Abstract
Naïve Indian rhesus macaques were immunized with a mixture of optimized plasmid DNAs expressing several SIV antigens using in vivo electroporation via the intramuscular route. The animals were monitored for the development of SIV-specific systemic (blood) and mucosal (bronchoalveolar lavage) cellular and humoral immune responses. The immune responses were of great magnitude, broad (Gag, Pol, Nef, Tat, Vif), long-lasting (up to 90 weeks post 3rd vaccination) and were boosted with each subsequent immunization, even after an extended 90 week rest period. The SIV-specific cellular immune responses were consistently more abundant in BAL than in blood, and were characterized as predominantly effector memory CD4+ and CD8+ T cells in BAL and as both central and effector memory T cells in blood. SIV-specific T cells containing Granzyme B were readily detected in both blood and BAL, suggesting the presence of effector cells with cytolytic potential. DNA vaccination also elicited long-lasting systemic and mucosal humoral immune responses, including the induction of Gag-specific IgA. The combination of optimized DNA vectors and improved intramuscular delivery by in vivo electroporation has the potential to elicit both cellular and humoral responses and dissemination to the periphery, and thus to improve DNA immunization efficacy.
Keywords: DNA vaccine, AIDS vaccine, rhesus macaque, BAL, PBMC, rectal mucosa, humoral immune response, cellular immune response, IFN-γ, TNFα, IL-12, adjuvant, immunology
1. Introduction
HIV-1 infection occurs mostly through sexual contact. Innate and adaptive immune responses at the site of viral entry provide an early and important barrier against disseminating HIV infection (for reviews see [1–4]). Therefore, vaccination strategies able to induce potent systemic as well as mucosal immune responses are likely to be the most successful in preventing HIV-1 infection and progression to AIDS. Different mucosal vaccination methods have been examined to test the hypothesis that mucosal delivery of the antigen is important for the generation of mucosal immunity (for reviews see [5–7] and references therein). Several groups have explored vaccination modalities in macaques able to induce mucosal immunity. These include DNA immunization via intradermal and mucosal delivery, DNA-prime combined with recombinant virus or bacterial vector boost, use of recombinant viruses, virus particles, or peptides [8–29]. Vaccination with DNA alone via the intramuscular route in the absence of systemic or mucosal recombinant virus or protein boost did not result in detectable mucosal immune responses [30–33].
The use of DNA alone as vaccination method is a promising immunization strategy that has advantages (production, stability, repeated use) over other vaccination modalities. DNA vaccination was reported to induce systemic and mucosal immune responses when co-administered intradermally and intrarectally in rhesus macaques [16, 20, 22]. Upon intramuscular (IM) DNA administration in chimpanzees [14], the induction of HIV-1 Gag-specific IgA was reported in breast milk but not in mucosa. Intramuscular DNA vaccination by needle and syringe in the absence of a heterologous boost has resulted in relatively low levels of systemic immune responses in primates and especially humans [4, 34–40]. Despite the low immune responses, protection resulting in reduced viremia has been reported in DNA vaccinated macaques challenged with SIV or SHIV [40–46]. Substantial improvement in gene expression and immunogenicity of DNA vaccines has been achieved by in vivo electroporation [47–57], especially in macaques [53–58]. Upon high dose challenge with pathogenic SIVmac251, we found improved control of viremia both in the acute and chronic phase in animals vaccinated using in vivo electroporation as DNA delivery method compared to the previously used needle/syringe DNA delivery [40, 58].
The purpose of the present work was to examine the ability of intramuscular vaccination, using the in vivo electroporation as DNA delivery method, to induce cellular and humoral immune responses and, importantly, to monitor their dissemination to mucosal sites. The animals described in this study were immunized in order to generate SIV-specific T-cell clones for adoptive transfer studies [59], and thus, were not available for virus challenge. In addition to the induction of abundant and broad SIV-specific immune responses, we report a significantly higher frequency of responses in bronchoalveolar lavage (BAL) compared to blood, that these responses were long-lasting (~2 years) and could be further boosted after an extended rest period of 90 weeks. Thus, IM DNA delivery is able to induce both systemic and mucosal SIV-specific immune responses.
2. Materials and Methods
2.1 DNA vectors
RNA optimized (referred to also as “codon optimized”) [60–63] SIVmac239 genes were inserted into pCMV.kan [40]. pCMVgagDX (1S) expresses the native myristoylated p57gag; MCP-p39gag (21S) expresses a secreted p39gag protein generated by N-terminal fusion with IP10-MCP3 [40, 55, 64]. SIV pol (103S) and the Nef-Tat-Vif fusion protein (147S) were expressed in pCMVLAMP.kan as fusion proteins with the lysosomal associated membrane protein 1 (LAMP-1) [65] (V. Kulkarni et al., in preparation). Rhesus macaque IL-12 plasmid (AG3-WLVrmIL12) was co-injected as molecular adjuvant. This plasmid is similar to the previously reported IL-12 plasmid [66], but expresses RNA optimized IL-12 p35 and p40 subunits, which result in higher levels of secreted cytokine (R. Jalah et al, in preparation).
2.2 Immunization and electroporation
The Indian rhesus macaques (Macaca mulatta) were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International. The DNA mixture contained a total of 2 mg of plasmid DNA (250 µg of each of the two SIV gag plasmid, 500 µg each of the pol and nef-tat-vif plasmids, and 500 µg of the IL-12 plasmid) in 1 ml water. These DNAs were injected intramuscularly (0.5 ml per injection) into the left and right thighs using the CELLECTRA® adaptive constant-current electroporator (Inovio).
2.3 Analysis of antigen-specific cellular immunity
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll separation. BAL fluid was collected after perfusion of 50 ml PBS into the lungs and cells were recovered by centrifugation, followed by two rinses with PBS. Cells were incubated at 1.5×106 cells/ml in complete RPMI-1640 in the presence or absence of pools of overlapping peptides (15-aa peptides overlapping by 11 aa; Infinity Inc. Biotech Research and Resource, Aston, PA) derived from SIV Gag, Pol, Nef, Tat, and Vif at a final concentration of 1 µg/ml for each peptide. Cells were cultured for 12 hours with monensin (Golgi Stop, BD Pharmingen, San Jose, CA) to inhibit cytokine secretion.
