whether it be PET, MRI, electrophysiological, or magnetoencephalographic, is a stimulus-rich event in its own right, and it appears reasonable to control for the effects of the scanning environment to enhance the signal of interest resulting from the experimental manipulation. One approach to this situation has been the use of a resting state during which subjects are scanned in the absence of experimental stimulation, tasks, or responses. Since the scanning environment during the resting state is identical to that present during the experimental state, it seems reasonable to attribute differences in the two states to the experimental conditions. The resting state, an early concern in the infancy of PET (Duara et al., 1987; Horwitz, Duara, & Rapoport, 1984; Junck et al., 1988; Metter, Riege, Hanson, Phelps, & Kuhl, 1988), has experienced renewed interest (e.g., Biswal, Yetkin, Haughton, & Hyde, 1995; De Luca, Beckmann, De Stefano, Matthews, & Smith, 2006; Fransson, 2005; Grecius, Krasnow, Reiss, & Menon, 2003; Gusnard & Raichle, 2001; Mazoyer et al., 2001; Raichle et al., 2001; Sidtis et al., 2004). The range of views as to what the resting state may represent will not be reviewed here, but the effects of applying a resting state contrast to the imaging data associated with syllable repetition will be considered. Table 1 ranks normalized blood flow data from highest to lowest in 22 regions of interest during syllable production and after resting state values have been subtracted (Sidtis et al., 2003). Both lesion data and performance-based analysis (PBA), reviewed in a subsequent section, identify the left inferior frontal gyrus, where Broca's area can be found, as playing a significant role in speech production (Sidtis et al., 2006, 2003). However, average blood flow values during syllable repetition do not identify the left inferior frontal region as an area of peak activity. It is tenth on the list of twenty-two regions ranked by flow rates, lagging behind the right inferior frontal region in sixth place. Does subtracting a rest state help? Table 1 shows that not only does the left inferior frontal area drop to fifteenth place after the resting state has been subtracted, but the right inferior frontal region has moved up to fifth place. The contrast with a resting state produces a significant left/right asymmetry, but it is in the wrong direction (Fig. 1). At least during syllable repetition, then, a classic speech area does not produce peak blood flow activity. Contrasting a rest state not only moves the speech area farther from peak status, but it also introduces a significant hemispheric asymmetry in the wrong direction. The absence of strongly lateralized activation effects during speech is common in functional imaging studies (see discussion in Sidtis et al., 2006).
Table 1.
Regions of interest ranked from highest to lowest blood flow values during speech production alone and after the resting state values are subtracted
| Rank | Brain region (speech) |
Brain region (speech minus rest) |
|---|---|---|
| 1 | L Thalamus | L Inferior cerebellum |
| 2 | L Putamen | L Mid cerebellum |
| 3 | R Putamen | R Transverse temporal gyrus |
| 4 | R Thalamus | L Superior cerebellum |
| 5 | L Mid cerebellum | R Inferior frontal gyrus |
| 6 | R Inferior frontal gyrus | R Inferior cerebellum |
| 7 | R Superior temporal gyrus | R Superior cerebellum |
| 8 | L Superior temporal gyrus | L Thalamus |
| 9 | L Transverse temporal gyrus | R Mid cerebellum |
| 10 | L Inferior frontal gyrus | R Superior temporal gyrus |
| 11 | L Superior cerebellum | L Transverse temporal gyrus |
| 12 | R Mid cerebellum | R Thalamus |
| 13 | R Transverse temporal gyrus | R Supplementary motor area |
| 14 | L Inferior cerebellum | R Putamen |
| 15 | L Caudate nucleus | L Inferior frontal gyrus |
| 16 | R Inferior cerebellum | L Putamen |
| 17 | R Caudate nucleus | L Superior temporal gyrus |
| 18 | R Superior cerebellum | R Sensorimotor area |
| 19 | L Supplementary motor area | L Sensorimotor area |
| 20 | L Sensorimotor area | L Supplementary motor area |
| 21 | R Supplementary motor area | L Caudate nucleus |
| 22 | R Sensorimotor area | R Caudate nucleus |
Both speech and resting values are normalized for global flow rates in the same way. The left inferior frontal gyrus is in bold. Note that subtracting the resting state actually reduces the activation in the left inferior frontal gyrus, which is involved in speech production based on the lesion data and the results of performance-based analysis.
Fig. 1.

Mean volume normalized cerebral blood flow (vnCBF) values for the left and right inferior frontal gyrus during syllable repetition by normal speakers (top), and these values following the subtraction of a resting state (bottom). Left and right side average flow values do not differ but subtracted flow values are significantly greater on the right side compared to the left side [t (51) = −3.283; p = .002].
The effect of a resting state contrast is not simply that the gap between functional imaging and lesion results is widened—information is lost. A PBA demonstrated that syllable repetition rates could be predicted by a linear combination of blood flow values obtained during task performance, normalized for global effects. When the PBA using speech rate was performed on the same data following subtraction of a matched resting state, no relationship between regional blood flow and performance could be found (Sidtis et al., 2003).
