Abstract
Craniofacial injuries require a variety of different cell types to repopulate areas of bone, cartilage, tendon, and fat. Mesenchymal stem cells (MSCs) provide a multipotent cell source for tissue engineering of this area, particularly when the cells are delivered via a 3D hydrogel environment. MSC differentiation into cartilage, bone, and fat has been investigated through a variety of techniques, some of which include the use of synthetic hydrogel scaffolds, integration of extracellular matrix components and other natural gel chemistries, microparticle delivery of growth factors, simultaneous mechanical stimulation, and the delivery of microRNA. This review aims to summarize the most recent studies involving the synthesis and application of 3D hydrogels to induce the differentiation of encapsulated MSCs and their subsequent matrix production.
Keywords: mesenchymal stem cells, hydrogels, chondrogenic differentiation, osteogenic differentiation, craniofacial repair
Introduction
Craniofacial injury encompasses a wide variety of cell types, tissues, and functional properties. Tissue types needed for repair of these injuries include cartilage, bone, fat, and ligaments. Therefore, complete surgical reconstruction of tissues in this area is often difficult to achieve due to its high complexity. An alternative to reconstructive surgeries, and in an effort to regenerate damaged tissues left behind after disease or trauma, the field of tissue engineering has recently become a widely studied area. Generally, tissue engineering makes use of cell types that are known for recapitulating the matrix and tissue components needed to replace the damaged area. Scaffolds are currently designed and surgically implanted with regard to their conductive or inductive properties, allowing for the in-growth of the surrounding tissue. Often, primary cells isolated from the affected individual are grown in culture and delivered back into the body in an effort to regenerate the tissue (Centeno et al., 2008; Saris et al., 2008). However, this procedure often leads to multiple surgeries and creation of another trauma site near the biopsy point, and provides a limited cell source for re-implantation (Jones and Peterson, 2006). Therefore, a major drive toward finding an alternative cell source for craniofacial tissue-engineering applications has led scientists to discover the many advantages of using adult human mesenchymal stem cells.
Mesenchymal stem cells (MSCs) have been widely studied over the past decade (Caplan, 1991; Pittenger et al., 1999; Caterson et al., 2002; Barry and Murphy, 2004) and have been found to be multipotent, having the capacity to differentiate into cartilage-, bone-, fat-, and endothelium-producing cells, all of which are useful for a variety of applications, including craniofacial diseases. Furthermore, the direct injection of MSCs for the treatment of diseased or damaged tissue has shown success in promoting regeneration; however, providing MSCs with a material environment that more closely recapitulates the tissue’s extracellular niche is likely to play a crucial role in the extent of healing. Thus, development of a material platform—one that could provide signals necessary to maintain the differentiation potential, growth rates, phenotypic changes, and the milieu of cues necessary to cause MSCs to transform into the cell types found in bone, fat, cardiac tissue, pancreatic cells, or cartilage—is the subject of intense research.
This review focuses specifically on recent efforts in the development of hydrogel-based materials for MSC delivery. Hydrogels are of great interest, since they can be used to deliver cells easily and non-invasively to a site of tissue damage through simple injections, and upon gelation, they form elastic materials with many tissue-like properties. The key to designing hydrogel systems for MSC delivery is a better understanding of the necessary components that must be integrated into the gel for the intended applications. For example, does the gel need to meet certain loading requirements? What biological functionalities, single or multiple, are necessary to direct the desired cellular functions? or How might the gel contribute to the hierarchical structure of a complex tissue? With these questions and others in mind, we briefly review common culture methods for MSCs and their differentiation, followed by a discussion of important hydrogel properties and their manipulation for cell-culture niches, and then place this all in the context of the current state of the art in hydrogel systems for engineering craniofacial tissues with MSCs.
Mesenchymal Stem Cells
The interest in mesenchymal stem cells (MSCs) as a cell source for craniofacial tissue engineering, especially the regeneration of bone and cartilage, has heightened within the last five years. Mesenchymal stem cells are multipotent cells of the bone marrow stroma, which have the ability to serve as long-lasting progenitor cells to bone, fat, muscle, and cartilage tissue (Prockop, 1997). The major differentiation pathways for MSCs are shown in Fig. 1. These cells are of great interest for cell-based therapies, due to their ease of isolation, high proliferation rate, differentiation potential, and sustained capacity for any age group (Johnstone et al., 1998; Pittenger et al., 1999; Katritsis et al., 2005).
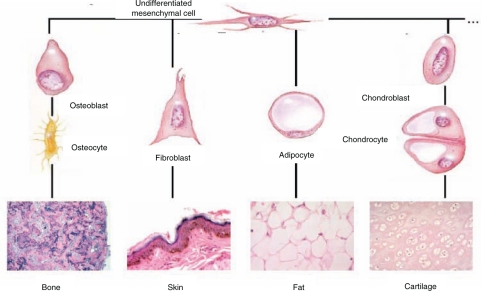
Figure 1.
The pathways of differentiation for a mesenchymal stem cell include bone, fat, nerve, heart, and cartilage tissue as well as others. This image depicts the rounded cell morphology indicative of chondrogenic differentiation, the star-like morphology of an osteoblast, and the elongated, spindly morphology of an undifferentiated MSC (Barry et al., 2001a).