2.4 Flow cytometric analysis
Immunostaining and flow cytometric analysis was performed as described [55, 58]. Briefly, cell surface staining was first performed using the following antibody cocktail: CD3-APCCy7, CD4-PerCPCy5.5, CD45RA-PE, CD28-Biotin (BD Pharmingen) and CD8-AF405 (Invitrogen, Carlsbad, CA). Cells were washed with wash buffer containing 0.2% human AB serum (Atlanta Biologicals) in Dulbecco’s Phosphate-Buffered Saline without calcium and magnesium (Mediatech Cellgro), stained with streptavidin APCCy5.5 (Invitrogen), washed, fixed, and permeabilized with the BD Cytofix/Cytoperm kit (BD Pharmingen). Intracellular cytokine staining was performed using IFN-γ-FITC, IL-2-APC and TNFα-PECy7 antibodies (BD Pharmingen). To determine cytotoxic T cells, staining was performed using the following labeled antibodies against cell surface molecules: CD3-APCCy7, CD4-AmCyan, CD8-AF405, CD28-PerCPCy5.5, CD45RA-AF700, CD95-FITC and CCR7-APC. After fixing and permeabilizing the cells, intracellular staining was performed using anti-IFN-γ-PECy7 (BD Pharmingen) and anti-Granzyme B-PE (Invitrogen) monoclonal antibodies. After intracellular staining, the cells were washed and samples were acquired on a FacsAria or a LSRII (BD Pharmingen). Data analysis was performed using the FlowJo platform (Tree Star Inc., Ashland, OR) and all antigen specific responses were reported after subtracting values obtained from incubating samples with medium alone.
2.5 Humoral responses
The end-point antibody titers against SIV Gag and Nef were determined by ELISA, using duplicates of serially diluted plasma samples. The mean absorbance (A450) plus 3 standard deviations (SD) obtained with non-immune rhesus macaque plasma were applied as cut-off values [40]. SIVgag-specific IgG and IgA antibodies in plasma and BAL were measured by an ELISA similar to that described previously [20, 22, 67]. Plates were coated overnight at 4°C with 200 ng Gag/well and the wells were then washed and blocked with 1% BSA in deionized water for 2 hrs at 37°C. The samples (plasma diluted 1:5; undiluted BAL fluid) were added to duplicate wells and incubated for 1 hr. The plates were washed with 1X wash buffer (cat no. 50-63-00, KPL, Gaithersburg, MD) and incubated with horse radish peroxidase-conjugated anti-monkey immunoglobulin IgA or IgG (Alpha Diagnostics International, San Antonio, TX) diluted 1:10,000 in blocking solution for 1 hr, followed by washing with wash buffer and development with TMB (3,3’, 5,5’-tetramethylbenzidine, SIGMA) peroxidase substrate at room temperature until color developed. The reaction was stopped using 2 M sulfuric acid and the absorbance was read at A450 nm. The background of the assay was determined using samples from 4 naïve animals. The mean absorbance A450 values obtained for SIV-specific IgA or IgG from the vaccinated and naive animals were plotted. Similarly, total IgG and IgA levels were determined as described [67] and compared to standard curves of known antibody concentrations. The specific activity of the antibody responses was calculated by dividing the amount of Gag-specific antibody (in ng/ml) by the amount of total IgG or IgA (in µg/ml) in each sample.
3. Results
3.1 SIV-specific cellular immune responses in blood
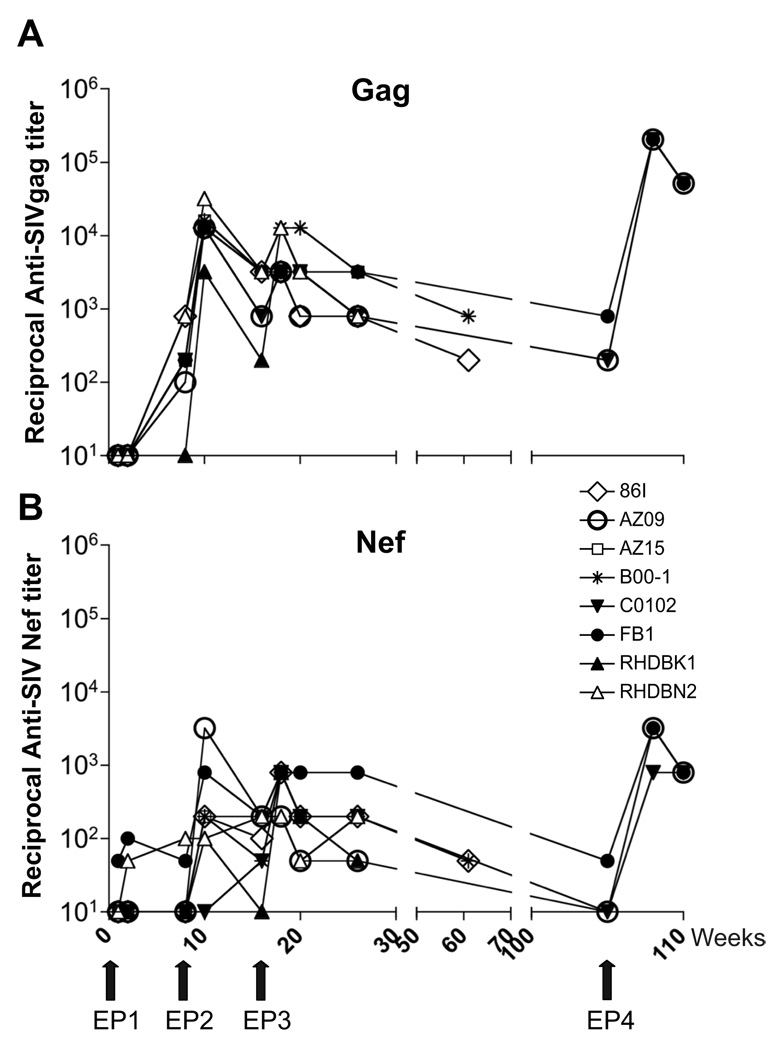
Eight naïve Indian rhesus macaques received 3 DNA vaccinations at weeks 0, 8, 16 as outlined in Fig. 1A. The study was terminated at week 26 (10 weeks post EP3) for 3 animals (AZ15, RHDBK1, RHDBN2). Two of the animals (86I and B-001) were monitored after another 35 weeks (week 45 post EP3). Three of the animals (FB1, AZ09, C0102) were available 90 weeks post EP3 for a 4th vaccination. The vaccine included a mixture of optimized DNA plasmids producing several SIVmac239 proteins (Gag, Pol, Nef, Tat and Vif) and also contained an optimized rhesus IL-12 expression plasmid as molecular adjuvant, previously shown to augment immune responses [57, 66, 68, 69]. The animals were monitored for the development of SIV-specific cellular and humoral immune responses both in blood and bronchoalveolar lavage (BAL). The analysis of BAL provides evaluation of adaptive immune responses at mucosal effector sites, and serves as an easily accessible extra-lymphoid mucosal compartment [70, 71].