The results of two different approaches (subtraction and covariance) to contrasting imaging data obtained during rest and task performance make another point about such contrasts. In exploring the relationships between performance and different measures derived from the same image data, the resting state was contrasted with syllable repetition using both simple subtraction and a covariance approach (Sidtis et al., 2003). The regional profiles associated with each approach are presented in Fig. 2. As the profiles produced by subtraction and a covariance analysis are strikingly similar, they likely represent the relative regional differences in blood flow when syllable repetition is compared to its resting state, but they are not informative regarding the functional anatomy of speech production. Neither profile represents data that are predictive of speaking rate and neither profile identifies classical speech areas as regions of peak activity. The problem is not in how contrast is performed, but the contrast itself. With respect to brain activity, such contrasts are likely much more like comparing proverbial apples and oranges than identifying a specific neural circuit in the on and off state.
Fig. 2.
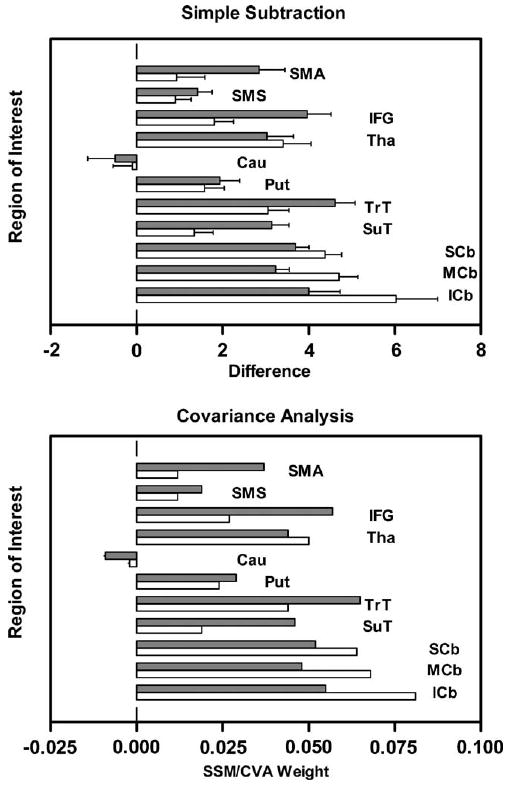
Region-of-interest profiles of the difference between syllable repetition and the resting state (top) and the covariance pattern that distinguishes these two states (bottom). Data for right-sided regions are represented by dark bars; left-sided regions are represented by light bars. The following regions are included: supplementary motor area (SMA), sensorimotor strip (SMS), inferior frontal gyrus (IFG), thalamus (Tha), caudate nucleus (Cau), putamen (Put), transverse temporal (TrT), superior temporal (SuT), and transverse regions of the cerebellum at the superior (SCb), middle (MCb), and inferior (ICb) levels (after Sidtis et al., 2003).
4. The resting state, set, imitation, and the mirror-neuron system
One reason that resting state contrasts fail to enhance the relationship between functional image data and performance data was demonstrated in an analysis of resting state data obtained from the same group of subjects across four different studies performed in the same scanner, using the same methods, but employing four different behavioral tasks (Sidtis et al., 2004). Each study was conducted at a different scanning session, and every scanning session took the form of alternating resting and task scans, repeated four times in a block design. Analyses of the rest data from the four studies revealed significant differences in resting states as a function of the tasks with which they were associated. This effect was not uniform. Different tasks affected different regions during their associated resting states, and some brain regions were influenced more during rest than others. In general, for any given region, blood flow during a resting state was more highly correlated with blood flow during the associated task than with blood flow during the other resting states. The correlation between rest and task was particularly high for the inferior frontal gyrus, an area that appears to be activated in many experimental conditions. The average correlations between blood flow values during rest and task conditions are presented in Fig. 3. The use of a resting state as a contrast condition risks minimizing the magnitude of a blood flow response, at best. At worst, such contrasts can introduce spurious activations and ignore real effects due to the non-uniform influence of different task contexts on different regions.
Fig. 3.
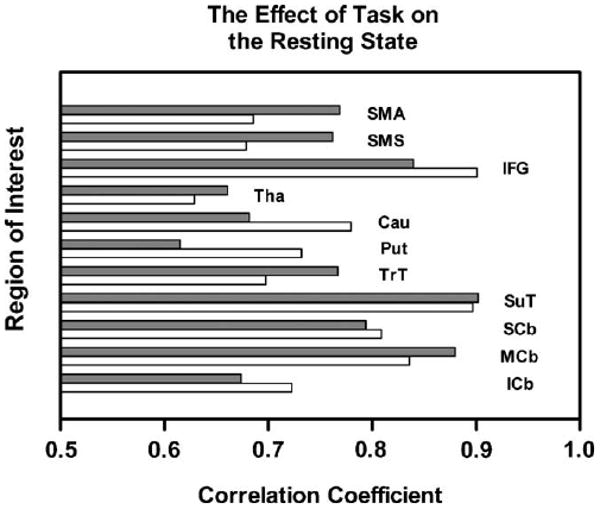
Average correlation values between blood flow measured during task performance and an associated resting state across four studies that employed different tasks. As in Fig. 2, correlation coefficients for right-sided regions are represented by dark bars; left-sided regions are represented by light bars. The following regions are included: supplementary motor area (SMA), sensorimotor strip (SMS), inferior frontal gyrus (IFG), thalamus (Tha), caudate nucleus (Cau), putamen (Put), transverse temporal (TrT), superior temporal (SuT), and transverse regions of the cerebellum at the superior (SCb), middle (MCb), and inferior (ICb) levels (Sidtis et al., 2004).