Adult MSCs are most often isolated from an aspirate of bone marrow collected from the iliac crest (Pittenger et al., 1999). Whole-bone-marrow aspirates have also been harvested from bone marrow compartments contained in the tibia and femur (Oreffo et al., 1998; Murphy et al., 2002), as well as from the thoracic and lumbar spine (D’Ippolito et al., 1999). Characterization of mesenchymal stem cells is often performed through investigation of their adhesive and proliferative capacity in culture, maintenance of specific cell-surface markers, as well as their ability to differentiate consistently into multiple cell types when presented with specific culture conditions (Pittenger et al., 1999). Morphologically, attached MSCs appear spindly and spread on a surface (Fig. 2A), whereas MSCs differentiated to osteoblasts appear more star-like and dense (Fig. 2B).
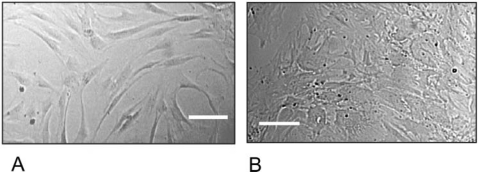
Figure 2.
Brightfield image depicting the morphology of plated hMSCs in culture (A) and those of differentiated MSCs that have become osteoblasts (B). These MSCs are adherent and attach and proliferate in a spindle shape. When provided with the appropriate cues to direct their differentiation, as in the case of osteogenic soluble factors, the cells become more star-like. Scale bar = 100 µm.
Cell culture conditions to induce differentiation of MSCs include media additives such as β-glycerol-phosphate, ascorbic acid-2-phosphate, dexamethasone, and fetal bovine serum (FBS) (Bruder et al., 1997; Barry et al., 2001a), as well as growth factors, namely, TGFβ (Johnstone et al., 1998; Mackay et al., 1998; Barry et al., 2001a; Worster et al., 2001; Caterson et al., 2002), BMP-2 (Lutolf et al., 2003), and IGF-1 (Longobardi et al., 2003). Providing MSCs with such additives or growth factors has been shown to increase their differentiation potential and aid in the deposition of the extracellular matrix components necessary for tissue-engineering applications. Those necessary ECM components include collagen type II, calcium deposits, or glycosaminoglycans (Caterson et al., 2002). Furthermore, MSCs have shown promising differentiation potential when cultured in conditioned media of a specific cell type (Hwang et al., 2007). Some of the most common soluble factors added to media to induce mesenchymal stem cell differentiation down osteogenic, chondrogenic, or adipogenic pathways are summarized in Table 1.
Table 1.
Soluble Factors Commonly Added to Culture Media to Induce Differentiation of MSCs Directed toward Specific Cell Types
| Cell Type | Soluble Factors | References |
|---|---|---|
| Osteoblasts | β-glycerol-phosphate, ascorbic acid-2-phosphate, dexamethasone and fetal bovine serum | Bruder et al., 1997; Barry et al., 2001a |
| Chondrocytes | o-Transforming growth factor β 1 and 3, bone morphogenetic protein-2, insulin-like growth factor, dexamethasone | Johnstone et al., 1998; Mackay et al., 1998; Barry et al., 2001a; Worsteret al., 2001; Caterson et al., 2002 |
| Adipocytes | isobutylmethylxanthine | Suzawa et al., 2003 |
Collectively, this information provides evidence that MSCs are directly induced through interactions with their surrounding environment, whether proteins, growth factors, or a vast array of other cues that can direct their growth rate and function. Knowledge of the complex cellular environment and cues needed to initiate differentiation pathways is critical to guide future clinical applications. While a better understanding of the induction potential of solubly delivered cues on MSCs is certainly critical, an equally important aspect relates to the influence of the surrounding material microenvironment or niche on MSCs.
Hydrogels as Cell Culture Systems
Hydrogels are polymers that are crosslinked to form a network that is rendered insoluble, but absorbs large amounts of water and swells (Park and Lakes, 1992; Peppas et al., 2006). Hydrogels can be classified by their chemistry (e.g., synthesized from naturally based vs. synthetic precursors) or the mechanism of the crosslinking reaction. Of further note, many hydrogel systems ‘gel’ under physiological conditions; this enables injectable cell delivery vehicles to be developed. With respect to this latter point, the mechanism of the crosslinking reaction becomes important in the design of in situ- forming gel systems.
In hydrogel synthesis, chain interactions and subsequent network formation can occur via physical, ionic, or covalent crosslinking (Prestwich et al., 1998). Physical networks form through chain entanglements, hydrogen bonding, or hydrophobic interactions such as those that occur during the gelation of poloxamers, known generically as Pluronics, or poly(N-isopropylacrylamide) [P(NIPAAm)] (Prestwich et al., 1998). Ionic hydrogels are created via multivalent interactions between macromolecular polymer chains and can be altered by changes in the ionic strength and/or pH of the system. The most common ionic hydrogel system that is often used as a cell delivery system is alginate, which is gelled by the addition of calcium ions. Both physical and ionic gels are reversibly crosslinked, so they tend to have relatively low mechanical properties. In contrast, covalently crosslinked networks are ‘permanent’ gels. For example, poly(vinyl alcohol) (PVA) gels are often synthesized by the addition of a low-molecular-weight crosslinker, such as glutaraldehyde. However, the cytocompatibility of the crosslinking molecule has limited its application for cell encapsulation. Alternatively, a gel system of increasing interest for tissue engineering is the chain polymerization of (meth)acrylate-modified poly(ethylene glycol) (PEG) chains, often via photopolymerization (Peppas et al., 2000). This method is frequently used to encapsulate cells, due to the ease of cell entrapment, short time-course for gelation and complete polymerization, and the high level of cytocompatibility (Bryant et al., 2000).