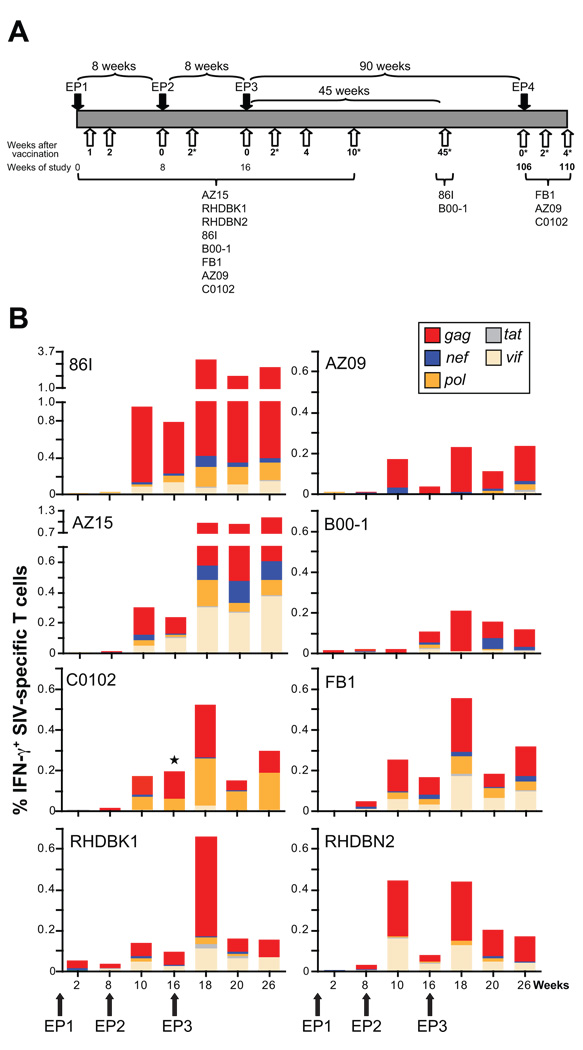
Fig. 1.
DNA vaccination induces SIV-specific responses. (A) Outline of study. Indian rhesus macaques were vaccinated using in vivo DNA electroporation three times (EP1, EP2, EP3) at weeks 0, 8 and 16. Some of the animals were subjected to a 4th vaccination (EP4) at week 106. The animals were monitored for cellular and humoral immune responses in blood (open arrows) and BAL (indicated by *). All animals were monitored up to week 26 post EP3. Two animals were also evaluated at week 61 (45 weeks post EP3) and 3 animals were evaluated at week 106 (90 weeks post EP3) at which point they received EP4. (B) Frequency and distribution of SIV-specific IFN-γ+ T cells in blood. The antigen-specific IFN-γ production for all 8 animals was obtained by measuring the frequency of IFN-γ+ T cells obtained upon incubation with the indicated peptide pools. ⋆, vif responses for animal C0102 were not determined at week 16 due to limited sample availability. Note that different scales were used for the different animals.
The frequency of antigen-specific IFN-γ secreting T cells in the blood of the 8 vaccinated macaques was measured upon stimulation with peptide pools spanning five individual SIV proteins (Gag, Pol, Nef, Vif and Tat) using polychromatic flow cytometry (Fig. 1B). SIV-specific IFN-γ producing T cells were detected at a low frequency by week 2 post EP1 in some animals (range <0.01 to 0.06% of the circulating T cells with a median of 0.01%). These responses were increased in most animals by week 8 post EP1 (median of 0.03%). The 2nd vaccination administered at week 8 (EP2) led to marked increases of the circulating T cells in response to multiple antigens (EP2 week 2; range 0.02–0.95%, median of 0.22%). The elevated responses persisted for the following 8 weeks. EP3 led to a further boosting of the cellular immune responses (EP3 week 2, range 0.21–3.2%; median of 0.54%). All animals maintained high levels of immune responses up to week 26 (10 weeks post EP3; range 0.12–2.63%; median of 0.27%). Peak responses for most animals were found at 2 weeks post EP3 with a range of 0.21–3 % of the circulating T cells. Circulating SIV-specific IFN-γ producing T cells were detected at significant frequencies (0.1–2.6%) up to week 10 post EP3, demonstrating duration of the vaccine effect. In fact, animals monitored for up to week 90 post EP3 had detectable SIV Gag-specific IFN-γ+ T cells in blood (see below, Fig. 2).
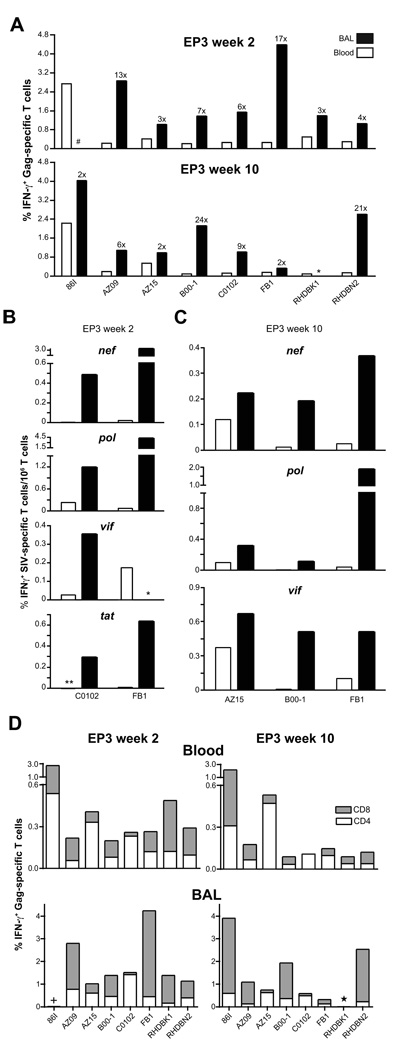
Fig. 2.
Induction of cellular and mucosal immune responses. (A) The frequency of SIV Gag-specific IFN-γ producing T cells in BAL (filled bars) and blood (open bars) at weeks 2 and 10 post EP3 is shown. The numbers indicate the fold increases in BAL compared to blood. *, BAL sample not available for RHDBK1 at week 10 post EP3. #, animal 86I had very low Gag responses at week 2 post EP3. (B) Cellular immune responses to other antigens. Comparison of the frequency of SIV (nef, pol, vif and tat)-specific IFN-γ producing T cells in BAL (filled bars) and blood (open bars) for animals C0102 and FB1 at week 2 post EP3. *, no Vif responses were measured in BAL from animal FB1 due to insufficient cell availability. **, Tat responses in blood for animal C0102 not detected. (C) Frequency of SIV (nef, pol, and vif)-specific IFN-γ producing T cells in BAL (filled bars) and blood (open bars) at week 10 post EP3 for animals AZ15, B00-1, and FB1. (D) Frequency and distribution of Gag-specific IFN-γ+ T cell subsets in blood and BAL at weeks 2 and 10 post EP3. Gag-specific T cells CD4+ (white bars) and CD8+ (grey bars) are plotted. *, BAL not available for RHDBK1. +, very low Gag responses for animal 86I at week 2 post EP3 in BAL.