The effects of the experimental task on the resting state blood flow can be interpreted as evidence of the psychological process referred to as set, a state of readiness to process information in a certain context or to perform a specific task. Set has been characterized as a state of readiness that occurs as a task becomes familiar and performance requires less conscious awareness (Woodworth, 1938). The influence of set was originally described as an unconscious determining tendency (Ach, 1905; Boring, 1929; Miller, 1951). It could be said that the determining tendency for each task in the imaging study was represented in the cerebral blood flow patterns in specific brain regions during the resting states (Sidtis et al., 2004).
The regions most influenced in the resting state by associated tasks have also been identified as part of the imitation (Iacoboni et al., 1999) and mirror-neuron systems (Rizzolatti & Craighero, 2004). These systems have been shown to respond to the observation of actions using a variety of techniques under a range of conditions (Rizzolatti, Fogassi, & Gallese, 2001). Areas in the left inferior frontal gyrus and the superior temporal sulci have been shown to respond when subjects simply observe an action (Grèzes, Armony, Rowe, & Passingham, 2003; Heiser, Iacoboni, Maeda, Marcus, & Mazziotta, 2003; Iacoboni et al., 2001; Iacoboni et al., 1999; Molnar-Szakacs, Iacoboni, Koski, & Mazziotta, 2005; Rizzolatti & Craighero, 2004) as has the right supplementary motor area (Koski, Iacoboni, Dubeau, Woods, & Mazziotta, 2003). These are the regions with some of the highest correlations between blood flow in the resting and task conditions. The resting state effects demonstrate that these regions can maintain an altered physiologic response that may reflect a mental set even in the absence of direct observation. Set, observation, and imitation may be different facets of a system for maintaining mental representations that facilitate behavior. To that extent, it may be important to look at the roles of the cerebellum and caudate nucleus in the imitation system. As will be shown in the next section, an activation approach will likely fail in the case of the caudate nucleus.
5. Are important brain regions eliminated by contrasts?
The analysis of the resting state data revealed another serious problem with the activation approach to identifying brain-behavior relationships. One region, the caudate nucleus, responded differently across experimental tasks, and has been shown to be significantly related to speech rate in performance-based analyses in normal (Sidtis et al., 2003) and ataxic speakers (Sidtis et al., 2006) (Fig. 4), but this brain area maintains similar levels of activity during task performance and rest in both groups. Blood flow in the caudate nucleus was not significantly different when the resting state was compared to performance conditions. Because of this, it was suggested that the caudate nucleus may play a role in maintaining a set for experimental performance conditions during the resting state (Sidtis et al., 2004). In the activation approach, contrasts between the resting and task conditions would eliminate the caudate nucleus from consideration as an important brain area very early in the analysis because there is no difference in contrasting conditions. Any subsequent account of the functional anatomy of a task in which the caudate nucleus or other brain region that behaves in this manner would be flawed, regardless of whether the account is offered in term of individual activation sites or an activation network. The exclusion of functionally significant brain areas by a contrast procedure could be considered a fatal flaw for functional mapping at a systems level. Understanding a brain system, even with a simple model like that depicted in Fig. 4, requires characterizing regional brain activity in one brain region in the context of the activity of the other regions in the system (e.g., Eidelberg, 2007). If the PBA of regional blood flow during syllable repetition was restricted to activated regions, the caudate nucleus would be excluded and the role of Broca's area would be distorted.
Fig. 4.
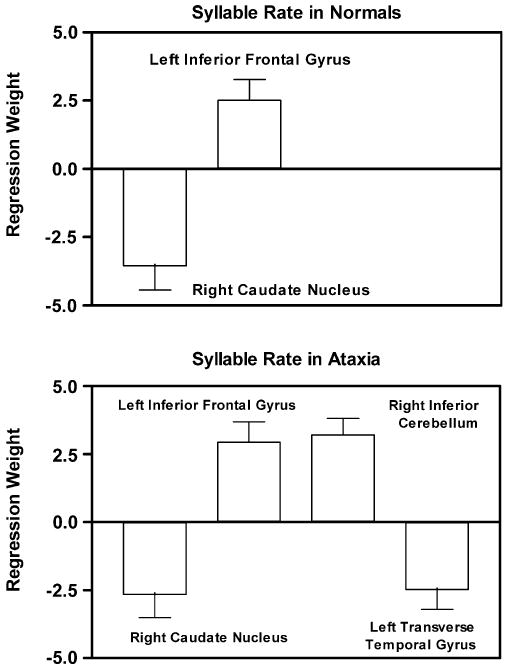
The linear regression weights (±one SEM) for brain regions that predict syllable repetition rates during scanning using a performance-based analysis (PBA). In the normal subjects (top), increased repetition rates are associated with increased blood flow in the left inferior frontal gyrus and decreased flow in the right caudate nucleus. This relationship was replicated in the ataxic speakers (bottom) in whom the model also included the right cerebellum and the left transverse temporal region (after Sidtis et al., 2006).