Beyond the method of crosslinking, another type of classification for gels is dependent upon the origin of the gel precursor molecules. Naturally occurring components are often used to create hydrogels for cell encapsulation purposes, since they provide essential cues that are found within the cell’s native environment (Yoneno et al., 2005). Examples of naturally based gels include collagen, fibrin, and matrigel. Those cells entrapped in a natural gel carrier will readily interact via integrin-ligand interactions, charged interactions, and as with the gel precursor molecules (Karageorgiou et al., 2004; Donzelli et al., 2007). Direct interactions between the natural extracellular matrix molecules of the gel system with the cells generate cell-signaling cascades that lead to the promotion of viability, spreading, and new matrix deposition (Garcia et al., 1999). Further, cells secrete various proteases and enzymes that allow for local remodeling and degradation of the material environment. Natural gels are considered to be a promoting type of material, since cells interact with them in a complex way and receive a plethora of signals from this type of environment.
In contrast, synthetic materials are also used to encapsulate cells, since they provide a material with properties that are readily tuned and manufactured reproducibly. For example, synthetic gels can be designed with a certain stiffness or degradation rate, and are often based on macromolecules such as poly(ethylene glycol), poly(hydroxyethyl methacrylate), poly(vinyl alcohol), or poly(acrylic acid). Synthetic gels are often considered permissive cell niches, since their properties allow for basic cell functions, but provide no specific cues to direct cellular interactions, other than the potential for indirect interactions through non-specifically absorbed proteins from the surrounding medium (Cook et al., 1997; Hern and Hubbell, 1998). The advantages and disadvantages of natural vs. synthetic hydrogels are listed in Table 2.
Table 2.
Advantages and Disadvantages of Natural vs. Synthetic Hydrogels
| Type of Hydrogel | Advantages | Disadvantages | References |
|---|---|---|---|
| Natural | -innate physical characteristics of ECM | -material properties not easily tunable | Li and Tuan, 2005; Pier et al., 1994; Cook et al., 2003 Lee et al., 2001 |
| -cells readily interact with material | -degradation products lead to immune response | ||
| -direct cellular function | |||
| Synthetic | -can fine-tune properties | -little cell interaction | Anseth et al., 1996; |
| -easily produced in large quantities with little variation | -must be tailored to direct cellular function | Peppas et al., 2006; Bryant and Anseth, 2001; Park and Lakes, 1992 |
From the perspective of their physical structures, natural and synthetic gels have many similarities and differences. These differences lie in the material precursors, charged species, and size and composition of the gels, whereas the similarities exist in the fact that both gel systems can be polymerized in the same fashion. As described above, both gel systems form crosslinks via a variety of interactions, such as ionic, physical, or covalent. The number and strength of the interactions dictate the crosslinking density of a gel, thereby affecting properties including the water content, as measured through the equilibrium swelling ratio (Q), the gel mechanical properties such as stiffness [e.g., compressive modulus (K)], and the transport of macromolecules, which is related to the mesh size (ξ) and ultimate diffusivity (D) (Anseth et al., 1996). A general depiction of the molecular structure of a hydrogel system can be seen in Fig 3A. Variations in the gel structure and crosslinking density can drastically alter the gel properties. For instance, decreasing the crosslinking density (ρx) increases the average mesh size, resulting in a higher degree of swelling, a decreased equilibrium modulus, and a shorter time scale for diffusion of important molecules into and out of the gel (Anseth et al., 1996). General trends in the gel properties as a function of the crosslinking density are depicted in Fig. 3B. The gel properties are extremely important for tissue-engineering applications, since the mesh size can limit nutrient or waste diffusion, while the compressive modulus or stiffness of the gel has been shown to provide mechanical stimulus to encapsulated cells (Bryant and Anseth, 2001) (as will be discussed later in this review). Therefore, understanding the differences in gel properties with regard to natural vs. synthetic components, the variety of gel precursor molecules, and charge effects provides a vast amount of knowledge as to how or why the cells encapsulated within these gels receive signals from their microenvironment and respond. The various degrees of control over gel properties make them an attractive source for MSC delivery and tissue regeneration, but incorporating all of the unique qualities of a native tissue into a single hydrogel material is often a difficult task. Thus, an emerging area of interest focuses on combining synthetic gel chemistries with natural gel components to achieve a material system that possesses specific advantages of each.
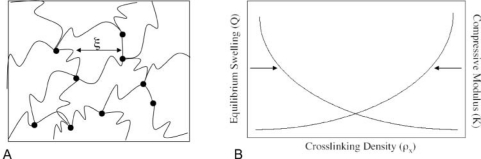
Figure 3.
Generic representation of hydrogel network structures and their properties. (A) Schematic structure for covalently crosslinked networks composed of multifunctional macromers. Here ( ) represents a kinetic chain crosslinked at a node (•). The mesh size (ξ) depicts the physical distance between crosslinks and can be correlated to the equilibrium swelling ratio (Q) (Canal and Peppas, 1989). (B) Generalized depiction of relationship among equilibrium swelling, compressive modulus, and crosslinking density with regard to hydrogel properties. As the crosslinking density increases, the swelling decreases, while the compressive modulus of the hydrogel increases.
) represents a kinetic chain crosslinked at a node (•). The mesh size (ξ) depicts the physical distance between crosslinks and can be correlated to the equilibrium swelling ratio (Q) (Canal and Peppas, 1989). (B) Generalized depiction of relationship among equilibrium swelling, compressive modulus, and crosslinking density with regard to hydrogel properties. As the crosslinking density increases, the swelling decreases, while the compressive modulus of the hydrogel increases.