We noted an animal-to-animal variability in the overall extent of cellular immune responses as well as differences in the ability to respond to individual antigens. Comparison of the cellular responses against the different viral proteins revealed that Gag was the most immunogenic antigen, and Tat elicited the weakest immunity. Strong responses to Vif were found in 5 of the animals (monkeys 86I, AZ15, FB1, RHDBK1, RHDBN2). Responses to Pol and Nef were found in all the animals, with responses to Pol being stronger. These data demonstrate great induction of de novo systemic cellular immune responses to the different antigens produced by the vaccine mixture and corroborate our recent finding [58], where we reported high levels of systemic cellular immune responses upon DNA-only vaccination by EP using different SIV DNA vaccine mixtures.
3.2 Detection of higher levels of SIV-specific responses in BAL compared to blood
To examine mucosal dissemination of the SIV-specific immunity following in vivo DNA electroporation via the intramuscular route, lymphocytes isolated from BAL fluid at selected time points (see Fig. 1A) were examined for the presence of SIV-specific cellular immune responses. Responses to antigens other than Gag could not be addressed consistently in all animals due to variable yields of T lymphocytes. High frequencies of long-lasting Gag-specific IFN-γ T cells were detected in the BAL for all the animals at week 2 and 10 post EP3 (Fig. 2A). Comparisons with the corresponding Gag-specific responses in the blood revealed that the frequency of the T cell responses in BAL was greatly elevated (ranging from 2–24× at weeks 2 and 10 post EP3). The animals were also analyzed for the presence of Nef-, Pol-, Tat- and Vif-specific responses in BAL, (Fig. 2B and C). Fig. 2B shows the data for 2 of the animals (C0102 and FB1) indicating that the responses to these antigens were also elevated in BAL compared to blood and these increased responses in BAL to multiple antigens persisted over long periods of time (Fig. 2C, data from week 10 post EP3; AZ15, B00-1, FB1). Thus, DNA vaccination by EP resulted in the generation of broad cellular immunity against all administered antigens, both systemically and in a mucosal effector site.
The frequency of CD4+ and CD8+ T cell populations within the SIV Gag-specific response was also monitored in blood and BAL (Fig. 2D). Analysis of blood from all 8 vaccinated animals (Fig. 2D, upper panel) demonstrated the presence of both CD4+ and CD8+ Gag-specific T cells at weeks 2 and 10 post EP3. Analysis of BAL (Fig. 2D, lower panel) showed a greater proportion of CD8+ Gag-specific T cells compared to that in blood and demonstrated that intramuscular DNA vaccination using electroporation could elicit mucosal SIV-specific immunity consisting predominantly of CD8+ T cells.
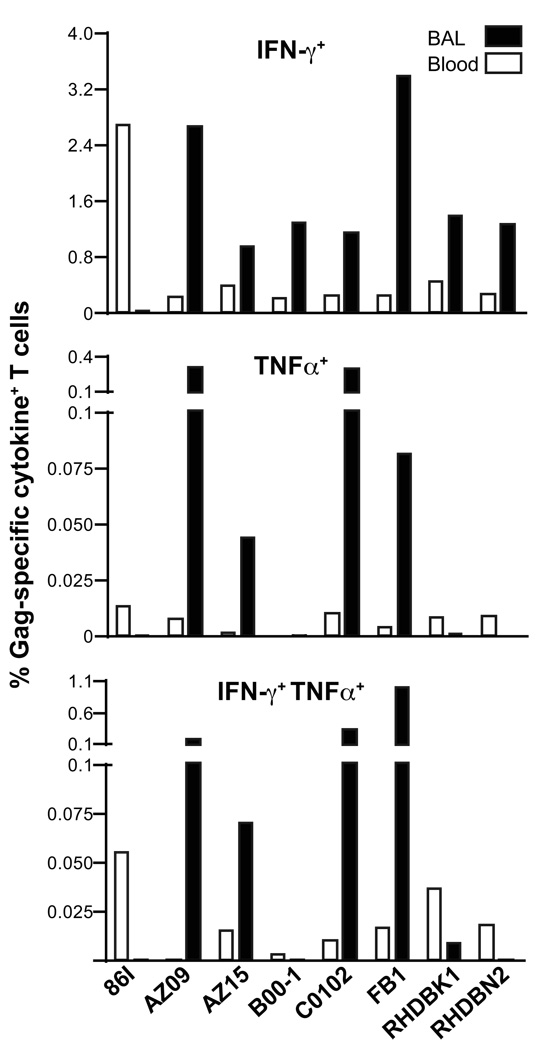
To further characterize the cellular immune responses, the SIV-specific T cells were examined for their ability to produce other cytokines in addition to IFN-γ. T lymphocytes, isolated from BAL and blood, were analyzed by intracellular staining for their ability to secrete IFN-γ, TNFα and IL-2 upon SIV peptide stimulation (Fig. 3). SIV-specific cells producing IL-2 were not found, and this may be related to IL-12 DNA co-administration [68]. The majority of the Gag-specific T cells were IFN-γ+, although low frequency of TNFα+ single or dual cytokine (IFN-γ+ TNFα+) positive cells were found in both peripheral blood and T cells recovered from BAL fluid.
Fig. 3.
SIV-specific responses in blood and BAL. T cells isolated from blood and BAL were analyzed by polychromatic flow cytometry for the production of IFN-γ and TNFα only or the combination thereof upon stimulation with the SIV Gag peptide pool. Note the different scales for the plots. Data shown is for EP3week2.