6. A final word about contrasts
Even if one is to accept the possibility that contrasting images from different conditions makes neurobiological sense, the results of a contrast procedure should still be treated with caution. The results of the common-activated-voxel-across-subjects constraint has been likened to the intersection area of a Venn diagram by one reviewer. The functional significance of small anatomical areas of similarity in the context of large anatomical areas of difference should be an empirical question rather than a starting point of an analytic approach. Rather than assuming that all differences following a contrast are functionally significant, it is worth considering the conditions under which functional imaging data are providing the equivalent of gross functional anatomy and those under which higher resolution localization of specific functions can be studied. Issues of measurement and scale properties are related to this point. It is highly unlikely that blood flow or hemodynamic responses or difference scores with either measure have uniform metric properties across brain regions or across tasks (Sidtis et al., 1999). For example, does a two-percent change in primary visual cortex have the same functional significance as a two-percent change in the inferior frontal gyrus or the hippocampus? During a visual task? During a speech task? During a memory task? Is the response range for a given brain area the same under all conditions? Some perspective on the magnitude and extent of blood flow changes associated with language deficits in cerebrovascular disease can be found in the work on magnetic resonance perfusion imaging by Hillis (2007).
Questions about the functional significance of small areas of difference following image contrasts also loom over the processes of image alignment and warping for voxel-based analyses. For a viable contrast to yield meaningful results, these processes must accurately overcome intersubject anatomical variability without distorting the functional information in the signal related to the behavior under study. This is not a trivial issue. In Broca's area, for example, there are significant intersubject cytoarchitectural differences in Brodmann's areas 44 and 45 and intersubject volume differences in Brodmann's area 44 as great as 10-fold (Amunts et al., 1999). Whereas there is significant progress in characterizing anatomical variability in structural images (e.g., Mazziotta et al., 2001), common spatial normalization schemes do not adequately accommodate the cortex, and intersubject variability in anatomy often remains even after scans have been transformed into a stereotaxic system (Toga & Thompson, 2002). Further, it is not clear that transformations that are appropriate to produce visually acceptable anatomical images are appropriate to lower resolution functional data or to the remapping of functional images to anatomical images. A large difference can be produced by a contrast between two brain regions in which the signal is high relative to the background if there is a misaligned boundary. The misalignment may represent a relatively greater extent of blood flow in one of two largely overlapping regions, which may reflect a true difference in the size of the area engaged in the neural processing, or it may reflect secondary physiological effects (e.g., differences in difficulty, cardiac or respiratory rates), or correlated motion, or image alignment problems, just to pose a few possibilities. A contrast will emphasize differences in such a situation even when the blood flow patterns in the two regions are largely similar.
If expectations are limited to gross functional anatomy rather than detailed localization, the use of a thresholded region-of-interest approach can be used to avoid some of the pitfalls of voxel-based contrasts. Unlike voxel-based analyses that determine activation based on contrast data in common voxels aligned across subjects, the thresholded region-of-interest approach is more likely to tolerate the well-known anatomical variability across subjects as well as the anatomically distorting effects of pathology since regions can be irregular in shape, adjusted in size and location to accommodate individual differences, and a thresholding procedure can estimate regional activity without the constraint of voxel alignment (Sidtis et al., 2006). Both voxel-based and region-of-interest approaches have advantages and disadvantages, but it should be noted that functional activity can be measured without relying on voxel-based activation contrasts.
7. Performance-based analysis (PBA)
Given the divergence between lesion data and activation sites in functional imaging studies in mapping the functional anatomy of speech production, the question of whether any form of the imaging data could predict performance during scanning was addressed (Sidtis et al., 2003). Multiple linear regression with a stepwise method of independent variable inclusion was used to determine the combination of regions, if any, that predicted syllable repetition rate in the task that required subjects to repeat the target syllables as quickly as possible. The aim was to predict performance from brain activity rather than to correlate a behavioral measure with the results of an image analysis. When regional data were normalized to reduce the effects of global differences across subjects, an increased syllable repetition rate was associated with increased flow in the left inferior frontal area and decreased flow in the right caudate nucleus measured during the speech production scans (Fig. 4, top). This relationship was not found when no global normalization was applied to the regional data, nor was it found when the data obtained during speech were contrasted with resting data, either by subtraction or by covariance anaylsis (see Fig. 2).
The presence of the left inferior frontal gyrus in the syllable rate model is consistent with the lesion literature on damage to Broca's area and can be considered a marker for the integrated activity of the left hemisphere cortico-striatal motor control system. The role of the right caudate is less obvious, but it is consistent with clinical (Caplan et al., 1990) and functional imaging (Metter et al., 1988) studies of the role of this area in fluency. Decreased hemodynamic responses in the basal ganglia have also been found to be associated with increased syllable repetition rate with fMRI (Riecker et al., 2006; Riecker et al., 2005; Wildgruber et al., 2001). It has been suggested that the basal ganglia provides timing cues for the initiation of speech segments (Alm, 2004). It may be that the decreased activity in the right caudate nucleus associated with repetition rate reflects a disengagement of right hemisphere motor systems from motor speech control (Sidtis et al., 2006).