Incorporation and Delivery of MSCs in Hydrogels
Natural Gels
Natural gels are often used for cell encapsulation and tissue regeneration, due to their innate biological characteristics that capture aspects of the native extracellular matrix (ECM). Cellular interactions with their microenvironment can readily direct their function. Collagen gels are used most often, since they are a widely abundant ECM component found as 19 various isoforms (Lee et al., 2001). Among the natural gels used for the encapsulation of MSCs is fibrin, a protein polymerized into a gel, which is collected from native fibrinogen, a main component in wound healing (Worster et al., 2001). Matrigel©is a commonly used gelatinous protein mixture derived from the basement membrane, and has been used to encapsulate successful MSCs. Also, polysaccharide gels formed from alginate or agarose, components collected from seaweed (Caterson et al., 2001), have been used as natural gels for MSC encapsulation. Further, important ECM components, such as gelatin, collagen, chondroitin sulfate, and others, are found to induce the growth of entrapped MSCs (Fukumoto et al., 2003).
Gels based on extracellular matrix components or those taken from nature, though not found within the ECM, have been shown to direct specific cellular functions, including proliferation, differentiation, and matrix development. For example, in an attempt to develop a gel system to promote adipocytic differentiation of MSCs, Mauney et al. compared the use of collagen gels with poly(lactic acid) (PLA) and silk scaffolds (Mauney et al., 2007). Their results revealed that the traditional collagen material supported high levels of cell adhesion of lipid-accumulating cells, while the silk scaffold seeded with MSCs and implanted into a rat muscle remained intact and allowed for the differentiation of MSCs into adipocytes. Furthermore, the evolution of cartilage tissue from differentiating MSCs in agarose gels has been investigated and compared with that of native tissue (Mauck et al., 2006). Bovine MSCs were encapsulated in agarose and induced to differentiate through the use of soluble factors found in the differentiation media. Mechanical and biochemical properties of the evolving tissue were measured over a 10-week period, and results indicated that the generated cartilage matrix of the differentiated MSCs was significantly less than that of native cartilage tissue. In studies investigating the effects of a natural gel component on cartilage regeneration, chondroitin sulfate, an extracellular matrix component of cartilage, was used successfully to direct MSCs down the chondrogenic pathway (Bryant et al., 2005; Varghese et al., 2008). Collectively, these results indicate that while natural gels often provide the cues needed to induce MSCs down a differentiation pathway, limitations exist in the difficulty of fine-tuning mechanical properties and consistently forming gels with similar characteristics.
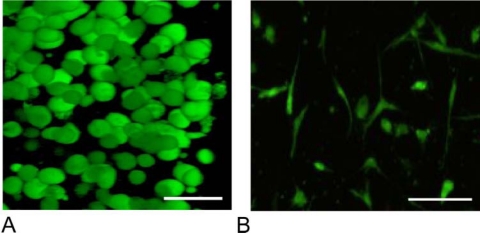
While natural materials are advantageous, in that they capture many aspects of the biochemical properties and biological signals of the native ECM, the inability to manipulate the mechanical properties, synthesize gels with reproducible properties, and control the resorption rate has led researchers to the further exploration of synthetic gels for delivery of MSCs. A striking difference of how MSCs interact and function in these two very different gel systems is shown in Fig. 4. A representative image of MSCs entrapped in a synthetic, PEG gel, where cells exhibit a rounded morphology as a result of limited matrix interactions and the inability to degrade and remodel the material niche appears in Fig. 4A. MSCs in a collagen gel, where cell integrins attach to the material and enzymes remodel the gel in a complex manner, is shown in Fig. 4B.
Figure 4.
MSCs entrapped at 10 million cells/mL in a synthetic scaffold (PEG) (A) and a collagen gel (B). Morphologically, the MSCs are confined and form a rounded shape in a non-degrading PEG gel (A), while they are able to degrade away and spread in the natural, collagen gel (B). Scale bar = 25 µm.
Synthetic Gels
Just as gels based on natural precursors provide material niches that integrate important biological cues, synthetically derived gels provide materials with properties that are more easily controlled (e.g., degradation, mechanics) and reproducible. The most widely used synthetic gels for MSC encapsulation are based on poly(ethylene glycol) (PEG) or poly(vinyl alcohol) (PVA) (Bryant et al., 1999; Bryant and Anseth, 2001; Burdick and Anseth, 2002). PEG and PVA networks are easily tunable by varying the crosslinking density and subsequently altering the swelling and compressive modulus of the system. Although synthetic gels, in particular crosslinked PEG, have shown success in generating a cartilage matrix with entrapped primary chondrocytes, or a bone-like matrix with encapsulated osteoblasts, the encapsulation of MSCs in this hydrogel renders the cells unviable (Bryant et al., 2003; Nuttelman et al., 2005). MSCs are an adherent cell, before differentiation into a chondrocyte, osteoblast, or adipocyte, and thus, the extremely permissive PEG gel does not promote cell adhesion and leads to anoikis (i.e., apoptosis due to lack of matrix interactions) of MSCs with time. Tailoring these permissive materials by integrating signals/chemistries found in the native ECM combines the advantages of both the natural and synthetic systems. Incorporation of ECM components aids in directing proper cell function, while the tunability of the synthetic materials provides network properties comparable with those of the native tissue.