3.3 Induction of very long-lasting SIV-specific immune responses in blood and BAL that can be boosted after an extended rest period
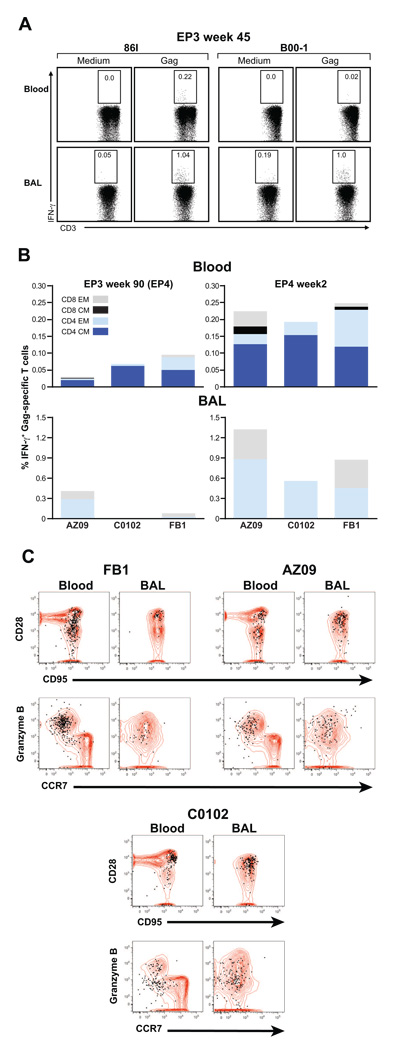
Some of the animals included in the study (see Fig. 1A) were monitored for a longer period (Fig. 4). We found persistence of Gag-specific responses in BAL and blood in two macaques, 86I and B00-1, analyzed 45 weeks post EP3 (Fig. 4A). In addition, the vaccine-induced IFN-γ+ memory T cells were still detectable at ~2 years after the last immunization (90 weeks post EP3) both in blood (all 3 macaques) and BAL (2 of 3 macaques, see Figure 4B, left panels). Responses to the other SIV antigens included in the vaccine were not detected at this time (data not shown).
Fig. 4.
SIV-specific responses in blood and BAL are long lasting and can be boosted after a ~2 years rest period. (A) Detection of SIV Gag-specific T cells in blood and BAL in animals 86I (left) and B00-1 (right) at week 45 post EP3. The frequency of the SIV Gag-specific IFN-γ+ T cells in blood (upper panel) and BAL (lower panel) are shown. (B) Frequency and distribution of Gag-specific IFN-γ+ T cell subsets (CD4+ and CD8+ effector (EM) and central memory (CM) cells) in blood and BAL of AZ09, C0102, and FB1 at 90 weeks post EP3 (EP4) (left panel). Induction of cellular immune responses in blood and BAL upon EP4 were measured 2 weeks later (right panel). Note that different scales were used for the analysis of blood and BAL. (C) The plots show overlays of the total T cell population (red contours) in blood and BAL with the Gag-specific cells as measured by IFN-γ production upon peptide stimulation (black dots). The cells were analyzed for memory markers (CD28, CD95, upper panel). The naïve population, defined by CD28+ CD95low / negative, does not contain any IFN-γ+ cells and is absent in BAL. The functionality of the Gag-specific CCR7− memory cell population (lower panel) was assessed by testing for their ability to produce Granzyme B as shown for animals FB1, AZ09, C0102.
These animals were subjected to a 4th immunization 90 weeks after the 3rd (EP4, as outlined in Fig. 1A). Analysis of blood and BAL samples obtained two weeks after EP4 demonstrated a boost in Gag-specific responses with higher frequencies of antigen-specific cells in BAL than in blood, as noted for the previous immunizations (Fig. 4B, right panels). Cell surface staining combining monoclonal antibodies against CD4, CD8, CD28, CD45RA, CD95 and CCR7 demonstrated that the Gag-specific T cells in BAL were mostly EM T cells, whereas in blood both CM and EM CD4+ and CD8+ SIV-specific T cells were detected. We evaluated the phenotype and functionality of the vaccine-induced cell populations at 4 weeks post EP4 (Fig. 4C). These Gag-specific IFN-γ+ T cells were CD28+CD95+ and CD28−CD95+ in blood and mainly CD28+CD95+ in BAL (Fig. 4C; upper panel). Further analysis showed that these cells lack CCR7, indicating an effector memory phenotype (lower panel). This finding is consistent with enrichment for terminally differentiated effector memory T cells in the lung, an extralymphoid effector site [70]. The CCR7− memory T cell population was further analyzed for their ability to produce Granzyme B. Figure 4C (bottom panel) shows that these cells produced Granzyme B, demonstrating that they have cytolytic potential (animals FB1, AZ09, C0102; Fig. 4C). Therefore, DNA-only vaccination may induce functional cytotoxic effector T cells both in blood and BAL from a long-lasting pool of antigen-specific memory cells detectable for ~2 years post vaccination. The possibility that effector cells are maintained (Fig. 4B) and readily boosted by DNA vaccination is interesting in view of the important role of effector cells in the rapid containment of virus early after infection [72].
3.4 Induction of long-lasting humoral responses in blood and BAL
Plasma and BAL fluids were examined for the presence of humoral responses to Gag and Nef (Fig. 5). The vaccinated animals (7 of 8) showed anti-SIV-Gag antibodies (IgG) in the plasma after the first DNA vaccination (week 8 post EP1; Fig 5A). These responses were boosted by EP2 and EP3 in all 8 animals reaching peak titers ranging from 1:3,200–1:32,000 (median titer 1:12,800). The humoral responses against Gag persisted, although to a lower level, with titers ranging from 1:800 to 3200 at week 10 post EP3. At this time point, the analysis of the BAL fluid demonstrated the presence of Gag-specific IgG in all the vaccinated animals (data not shown). Some of the animals were also analyzed at later time points such as week 45 post EP3 (animals 86I and B00-1) and week 90 post EP3 (animals FB1, AZ09, C0102). Figure 5A shows that these animals were persistently positive with no significant changes in the Gag antibody titers. Following EP4, we found a rapid increase of Gag responses to very high titers (~1:200,000) in all the vaccinated animals. We noted that the antibody titers obtained after EP4, administered after the extended rest period of 90 weeks, were higher than those obtained during the preceding immunizations.
Fig. 5.
DNA vaccination induced significant SIV-specific humoral responses. Anti-SIV-Gag (A) and anti-SIV-Nef (B) antibody titers were measured in plasma and the end-point dilution titers at indicated time points are shown for all animals. Animals 86I and B00-1 were available for analysis up to week 45 post EP3. Animals FB1, AZ09, C0102 were available at week 90 post EP3, at which time point they were subjected to a fourth DNA Electroporation (EP4). End-point dilutions were obtained upon testing serial dilutions of the plasma samples starting at EP1 week 1. Titers are reported as the reciprocal of the highest positive dilution.