The same PBA was applied to regional blood flow data from subjects with hereditary ataxia engaged in the syllable repetition task. The results replicated the inverse relationship between the left inferior frontal gyrus and right caudate and speech rate (Fig. 4, bottom; Sidtis et al., 2006). The performance-based analysis of blood flow in the ataxic speakers not only replicated the functional model of syllable rate in normal speakers, it also introduced two additional regions: the left transverse temporal area and the right cerebellum (Fig. 4, bottom).
8. Partial accounts of complex systems
The presence of the transverse temporal region in the PBA model for speech rate in the ataxic subjects suggests an increased role in auditory feedback in this group. The inverse relationship between blood flow and syllable repetition rate suggests that lower speech rates and more severe dysarthria may be associated with greater use of auditory feedback (higher flow) in ataxic speakers. Auditory feedback is believed to play a role in maintaining an internal model of the relationship between articulation and produced speech sounds to ensure intelligibility (Perkell et al., 1997), a function that may be related to the phenomena previously identified with set, imitation, and mirror image responses.
Activity in the temporal lobe has also been identified as significant in studies of developmental stuttering. Watson, Pool, Devous, Freeman, and Finitzo (1992) reported reduced temporal lobe blood flow in stutterers using single photon emission computed tomography (SPECT), a precursor to PET and fMRI. Using an activation measure, De Nil et al. (2000) found smaller increases in the left superior temporal gyrus in stutterers compared to non-stutterers while Van Borsel, Achten, Santens, Lahorte, and Voet (2003) found similar effects bilaterally. Examining brain-behavior relationships more directly, Braun et al. (1997) found a negative correlation between dysfluency scores and blood flow in auditory areas in the right hemisphere. Fox et al. (2000) reported similar effects bilaterally with negative correlations between stutter rates and blood flow in the superior temporal gyrus. It was argued that the negative correlations represented inhibition in this area suggesting a reduced auditory component in speech production in stutterers. In the ataxic group, it appeared that blood flow in auditory areas increased with increasing difficulty with repetition. These opposite effects may reflect different adaptations to problems with motor speech control stemming from a developmental problem compared to an adult-onset neurodegenerative disease, to different pathophysiological processes, or to different types of dysfluency. They also represent performance-based results that lead to empirical questions relevant to functional brain organization without relying on the notion of activation, which always implies a contrast.
The appearance of the right cerebellum in the PBA model for speech rate in the ataxic subjects is also significant with respect to the lesion experience. While neither the average blood flow nor the results of a resting state contrast reflect the correct cerebellar lateralization for motor speech control, the result of the PBA in ataxic subjects did, just as the PBA correctly identified Broca's area in data where a resting state contrast demonstrated significantly greater flow in the right inferior frontal gyrus.
An examination of the syllable rate models for normal (Fig. 4, top) and ataxic subjects (Fig. 4, bottom) suggests that at the present level of methodological and technical resolution, functional imaging results may best be viewed as providing a surrogate measure of more complicated neural systems. The neurology of motor speech control involves more than the regions identified in the PBA studies described here. Further, a comparison of the normal and ataxic results raises a concern about the claim that functional imaging has the potential for mapping brain function without the need to study of the effects of lesions. We know from changes in speech following deafness that auditory feedback plays a role in normal speech, yet in the series of speech studies described here, the involvement of an auditory area is established in the ataxic speakers but not the normal speakers. Similarly, it is unlikely that the cerebellum plays a role in motor speech control only when it is damaged. Rather, it is more likely that many elements in this control system operate at a level of efficiency at which their contributions remain below the level of detection by current functional imaging techniques. This may be a property of specialized brain systems that works against the activation approach. When the system is stressed by disease, however, the response characteristics change revealing more of its workings (Sidtis et al., 2006). While the study of well-defined neurologic populations may add another layer of complexity to the study of brain-behavior relationships, it appears that an accurate account of functional brain organization will continue to require studies of different neurological diseases, even with functional imaging.
9. Conclusions
Finding the function in functional images requires success in several domains: the accurate measurement of neurobiologically meaningful signals, image processing that does not distort the signal, and the establishment of a relationship between these signals and the behavior under study. Using a clinically well-established fact about brain organization, the lateralization of speech and language, and accepted neurological anatomy of motor control, it has been argued that the activation approach fails to accurately map the functional anatomy of speech production. Contrasts between syllable repetition and other tasks or a resting state do not demonstrate significant blood flow increases in all of the relevant anatomical areas, but do show increases in spurious areas. Further, the magnitude of the differences resulting from such contrasts has no obvious relationship to the functional significance of brain areas to speech production, even in traditional speech areas.