Hybrid Synthetic Gels
Incorporation of signals found in the native ECM, which provide the adhesive motifs necessary to maintain MSC survival in synthetic gels, is important. Anchorage-dependent cell types, such as MSCs, require adhesive sites necessary to relay biomechanical and biochemical signals to the cell to induce proliferation, migration, or differentiation (Drumheller and Hubbell, 1994; Benoit and Anseth, 2005; Nuttelman et al., 2005). Lack of cell-matrix interactions forces cells to expend their energy source in an effort to maintain their survival, ultimately ending in programmed cell death, or apoptosis. Fibronectin, a widely studied adhesive ECM component, has been entrapped in synthetic gels and shown to provide a source of cell attachment and cues to maintain cellular survival (Burdick and Anseth, 2002; Nuttelman et al., 2005). While large proteins and growth factors have shown promise as entrapped components in a hydrogel system, these large molecules are often compromised or can alter the material properties of the gel. Entrapment processes can render some large ECM molecules biologically inactive, and more often are incorporated as a heterogeneous mixture throughout the gel environment.
Smaller molecules, such as peptides, are more stable and often more homogeneously distributed throughout the hydrogel, providing specific cues to the entire population of encapsulated cells. Therefore, experimentally determined peptide motifs that are incorporated into these gels can specifically target a desired cellular response without entrapment of a large protein. For instance, the tethering of RGD (arginine-glycine-aspartic acid), the major adhesive site found on fibronectin, within a hydrogel provides similar adhesion properties to the cell to mimic the fibronectin protein (Burdick and Anseth, 2002; Weber et al., 2007). Studies have demonstrated the induction potential of RGD functionalized gels on MSC differentiation into osteoblasts (Nuttelman et al., 2005; Yang et al., 2005). MSCs encapsulated in PEG gels containing covalently attached RGD were shown to differentiate readily into osteoblasts, forming a mineralized matrix at an optimal concentration of 2.5 mM RGD. While RGD provides an attachment site needed for the undifferentiated MSCs, this adhesive molecule has been shown to hinder the differentiation process into chondrocytes. Recent studies revealed that the incorporation of an enzymatically cleavable peptide sequence in line with the adhesive RGD sequence allows for initial cellular attachment prior to cellular degradation and release of the peptide (Salinas and Anseth, 2008). It has been demonstrated that chondrogenic differentiation and cartilage matrix evolution increased through the temporal removal of RGD, as dictated by cellular production of a matrix metalloprotease, which cleaved the RGD-containing peptide from the PEG network (Salinas et al., 2008). There are various methods for the incorporation of ECM-derived peptide sequences into PEG networks through controlled temporal and spatial polymerization techniques.
Incorporation and Delivery of Bioactive Factors in Hydrogels
Traditional 2D cultures of MSCs have led to the identification of numerous soluble factors and conditions to promote the differentiation of MSCs down specific pathways. Further, numerous growth factors are known to influence the proliferation and/or secretory properties of differentiated cells and are an important aspect of promoting the regeneration of new tissue. Thus, the incorporation of well-identified growth factors into MSC-laden hydrogels encompasses a major area of investigation. The inclusion of growth factors at the right concentration in the right context and released with the right profile in 3D gels containing encapsulated MSCs is a critical step in the development of a cell-directive delivery vehicle. As mentioned above, larger molecules, such as proteins, are often difficult to entrap in a stable manner and use in cell-laden materials. Entrapped growth factors can readily diffuse out of the gel before cellular uptake, and their bioactivity can be hindered through the encapsulation process. Due to these limitations, researchers have begun tailoring the release of growth factors through the use of degradable micro- or nanoparticles. The encapsulation of transforming growth factor beta-1 (TGFβ-1) in gelatin microparticles, which were subsequently encapsulated into an oligo(poly(ethylene glycol) fumarate) (OPF) gel containing MSCs, was shown to improve the chondrogenic differentiation and cartilage matrix deposition of the cells (Park et al., 2007). Further, studies investigated the release kinetics of TGFβ-1 and BMP-2 from calcium-phosphate-derived scaffolds and determined that their release profile improved with conjugation to PLGA (Jansen et al., 2005; Habraken et al., 2006). Controlling the degradation rate of the microparticle controls the release rate of the encapsulated growth factor, leading to a specifically designed drug delivery profile. The use of microparticles for the delivery of growth factors aims to mimic the secretion and transport of these cues to the MSCs over a specific time-course for the induction of particular cellular functions.
While growth factors have shown promise as drug-eluting microparticles, further research has also investigated the likelihood of MSC induction through immobilized factors. Studies revealed that immobilized bone morphogenetic factor 2 (BMP-2) on a silk scaffold led to the differentiation of MSCs into osteoblasts and the development of a mineralized matrix (Karageorgiou et al., 2004). Furthermore, the study claimed that soluble delivery of the BMP-2 to MSCs seeded on the silk scaffold hindered their ability to differentiate and produce bone matrix components. Other investigations demonstrated that the entrapment of BMP-2 into an enzymatically cleavable PEG gel led to cell recognition and activation as surrounding cells degraded and infiltrated the drug-laden scaffold (Lutolf et al., 2003). Surface-immobilized epidermal growth factor (EGF) has also been shown to improve MSC attachment and survival (Fan et al., 2007). Therefore, the immobilization of some growth factors leads to improved biological activity, as witnessed by the improved MSC survival and function, and allows for proper interaction with the growth factor through cell-protein interactions.
Just as immobilized growth factors have shown promise for directing MSC function, there is an increasing trend toward the identification of small-molecule drugs for cellular uptake and the promotion of bone or cartilage regeneration. For example, the synthesis of a statin-releasing gel was used to direct the differentiation of MSCs into osteoblasts, since statins have been shown to increase the production of BMP-2 in cultures. Fluvastatin was conjugated to a hydrolytically degradable lactide segment and incorporated into a cell containing PEG gel (Benoit et al., 2006). The degradation of the lactide tether led to fluvastatin release and MSC induction into an osteoblast cell type. Further, cells entrapped in a BMP-2-containing gelatin porous scaffold were seen to have increased osteoblast functionality and mineralized matrix deposition (Takahashi et al., 2005). Finally, studies revealed that PLGA scaffolds loaded with bone-inducing agents, such as ascorbic acid and dexamethasone, and MSCs achieved high levels of osteogenesis and bone matrix evolution (Kim et al., 2003).