All animals developed systemic anti-SIV-Nef binding antibodies (Fig. 5B), which were 1–1.5 logs lower than the Gag responses. The responses were detected after EP1 (2 of 8 animals) and were boosted in all animals upon the following vaccinations with peak titers ranging from 1:200–1:800 (median 1:800) at 2 weeks post EP3. The humoral responses against Nef were also detected in all the animals at week 10 post EP3. The two animals (B00-1, FB1), which showed Gag responses at week 45 post EP3, also maintained anti-Nef antibody responses at this time point. Analysis of the Nef responses at week 90 post EP3 showed that 1 of the 3 animals was still positive. EP4 vaccination led to rapid increases in anti-Nef responses (Fig. 5B), as observed for the Gag responses (Fig. 5A). Together, our data demonstrate that DNA-only vaccination induces long-term persistent humoral responses. The rapid and high induction of recall responses indicates the effective generation of immunological memory.
3.5 Induction of Gag-specific IgA
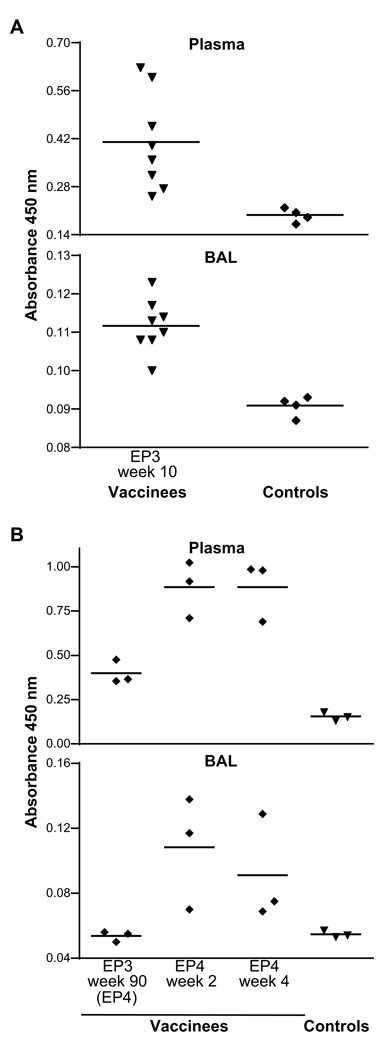
We also measured Gag-specific IgA in plasma and BAL fluid (Fig. 6). Ten weeks following EP3 (Fig. 6A), the eight vaccinated animals had detectable levels of Gag-specific IgA compared to the background levels determined in 4 naïve macaques, both in plasma and BAL (Fig. 6A). Upon resting three animals for 90 weeks after EP3, Gag-specific IgA was found to be persistent in plasma but not in BAL (Fig. 6B), and these animals immediately boosted the levels of Gag-specific IgA in both plasma and BAL after EP4 (Fig. 6B, 2 and 4 weeks after EP4). The same BAL samples from these monkeys also showed clearly detectable Gag-specific IgG at all these time points (data not shown). These data show that efficient DNA vaccination via the intramuscular route induces systemic SIV-specific immune responses as well as dissemination to mucosal sites.
Fig. 6.
Measurements of Gag-specific IgA in plasma and BAL by ELISA. (A) Analysis 10 weeks following EP3 in plasma and BAL washes. The difference between vaccinees and controls is significant (p=0.0041; t test). Mean absorbance values of SIV Gag-specific IgA are shown for all 8 vaccinated animals and 4 naïve controls. (B) Analysis 90 weeks after EP3 (day of EP4) as well as 2 and 4 weeks post EP4 for three vaccinated macaques compared to three naïve controls.
4. Discussion
In this study, we characterized the immune responses obtained after DNA vaccination via the IM route using in vivo electroporation, in the absence of mucosal vaccine administration or any heterologous boost. We [55] and others [57] have previously demonstrated that electroporation increased immune responses in Rhesus macaques compared to IM injection. The adjuvant effect of IL-12 DNA in combination with DNA vaccination has also been shown [57, 66, 68, 69]. Our goal here was to use DNA electroporation in the presence of IL-12 DNA as an adjuvant to further characterize the quality, longevity and dissemination of the immune response. The group of 8 macaques described in this manuscript was used successfully for adoptive transfer experiments [59]. DNA vaccination led to a great number of specific immune responses and facilitated the efficient isolation of MHC-restricted SIV specific cell clones. The logistics of the adoptive transfer experiments also allowed the study of immune responses in animals maintained for one and two years in the absence of any other intervention.
In the present study, we report efficient dissemination and long-term maintenance of cellular and humoral responses to an effector mucosal site, lung epithelium. Detection of immune responses both systemically and in mucosa up to 90 weeks after three vaccinations was particularly interesting as these results demonstrate the ability of intramuscular DNA immunization to induce high frequencies of antigen-specific cellular immune responses that were truly long-lasting. Furthermore, SIV-specific cellular responses, including IFN-γ+ TNFα+ dual positive T cells, were detected in BAL. Multifunctional antigen-specific T cells having the ability to secrete more than one cytokine have been shown to correlate with long-term non-progression in “elite controllers” infected with HIV [73]. Thus, the presence of the vaccine-induced IFN-γ and TNFα positive cells in mucosal effector sites may play an important role in the elimination or containment of the incoming virus. In BAL, anti-SIV responses were predominantly CD8 EM T cells, whereas in blood both CM and EM CD4+ and CD8+ SIV-specific T cells were detected. We demonstrated the induction of SIV-specific effector memory T cells with Granzyme B and the potential to kill infected cells both in blood and BAL. The durable mobilization of antigen specific T cells to peripheral effector mucosal sites, like the lung, suggests that these cells could have a sentinel function upon subsequent antigenic exposure. DNA vaccination was also able to induce, in addition to systemic anti-SIV Gag and anti-Nef antibody responses, humoral responses in BAL including Gag-specific IgA. The great induction of immune responses obtained immediately after EP4 indicates that recall responses can be rapid and potent upon re-exposure to the antigen, even after a long period (~2 years) post vaccination. These data show that in vivo electroporation of DNA in the absence of a heterologous boost is a potent vaccination method and is able to efficiently induce both systemic and mucosal immunity in rhesus macaques. These findings provide evidence that intramuscular in vivo DNA electroporation is able to overcome limitation of DNA intramuscular vaccination reported in previous studies [30–33], which failed to detect mucosal immune responses. In a recent DNA-only vaccination study in Indian rhesus macaques using intramuscular DNA delivery via EP, we reported induction of potent immune responses and significant decrease of viremia during the acute (1 log) and chronic (1.7 log) phases of infection after high dose SIVmac251 challenge [58]. Together, these studies show that the improved DNA delivery via EP is able to induce more efficacious immune responses both systemic and mucosal.