The need for a contrast is the heart of the activation approach in functional imaging, and it presents the most serious problem for this method. The contrast is intended to both decompose a mental process or behavior into a constituent component, and to isolate the brain area or areas responsible for the isolated component. In this approach, the brain-behavior relationship is established by the presence of a significant increase in blood flow or hemodynamic response when the contrasting images are compared. The activation approach not only assumes that mental processes and behaviors can be decomposed, but that neurological systems can also be decomposed in parallel, and further, that a functional isomorphism between brain and behavior can be maintained in the effort. Some of the problems with the decomposition of behavioral measures have been discussed, and there is a century of literature that can be referenced to gauge the difficulties with this effort. However, there is less likelihood of successfully decomposing neurological systems underlying complex behaviors with activation methods since relatively little is understood about their nature, especially as measured indirectly by blood flow or hemodynamic response. Further, Chen and Small (2007) have shown that task variations that alter ecological validity result in differences in functional imaging reliability across normal and patient groups, suggesting another dimension to the problem.
The activation criterion of significant change between conditions is inadequate for models of functional brain organization, if for no other reason, because this criterion excludes important brain areas and includes irrelevant ones. Rather than relying on the activation assumptions, a performance-based analysis reveals brain-behavior relationships in motor speech control that are not present in mean blood flow values or in the results of contrasts. This is a step toward deriving a model, albeit a simple one, of cortical–subcortical interactions during syllable repetition that is consistent with lesion work. The performance-based approach is not a correlation between a performance measure and the results of a contrast. It appears that any contrast has the potential for damaging the functional information in the imaging data. This approach is also not a correlation between a performance measure and the activity in every voxel, which will reflect the high degree of intercorrelation among brain regions in imaging data. The performance-based analysis described in this paper represents an effort to simplify the problem of finding the function in functional images, as is the use of regions-of-interest. Instead of assuming that function will emerge from decompositions of behavioral tasks and unknown brain systems, the performance-based approach seeks to determine the relationship between the behavior in the scanner and the brain data obtained while the behavior is performed. While the brain model for syllable rate is quite simple, it is consistent with lesion work, it is replicable in more than one subject population, and it generates testable hypotheses.
If one accepts the reservations about activation, then symmetrical contrasts (syllable repetition vs phonation and phonation vs syllable repetition), applying conjunction analysis, meta-analyses, and database repositories of activation sites does not ameliorate the problems. Important brain areas excluded by contrasts will not be recovered by these procedures, and areas included for a variety of secondary and spurious reasons will often remain included. Reliability and validity are different concepts, and the reliability of an activation pattern does not insure its validity as a representation of functional brain organization. With respect to decomposition, it is probably more defensible to decompose behavior rather than images until there is a better understanding of what is represented in the images (Sidtis, Anderson, Strother, & Rottenberg, 2001). Instead of contrasting tasks for the purposes of localization, contrasting clinical and normal groups, either at rest (Eidelberg, 2007) or while performing clinically relevant tasks (Sidtis et al., 2006), may provide insights into the properties of brain systems.
It is ironic that activation has been embraced by the cognitive neuroscience approach to the study of brain and behavior, including language. The cognitive side of this endeavor evolved from the cognitive counter-revolution, which accepted the importance of mental processes and rejected behaviorism's dismissal of mental life in favor of stimulus–response relationships (Miller, 2003). On the neuroscience side, though, the activation notion retains a behaviorist's approach to the brain with an assumed constancy between stimulus and response (e.g., articulation activates region X, phonation activates region Y, articulation + phonation activates regions X + Y). Although popular, the simplistic stimulus–response assumptions of the activation approach appear to be no more suited to understanding patterns of brain activity than they were to understanding language from a behaviorist perspective or to the decomposition of reaction times at the start of the last century. Unless by function, one merely means blood flow, the function in functional images must be demonstrated and not simply assumed. Functional imaging alone will not reveal the characteristics of speech and language processing, or any other mental process, because there is currently an inadequate understanding of the characteristics of the brain responses being imaged, at least with respect to brain-behavior relationships. Blood flow is a complex process (Baslow & Guilfoyle, 2007; Drake & Iadecola, 2007) that can increase locally, regionally, or globally to varying degrees in response to some conditions but not others. The relationship between cerebral blood flow measured by functional imaging and speech production strongly argues that blood flow or hemodynamic response increases cannot be the benchmark for the functional mapping of complex behaviors. While imperfect, the lesion experience should remain an important reference point in harnessing the potential of functional imaging for understanding brain-behavior relationships (Fellows et al., 2005; Rorden & Karnath, 2004). The lesion experience should not only be brought to bear when it agrees with a functional imaging result. Understanding the conditions under which the two approaches provide discordant answers will contribute even more to characterizing how behavior is represented in the brain.
Acknowledgments
This work was supported by the Parkinson's Disease Foundation, the Bob Allison Ataxia Research Center, and by NIH R01 DC007658 and P20 MH57180. The help of my collaborators in the studies reviewed in this paper is gratefully acknowledged, as are the thoughtful comments provided by the reviewers.