Tethering the drug or molecule of choice to the gel network through the use of a degradable linker allows for tuned release kinetics and delivery of the drug to the encapsulated MSCs. This method is quite useful for the predictable release of low-molecular-weight molecules that are potent at low doses. In contrast, microparticle encapsulation is more beneficial for the release of macromolecules with short half-lives and when high loading is required. In general, both techniques are easily tuned to vary the degradation rate and delivery of the targeted therapeutic, which provides numerous options for inducing encapsulated MSCs.
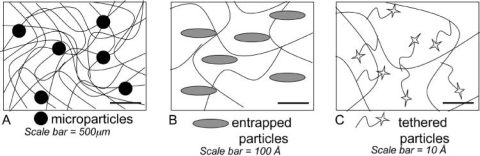
A general schematic of the delivery methods for micro- or nanoparticles, entrapped proteins or peptides, and tethered molecules is presented in Fig. 5. While the microparticles are easily delivered at higher loadings and protect molecules with short half-lives, this approach suffers from difficulties in achieving uniform loading, burst effects, and requires optimization for each individual protein/molecule of interest.
Figure 5.
Schematic of delivery methods for molecules to induce cellular behavior. (A) The entrapment of microparticles. (B) The encapsulation of larger molecules. (C) Covalent tethering of small molecules to the gel network.
Encapsulation of larger molecules is easily achieved during gelation and provides a simple way to entrap molecules of interest. However, this method is appropriate only for molecules in a limited molecular weight range (i.e., similar to the network mesh size) and those that are relatively stable and have a long half-life. Finally, smaller molecules, such as peptides, can be directly tethered to the gel, and the release profile can be tuned by the chemistry of the linker. Enzymatically degradable linkers can release the peptide or growth factor based upon a cell-mediated time line, whereas hydrolytically degradable linkers can be engineered in such a way as to generate a specific timed release profile. While the exact loading of the molecule is controlled during gel synthesis, one is often limited to low loadings, and the gel properties are directly influenced by the tethered component. Regardless, this can be a very versatile means for the reproducible and controllable release of low-molecular-weight molecules.
Degradation of the Hydrogel Environment and its Influence on MSCs
MSCs respond directly to the mechanical stiffness or structure of their surrounding environment to aid in their differentiation process (Kelly and Prendergast, 2005). Furthermore, the loss or degradation of this environment has been shown to have a dramatic and important effect on ECM deposition and the extent of matrix production (Rice and Anseth, 2004). Degradable gels have been in place for years to regulate the secretory properties of encapsulated cells and the amount of matrix deposited and tissue formed. Generally, cells entrapped in non-degradable gels are limited to matrix elaboration in proximity to the cell. As the gel degrades, this decreases the network crosslinking density, increases the mesh size, and allows for further matrix evolution, which ultimately facilitates the creation of a new tissue. Therefore, research has investigated numerous techniques to create a biodegradable matrix for the encapsulation and delivery of MSCs.
While degradable materials aid in the regeneration of tissue throughout the scaffold space, the time scale at which these materials degrade and the mechanism for degradation are of utmost importance in the design and application of the gels. The mass loss or degradation rate of an implanted cell-laden matrix must match the tissue evolution rate of the entrapped cells to achieve proper tissue regeneration and integration. One direction for degradation is that of chemical degradation of the hydrogel bonds, which is often achieved through hydrolysis of bonds, such as esters that crosslink the polymer chains. In gels, hydrolytic degradation is often a bulk process, whereby a material’s high water content leads to homogeneous degradation throughout. However, to reach complete degradation on a specific cellular time scale, a great deal of material fine tuning is necessary. Tuning of the gel properties can be achieved through a mixture of different gel precursor molecules that possess a variety of cleavage rates. Therefore, easily tunable synthetic gels can sometimes be used for better control of the rate of mass loss. For example, slowly degrading linkers such as ε-polycaprolactone are often mixed with more rapidly degrading linkers, like poly(lactic acid), at various different ratios to achieve the proper degradation rate for a PEG gel (Rice et al., 2006).
While these methods are frequently used in research today, numerous studies have involved the use of cell-directed degradation via the production of enzymes. As opposed to bulk and homogeneous degradation of a material, enzymatic or cell-mediated degradation can also occur. Through enzymatic cleavage or degradation, the cells can direct the time line of material loss and adjust their surrounding environment as needed for cellular growth, matrix deposition, and matrix re-organization. This provides specific advantages, since cell-dictated, enzymatic degradation primarily changes the local environment of the cell without affecting bulk properties.
Techniques to achieve cellularly regulated matrix degradation have focused on the use of matrix metalloproteases (MMPs). MSCs, during their differentiation process, will up- and down-regulate various MMPs in an effort to degrade, rebuild, and re-organize the surrounding tissue. Therefore, an understanding of the time line upon which these enzymes are up-regulated directs the design of these types of degradable gels. Researchers have described the integration of MMP linkers in a PEG hydrogel, whereby cells were able to degrade and migrate through the gel (Lutolf et al., 2003). These PEG-MMP hydrogels contained entrapped BMP-2, which induced cell migration into the material upon degradation of the MMP linkers and eventually led to the formation of bone matrix. Further studies demonstrated cellular infiltration into a drug-releasing scaffold via an MMP degradation mechanism (Seliktar et al., 2004). The use of an enzymatically cleavable linker to degrade MSC-containing gels may prove extremely useful in efforts to recreate craniofacial tissues.