Increased immune responses may provide a critical step in improving DNA vaccination efficiency in humans. Human trials indicate that the magnitude of immune responses after DNA vaccination remains low [4, 34–39, 51, 74–77] compared to levels reported in macaques. Therefore, the availability of more efficient DNA delivery methods, such as the method described here, may increase the efficiency of DNA vaccination and provide a critical advantage for future plasmid DNA vaccine studies in humans [78]. To our knowledge, this is the first report showing an induction of durable mucosal immunity against SIV antigens in non-human primates obtained by intramuscular vaccination with plasmid DNAs, in the absence of mucosal vaccine administration or any recombinant viral boost. These data support the concept of systemic priming leading to mucosal dissemination of effector cells (reviewed in [79]) and show that this can be achieved in primates using an efficient vaccination method, such as the DNA in vivo electroporation.
Acknowledgment
We thank D. Weiss, J. Treece, I. Kalisz and their staff at Advanced BioScience Laboratory Kensington, MD, V. Coalter for expert help, T. Demberg and P. Kozlowski for advice, and T. Jones for editorial assistance. This work was supported by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haynes BF, Shattock RJ. Critical issues in mucosal immunity for HIV-1 vaccine development. J Allergy Clin Immunol. 2008 Jul;122(1):3–9. doi: 10.1016/j.jaci.2008.03.036. quiz 10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008 Jan–Mar;10(1):36–46. [PubMed] [Google Scholar]
- 3.Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004 Mar;20(3):247–253. doi: 10.1016/s1074-7613(04)00053-6. [DOI] [PubMed] [Google Scholar]
- 4.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008 Oct;9(10):776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki Y, Nochi T, Kiyono H. Progress towards an AIDS mucosal vaccine: an overview. Tuberculosis (Edinb) 2007 Aug;87 Suppl 1:S35–S44. doi: 10.1016/j.tube.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006 Feb;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 7.Hinkula J. Clarification of how HIV-1 DNA and protein immunizations may be better used to obtain HIV-1-specific mucosal and systemic immunity. Expert Rev Vaccines. 2007 Apr;6(2):203–212. doi: 10.1586/14760584.6.2.203. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Hidajat R, Peng B, Venzon D, Aldrich MK, Richardson E, et al. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251) Vaccine. 2007 Nov 19;25(47):8021–8035. doi: 10.1016/j.vaccine.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Mercier GT, Nehete PN, Passeri MF, Nehete BN, Weaver EA, Templeton NS, et al. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007 Dec 17;25(52):8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SW, Cun AS, Harris-McCoy K, Ertl HC. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007 Mar 8;25(12):2187–2193. doi: 10.1016/j.vaccine.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007 Oct 10;367(1):156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Rasmussen RA, Nolan KM, Frankel FR, Lieberman J, McClure HM, et al. Live attenuated Listeria monocytogenes expressing HIV Gag: immunogenicity in rhesus monkeys. Vaccine. 2007 Oct 16;25(42):7470–7479. doi: 10.1016/j.vaccine.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J, et al. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J Virol. 2002 Nov;76(22):11659–11676. doi: 10.1128/JVI.76.22.11659-11676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagarazzi ML, Boyer JD, Javadian MA, Chattergoon MA, Shah AR, Cohen AD, et al. Systemic and mucosal immunity is elicited after both intramuscular and intravaginal delivery of human immunodeficiency virus type 1 DNA plasmid vaccines to pregnant chimpanzees. J Infect Dis. 1999 Oct;180(4):1351–1355. doi: 10.1086/314978. [DOI] [PubMed] [Google Scholar]
- 15.Leung NJ, Aldovini A, Young R, Jarvis MA, Smith JM, Meyer D, et al. The kinetics of specific immune responses in rhesus monkeys inoculated with live recombinant BCG expressing SIV Gag, Pol, Env, and Nef proteins. Virology. 2000 Mar 1;268(1):94–103. doi: 10.1006/viro.1999.0131. [DOI] [PubMed] [Google Scholar]
- 16.Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, et al. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. 2002 Apr;76(7):3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans DT, Chen LM, Gillis J, Lin KC, Harty B, Mazzara GP, et al. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003 Feb;77(4):2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mederle I, Le Grand R, Vaslin B, Badell E, Vingert B, Dormont D, et al. Mucosal administration of three recombinant Mycobacterium bovis BCG-SIVmac251 strains to cynomolgus macaques induces rectal IgAs and boosts systemic cellular immune responses that are primed by intradermal vaccination. Vaccine. 2003 Oct 1;21(27–30):4153–4166. doi: 10.1016/s0264-410x(03)00537-1. [DOI] [PubMed] [Google Scholar]
- 19.Pinczewski J, Zhao J, Malkevitch N, Patterson LJ, Aldrich K, Alvord WG, et al. Enhanced immunity and protective efficacy against SIVmac251 intrarectal challenge following ad-SIV priming by multiple mucosal routes and gp120 boosting in MPL-SE. Viral Immunol. 2005;18(1):236–243. doi: 10.1089/vim.2005.18.236. [DOI] [PubMed] [Google Scholar]
- 20.Wang SW, Kozlowski PA, Schmelz G, Manson K, Wyand MS, Glickman R, et al. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol. 2000 Nov;74(22):10514–10522. doi: 10.1128/jvi.74.22.10514-10522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeson P, Boyer J, Kumar S, Lewis MG, Mattias L, Veazey R, et al. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of alpha4beta7 integrin and an effector memory phenotype. Virology. 2006 Oct 25;354(2):299–315. doi: 10.1016/j.virol.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang SW, Bertley FM, Kozlowski PA, Herrmann L, Manson K, Mazzara G, et al. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res Hum Retroviruses. 2004 Aug;20(8):846–859. doi: 10.1089/0889222041725253. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008 Sep 15;181(6):4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001 Dec;7(12):1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 25.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006 Apr 15;107(8):3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, et al. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006 Apr;80(8):3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertley FM, Kozlowski PA, Wang SW, Chappelle J, Patel J, Sonuyi O, et al. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004 Mar 15;172(6):3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 28.Baig J, Levy DB, McKay PF, Schmitz JE, Santra S, Subbramanian RA, et al. Elicitation of simian immunodeficiency virus-specific cytotoxic T lymphocytes in mucosal compartments of rhesus monkeys by systemic vaccination. J Virol. 2002 Nov;76(22):11484–11490. doi: 10.1128/JVI.76.22.11484-11490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002 Aug 15;100(4):1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- 30.Belyakov IM, Ahlers JD, Nabel GJ, Moss B, Berzofsky JA. Generation of functionally active HIV-1 specific CD8+ CTL in intestinal mucosa following mucosal, systemic or mixed prime-boost immunization. Virology. 2008 Nov 10;381(1):106–115. doi: 10.1016/j.virol.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eo SK, Gierynska M, Kamar AA, Rouse BT. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J Immunol. 2001 May 1;166(9):5473–5479. doi: 10.4049/jimmunol.166.9.5473. [DOI] [PubMed] [Google Scholar]