References
- Ach N. Über die Willenstätigkeit und das Denken. Göttingen: Vandenhoeck & Ruprecht; 1905. [Google Scholar]
- Ackermann H, Vogel M, Peterson D, Poremba M. Speech deficits in ischaemic cerebellar lesions. Neurology. 1992;239:223–227. doi: 10.1007/BF00839144. [DOI] [PubMed] [Google Scholar]
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communicative Disorders. 2004;37:325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Rosengart A, DeWitt LD, Pessin MS, Caplan LR. Anterior inferior cerebellar artery territory infarcts. Archives of Neurology. 1993;50:154–161. doi: 10.1001/archneur.1993.00540020032014. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. The Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Guilfoyle DN. Using proton magnetic resonance imaging and spectroscopy to understand brain “activation”. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.06.119. this issue. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Boring EG. A history of experimental psychology. New York: The Century; 1929. [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering: an H215O positron emission tomography study. Brain. 1997;120:761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, et al. Caudate infarcts. Archives of Neurology. 1990;47:133–143. doi: 10.1001/archneur.1990.00530020029011. [DOI] [PubMed] [Google Scholar]
- Chen E, Small SL. Test–retest reliability in fMRI of language: group and task effects. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.04.015. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AE, Wada JA. Speech dominance and handedness in the normal human. Brain and Language. 1978;5:42–55. doi: 10.1016/0093-934x(78)90006-8. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2000;43:1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- Donders FC. Over de snelheid van psychische processen. Nederlandsch Archief voor Genees- en Natuurkunde. 1869;4:117–145. [Google Scholar]; Koster WG, translator. Acta Psychologica. Vol. 30. 1969. On the speed of mental processes; pp. 412–431. [DOI] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. Role of neuronal signaling in controlling cerebral blood flow. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.08.002. this issue. [DOI] [PubMed] [Google Scholar]
- Duara R, Gross-Glenn K, Barker WW, Chang JY, Apicella A, Lowenstein D, et al. Behavioral activation and the variability of cerebral glucose metabolic measurements. Journal of Cerebral Blood Flow and Metabolism. 1987;7:266–271. doi: 10.1038/jcbfm.1987.62. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. The assessment of neurological systems with functional imaging. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.06.010. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive neuroscience. Journal of Cognitive Neuroscience. 2005;17:850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production: a PET performance-correlation analysis. Brain. 2000;123:1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RSJ, Dolan RJ. The trouble with cognitive subtraction. NeuroImage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Grecius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. NeuroImage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Review Neuroscience. 2001;2:685–692. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC. The essential role of Broca's area in imitation. European Journal of Neuroscience. 2003;17:1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x. [DOI] [PubMed] [Google Scholar]
- Hansen LK. Multivariate strategies in functional magnetic resonance imaging. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.12.004. this issue. [DOI] [PubMed] [Google Scholar]
- Hillis A. Magnetic resonance perfusion imaging in the study of language. Brain and Language. 2007 doi: 10.1016/j.bandl.2006.04.016. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. Journal of Cerebral Blood Flow and Metabolism. 1984;4:484–499. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Human Brain Mapping. 2001;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proceedings of the National Academy of Sciences of United States of America. 2001;98:2526–2528. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Zamarripa F. Is overt stuttered speech a prerequisite for the neural activations associated with chronic developmental stuttering? Brain and Language. 2000;75:163–194. doi: 10.1006/brln.2000.2351. [DOI] [PubMed] [Google Scholar]
- Jennings JM, McIntosh AR, Kapur S, Tulving E, Houle S. Cognitive subtractions may not add up: the interaction between semantic processing and response mode. NeuroImage. 1997;5:229–239. doi: 10.1006/nimg.1997.0257. [DOI] [PubMed] [Google Scholar]
- Junck L, Gilman S, Rothley JR, Betley AT, Koeppe RA, Hichwa RD. A relationship between metabolism in frontal lobes and cerebellum in normal subjects studied with PET. Journal of Cerebral Blood Flow and Metabolism. 1988;8:774–782. doi: 10.1038/jcbfm.1988.132. [DOI] [PubMed] [Google Scholar]
- Kent RD, Tjaden K. Brain functions underlying speech. In: Hardcastle WJ, Laver J, editors. Handbook of phonetic sciences. Oxford: Blackwell; 1997. pp. 220–255. [Google Scholar]
- Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC. Modulation of cortical activity during different imitative behaviors. Journal of Neurophysiology. 2003;89:460–471. doi: 10.1152/jn.00248.2002. [DOI] [PubMed] [Google Scholar]
- Leeper R. Cognitive processes. In: Stevens SS, editor. Handbook of experimental psychology. New York: John Wiley & Sons; 1951. pp. 730–757. [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter JE, Riege WH, Hanson WR, Phelps ME, Kuhl DE. Evidence for a caudate role in aphasia from FDG positron computed tomography. Aphasiology. 1988;2:33–43. [Google Scholar]
- Miller GA. Speech and language. In: Stevens SS, editor. Handbook of experimental psychology. New York: John Wiley & Sons; 1951. pp. 789–810. [Google Scholar]