Mechanical Properties and Stimulation of MSC-Laden Gels
The mechanical properties of the various tissues that encompass the craniofacial region differ dramatically. Cartilage, bone, ligaments, and fat all possess different matrix compositions, which alter their compressive modulus, structure, and supportive properties. MSCs are known to respond differently to the environment in which they reside, taking on a bone phenotype in the presence of a porous, dense, bone-like scaffold (Dadsetan et al., 2008). Cartilage differentiation is achieved in the presence of a softer collagen-based material (Koga et al., 2007; Moioli et al., 2008). A great deal of research has been conducted in the field of mechanical signals or stimuli on MSCs. While this review is focused primarily on current research in gel systems of various chemistries, it is worthwhile to mention the equally important role of mechanotransduction. While numerous groups are developing advanced bioreactors that provide mechanical stimulation of various regimes (Lee et al., 2001; Williams et al., 2007; McMahon et al., 2008), here we briefly discuss how gel stiffness can be related to the induction of MSC differentiation. In particular, researchers have studied the phenomenon of MSC differentiation pathways induced by the mechanical stimulus felt by the cells when seeded or entrapped in a variety of matrices (Engler et al., 2006).
A great deal of investigation has been focused on the effects of mechanical stimulus on bovine chondrocytes. In general, articulating joints will undergo pressures not associated with other tissue types. Therefore, the cells that are to be used for joint repair must withstand the same forces as native cells in the area. Studies investigating the complexity of joint properties on chondrocytes have shown that the cells will rearrange their ECM to direct tensile forces (Asanbaeva et al., 2007), and that this re-arrangement, whether induced by the cells or by external mechanical forces, does not hinder the activity of the native chondrocytes (Williams et al., 2007). Interest in the mechanical stimulation of MSCs in 3D hydrogels has recently heightened. Chondrocyte matrix components were shown to have an increased effect relative to mechanical forces; therefore, scientists have begun to translate this into MSC differentiation through mechanical stimulation. The effects of mechanical forces on MSCs embedded in collagen gels have been investigated (McMahon et al., 2008). This research found that continuous tensile loading regulated the differentiation of MSCs into chondrocytes and their production of glycosaminoglycan ECM components. Furthermore, when MSCs were encapsulated in hydrogels and exposed to compressional loading over a three-week period, the gene expression profile for chondrogenic markers increased, as seen with loaded samples (Terraciano et al., 2007). These initial investigations showed that mechanical stimulation of MSCs in a 3D culture can influence their gene expression profile, as well as their deposition of cartilage matrix components.
Through the use of new techniques and materials, improvements have been made in an effort to induce the differentiation of MSCs and eventually recreate tissue structures. While research into new materials is ongoing, further investigations into the degradation of a cell-laden scaffold and its effects on cellular differentiation and ECM deposition are also a major area of exploration, as described in the section above. Further, numerous groups (Engler et al., 2006; Dadsetan et al., 2008) have focused on the use of altering gel properties in an effort to direct cell fate. It was found that the porosity of a matrix, mimicking that of bone, induced the differentiation of MSCs into osteoblast-type cells (Dadsetan et al., 2008). Further, studies involving the elasticity of a matrix demonstrated that the variations in mechanical stiffness and pore size of a scaffold influenced cell function and differentiation. It was found that the more elastic matrix led to neurogenic precursor cells, whereas the stiffer matrices led to bone formation (Engler et al., 2006). Scientists believe that these mechanical stimuli can act synergistically to induce MSC differentiation and cellular function.
Achieving Multiphase Gel Materials for Complete Tissue Repair
Since the ultimate clinical goal is often to regenerate areas of tissue damage that require multiple cells types and interfaces of different tissues, researchers have begun to investigate means to build multiphase tissues in one-scaffold systems. Tissues, in particular those at an interface with a different tissue type, are extremely complex in nature (Sharma and Elisseeff, 2004). Cartilage, for example, contains three major zones, each comprised of chondrocytes differing in morphology, phenotype, and gene expression profiles (Cohen et al., 1998). Deep-zone cartilage is often composed of hypertrophic chondrocytes, which are beginning to produce more bone-like ECM components. However, higher zones allow for the production of more hyaline cartilage, softer than that found in the deep zone (Freeman et al., 1997). Recapitulating the differences of not only one type of tissue, like cartilage, but also trying to encompass the interface between bone and cartilage has proven to be a difficult task. Yet research in this area is greatly needed. Traumatic injuries often involve multiple tissue types, and regenerating matrix in the entire site requires a complex delivery of cues.