- 32.Yang K, Whalen BJ, Tirabassi RS, Selin LK, Levchenko TS, Torchilin VP, et al. A DNA vaccine prime followed by a liposome-encapsulated protein boost confers enhanced mucosal immune responses and protection. J Immunol. 2008 May 1;180(9):6159–6167. doi: 10.4049/jimmunol.180.9.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyanathan M, Mu J, Kugathasan K, Zhang X, Damjanovic D, Small C, et al. Airway delivery of soluble mycobacterial antigens restores protective mucosal immunity by single intramuscular plasmid DNA tuberculosis vaccination: role of proinflammatory signals in the lung. J Immunol. 2008 Oct 15;181(8):5618–5626. doi: 10.4049/jimmunol.181.8.5618. [DOI] [PubMed] [Google Scholar]
- 34.Tavel JA, Martin JE, Kelly GG, Enama ME, Shen JM, Gomez PL, et al. Safety and immunogenicity of a Gag-Pol candidate HIV-1 DNA vaccine administered by a needle-free device in HIV-1-seronegative subjects. J Acquir Immune Defic Syndr. 2007 Apr 15;44(5):601–605. doi: 10.1097/QAI.0b013e3180417cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006 Dec 15;194(12):1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004 Apr;85(Pt 4):911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 37.Bansal A, Jackson B, West K, Wang S, Lu S, Kennedy JS, et al. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol. 2008 Jul;82(13):6458–6469. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008 May 23;26(22):2788–2795. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- 39.Wilson CC, Newman MJ, Livingston BD, MaWhinney S, Forster JE, Scott J, et al. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin Vaccine Immunol. 2008 Jun;15(6):986–994. doi: 10.1128/CVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005 Jul;79(13):8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006 Jun;80(12):5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barouch DH, Fu TM, Montefiori DC, Lewis MG, Shiver JW, Letvin NL. Vaccine-elicited immune responses prevent clinical AIDS in SHIV(89.6P)-infected rhesus monkeys. Immunol Lett. 2001 Nov 1;79(1–2):57–61. doi: 10.1016/s0165-2478(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 43.Egan MA, Charini WA, Kuroda MJ, Schmitz JE, Racz P, Tenner-Racz K, et al. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J Virol. 2000 Aug;74(16):7485–7495. doi: 10.1128/jvi.74.16.7485-7495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthumani K, Bagarazzi M, Conway D, Hwang DS, Manson K, Ciccarelli R, et al. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduce viral loads after pathogenic intrarectal SIV(mac)251 challenge in Rhesus Macaques. Vaccine. 2003 Jan 30;21(7–8):629–637. doi: 10.1016/s0264-410x(02)00571-6. [DOI] [PubMed] [Google Scholar]
- 45.Mossman SP, Pierce CC, Watson AJ, Robertson MN, Montefiori DC, Kuller L, et al. Protective immunity to SIV challenge elicited by vaccination of macaques with multigenic DNA vaccines producing virus-like particles. AIDS Res Hum Retroviruses. 2004 Apr;20(4):425–434. doi: 10.1089/088922204323048177. [DOI] [PubMed] [Google Scholar]
- 46.Koopman G, Mortier D, Hofman S, Mathy N, Koutsoukos M, Ertl P, et al. Immune-response profiles induced by human immunodeficiency virus type 1 vaccine DNA, protein or mixed-modality immunization: increased protection from pathogenic simian-human immunodeficiency virus viraemia with protein/DNA combination. J Gen Virol. 2008 Feb;89(Pt 2):540–553. doi: 10.1099/vir.0.83384-0. [DOI] [PubMed] [Google Scholar]
- 47.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998 Sep;16(9):867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 48.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999 Apr;6(4):508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 49.Prud'homme GJ, Glinka Y, Khan AS, Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr Gene Ther. 2006 Apr;6(2):243–273. doi: 10.2174/156652306776359504. [DOI] [PubMed] [Google Scholar]
- 50.Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. 1999 May 25;96(11):6417–6422. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004 Apr;11(8):711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 52.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164(9):4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 53.Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004 Jun 23;22(19):2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 54.Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J, et al. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine. 2006 May 22;24(21):4503–4509. doi: 10.1016/j.vaccine.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007 May;81(10):5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Patel V, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;06:15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minang JT, Trivett MT, Trubey CM, Estes JD, Li Y, Smedley J, et al. Distribution, persistence and efficacy of adoptively transferred central and effector memory-derived autologous SIV-specific CD8+ T-cell clones in rhesus macaques during acute infection. J Immunol. 2009 doi: 10.4049/jimmunol.0902410. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, et al. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol. 1994 May;68(5):2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN, et al. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997 Jul;71(7):4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992 Dec;66(12):7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992 Jan;66(1):150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Gegerfelt AS, Rosati M, Alicea C, Valentin A, Roth P, Bear J, et al. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J Virol. 2007 Feb;81(4):1972–1979. doi: 10.1128/JVI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chikhlikar P, Barros de Arruda L, Agrawal S, Byrne B, Guggino W, August JT, et al. Inverted terminal repeat sequences of adeno-associated virus enhance the antibody and CD8(+) responses to a HIV-1 p55Gag/LAMP DNA vaccine chimera. Virology. 2004 Jun 1;323(2):220–232. doi: 10.1016/j.virol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 66.Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006 May 22;24(21):4677–4687. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 67.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009 Jan;83(2):791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA+IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol. 2008 Jun 15;180(12):7969–7979. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- 69.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol. 2005 Oct;34(5–6):262–270. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 70.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002 Jan 1;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 71.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006 Jun;116(6):1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009 Feb 15;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008 Feb 20;26(8):1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorse GJ, Baden LR, Wecker M, Newman MJ, Ferrari G, Weinhold KJ, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008 Jan 10;26(2):215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 76.Eller MA, Eller LA, Opollo MS, Ouma BJ, Oballah PO, Galley L, et al. Induction of HIV-specific functional immune responses by a multiclade HIV-1 DNA vaccine candidate in healthy Ugandans. Vaccine. 2007 Nov 7;25(45):7737–7742. doi: 10.1016/j.vaccine.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 77.Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, Nason MC, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007 May 16;25(20):4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 78.Vasan S, Schlesinger SJ, Huang Y, Hurley A, Lombardo A, Chen Z, et al. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine. PLoS One. 2010;5(1):e8617. doi: 10.1371/journal.pone.0008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu Rev Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]