- Miller GA. The cognitive revolution: a historical perspective. Trends in Cognitive Sciences. 2003;7:141–144. doi: 10.1016/s1364-6613(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathological and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segration within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cerebral Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Murphy K, Cornfield DR, Guz A, Fink GR, Wise RJS, Harrison J, et al. Cerebral areas associated with motor control of speech in humans. Journal of Applied Physiology. 1997;83:1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Newman SD, Tweig DB, Carpenter PA. Baseline conditions and subtractive logic in neuroimaging. Human Brain Mapping. 2001;14:228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkell J, Matthies M, Lane H, Guenther F, Wilhelms-Tricaro R, Wozniak J, et al. Speech motor control: acoustic goals, saturation effects, auditory feedback and internal models. Speech Communication. 1997;22:227–250. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Meyer J, Dogil G, Haider H, et al. Articulartory/phonetic sequencing at the level of the anterior perisylvian cortex: a functional magnetic resonance imaging (fMRI) study. Brain and Language. 2000;75:259–276. doi: 10.1006/brln.2000.2356. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Gröschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. NeuroImage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, et al. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Dogil G, Haider H, Grodd W, Ackermann H. Hemispheric lateralization effects of rhythm implementation during syllable repetitions: an fMRI study. Neuroimage. 2002;16:169–176. doi: 10.1006/nimg.2002.1068. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Grodd W, Ackermann H. Reorganization of speech production at the motor cortex and cerebellum following capsular infarction: a follow-up functional magnetic resonance imaging study. Neurocase. 2002;8:417–423. doi: 10.1076/neur.8.5.417.16181. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Reviews of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schiff HB, Alexander MP, Naesser MA, Galaburda AM. Aphemia: clinical-anatomic correlations. Archives of Neurology. 1983;40:720–727. doi: 10.1001/archneur.1983.04050110038005. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Varge M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cerebral Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ. From chronograph to functional image: what's next? Brain and Cognition. 2000;42:75–77. doi: 10.1006/brcg.1999.1166. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Anderson JR, Strother SC, Rottenberg DA. Establishing behavioral correlates of functional imaging signals. In: Gjedde A, Hansen SB, Knudsen GM, Paulson OB, editors. Physiological imaging of the brain with PET. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Sidtis JJ, Gomez C. Maintaining speech in early neurodegenerative disease: Broca's activity increases while other areas decline. Brain and Language. 2003;87:201. [Google Scholar]
- Sidtis JJ, Gomez C, Groshong A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. NeuroImage. 2006;31:246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Anderson JR, Rottenberg DA. Are brain functions really additive? NeuroImage. 1999;5:490–496. doi: 10.1006/nimg.1999.0423. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. Predicting performance from functional imaging data: methods matter. NeuroImage. 2003;20:615–624. doi: 10.1016/S1053-8119(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. The effect of set on the resting state in functional imaging: a role for the striatum? NeuroImage. 2004;22:1407–1413. doi: 10.1016/j.neuroimage.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of United States of America. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. New approaches in brain morphometry. American Journal of Geriatric Psychiatry. 2002;10:13–23. [PubMed] [Google Scholar]
- Tognola G, Vignolo LA. Brain lesions associated with oral apraxia in stroke patients: a clinico-neuroradiological investigation with the CT scan. Neuropsychologia. 1980;18:257–272. doi: 10.1016/0028-3932(80)90122-0. [DOI] [PubMed] [Google Scholar]
- Urban PP, Wicht S, Vukurevic G, Fitzek C, Stoeter P, Massinger C, et al. Dysarthria in acute ischemic stroke. Neurology. 2001;56:1021–1027. doi: 10.1212/wnl.56.8.1021. [DOI] [PubMed] [Google Scholar]
- Urban PP, Marx J, Hunsche S, Gawehn J, Vucurevic G, Wicht S, et al. Cerebellar speech representation. Archives of Neurology. 2003;60:965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Van Borsel J, Achten E, Santens P, Lahorte P, Voet T. fMRI of developmental stuttering: a pilot study. Brain and Language. 2003;85:369–376. doi: 10.1016/s0093-934x(02)00588-6. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. Does functional neuroimaging solve the questions of neurolinguistics? Brain and Language. 2007 doi: 10.1016/j.bandl.2006.05.006. this issue. [DOI] [PubMed] [Google Scholar]
- Van Zandt T, Ratcliff R. Statistical mimicking of reaction time data: single process models, parameter variability, and mixtures. Psychonomic Bulletin & Review. 1995;2:20–54. doi: 10.3758/BF03214411. [DOI] [PubMed] [Google Scholar]
- Watson BC, Pool KD, Devous MD, Freeman FJ, Finitzo T. Brain blood flow related to acoustic laryngeal reaction time in adult developmental stutterers. Journal of Speech and Hearing Research. 1992;35:555–561. doi: 10.1044/jshr.3503.555. [DOI] [PubMed] [Google Scholar]
- Watt HJ. Experimentelle Beiträge zu einer Theorie des Denkens. Archiv für die gesamte Psychologie. 1905;4:289–436. [Google Scholar]
- Weiller C. Imaging recovery from stroke. Experimental Brain Research. 1998;123:13–17. doi: 10.1007/s002210050539. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. NeuroImage. 2001;13:101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Greene J, Büchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental psychology. New York: Henry Holt and Company; 1938. [Google Scholar]