Some progress has been made toward achieving a multiphase scaffold containing both differentiated cartilage and bone cells from MSCs. Cartilage formation into distinct layers has been studied in detail via several techniques. Initial research found that MSCs encapsulated in a collagen gel and implanted into an osteochondral defect were able to differentiate into the zonal layers of native cartilage (Koga et al., 2007). MSCs were characterized as having the hyaline phenotype, in the middle regions of the scaffold, and a hypertrophic phenotype, in regions closest to the surrounding bone. Further, when incorporating growth factors into a scaffold, investigators have shown that multiple tissue types are expressed. For example, the formation of a bone-soft tissue-bone composite created by MSCs seeded in a collagen carrier with microencapsulated TGFβ-3 was studied (Moioli et al., 2008). The release of TGFβ-3 used in this study is thought to mimic the native secretion of this growth factor to influence chondrocyte differentiation and ECM production. Cell-seeded scaffolds were implanted into a cranial defect, and, through signals from the surrounding environment and the release of TGFβ-3, explants clearly displayed bone-cartilage-bone interfaces. In another study, a polycaprolactone-β-tricalcium phosphate composite gel induced MSC differentiation into an osteochondral phase, the interface between bone and cartilage layers (Swieszkowski et al., 2007). Other efforts at designing a hydrogel to present matrix components for the direction of MSCs into bone and cartilage phases demonstrated that MSCs seeded into a hydroxyapatite/chitosan scaffold achieved bone and cartilage differentiation (Oliveira et al., 2008). Here, the bone differentiation was affected by the osteoconductive hydroxyapatite material, while cartilage differentiation was guided by the chitosan portion of the bilayered scaffold. The collection of these experiments demonstrates the need for the design of bilayered, multilayered, or even gradient scaffolds for the repair of complex injuries. Although research has begun investigating means to achieve the differentiation of multiple cell types within one system, more research must be focused on this portion of the field.
Future Directions in Gel Development for 3D Culture Environments for MSCs and Their Clinical Application
While research in the field of MSC differentiation in hydrogel carriers is growing, and reports of various successes are emerging, it has certainly highlighted the need for future research to address current limitations involving, but not limited to, gel degradation, cell-material interactions, cell function, and multiphase tissue development. These limitations are being overcome by the provision of new material chemistries, fabrication technologies, and translational approaches for clinical applications. The complex nature of differentiating MSCs into a variety of different cell types has driven many aspects of these investigations. Intricate signaling, manipulations of differentiation pathways, and multifaceted cues are the areas to which scientists are directing their focus in understanding and influencing cell behavior.
Another interesting avenue for research is the directing of MSC differentiation and function in 3D hydrogels by means of new biological signals in the form of microRNAs. This is a recent area of research, but microRNAs provide a vast amount of potential for this area of study. MicroRNAs are a class of non-coding RNAs that can mediate the function of mRNAs in translating a protein via their target region. Most microRNAs are specifically targeted toward particular mRNAs; therefore, the regulation of these mRNAs is extremely well-organized. While only a select number of microRNA targets have been identified, studies have shown that the use of microRNAs can direct cellular function. It has been demonstrated that microRNAs transfected into myoblasts were shown to stimulate cell viability, growth, and proliferation without the presence of serum components (Kim et al., 2006). Furthermore, the inhibition of these microRNAs was shown to affect the cells in a manner similar to the removal of serum components from a culture. Other studies showed that microRNAs transfected into MSCs were able to regulate neurotransmitters in an effort to direct neuronal cell differentiation (Greco and Rameshwar, 2007). Therefore, these investigations show promise in the area of directing MSC function and fate in a 3D hydrogel environment.
The complex nature of MSC differentiation, along with the complexity of the tissues that scientists are aiming to recreate, requires the use of multiple signaling cues. The effectiveness of growth factors in directing cellular function has been demonstrated for decades; however, the combinatorial effects of these factors are not as widely studied. The design of a rapid screening method would greatly improve the techniques that scientists currently use to screen potential molecules. Some advances have been made in the area of creating high-throughput techniques to characterize materials and their inductive properties on causing the differentiation of MSCs (Benoit et al., 2008). This method would provide insight into the types and concentrations of gel precursor components that are effective in directing MSC differentiation and function. Once identified, a larger-scale hydrogel system can be designed from those material components that are needed to force the cells down a directed pathway.
Researchers have also begun to show an increased interest in the use or delivery of multiple cues as needed to best mimic the milieu of cues that direct MSC differentiation natively. Studies using the combination of TGFβ-1 and either IGF-1 or BMP-6 showed that MSCs responded better to the combination of factors than to either factor alone, in directing cartilage matrix deposition (Indrawattana et al., 2004). Similar results were shown with BMP-2 and TGFβ-1 having synergistic effects on the chondrogenic differentiation of MSCs (Toh et al., 2005). In an effort to better mimic the up- and down-regulation of cues in a native environment, researchers found that the early delivery of FGF-2 extended pre-osteoblast differentiation of MSCs in culture, while the later delivery of BMP-2 furthered bone development (Maegawa et al., 2007). In general, there is a critical need for understanding these synergistic effects to improve the design of MSC 3D scaffolds for cell delivery and tissue-engineering applications.
Conclusions
Great strides have been made in the development of hydrogel environments for the 3D culture of MSCs, cues to induce their differentiation and matrix deposition. Researchers have investigated the use of microencapsulated growth factors, complex matrices comprised of natural cues and easily tunable synthetics, as well as the use of new technologies, such as microRNAs. These directions of research provide great potential, since the MSC cell type is easily isolated and differentiation of these cells can be achieved through a variety of methods. Furthermore, the encapsulation of MSCs into a 3D hydrogel system allows for the implantation of this cell-laden device into a site of trauma for in situ regeneration of damaged tissue. The research in this area is ongoing, and the field is ever expanding the knowledge base on the complexity of these cells and their differentiation pathways. New scientists will evolve better techniques for the induction of MSCs down a particular pathway, and new investigations will aid in the development of a more mechanically sound hydrogel for the encapsulation and delivery of MSCs into an articulating joint. The future contains a wide array of possibilities for MSC tissue-engineering applications.
Acknowledgments
The authors thank Michael Schwartz, Alexandra Halevi, Charles Nuttelman, and Danielle Benoit for their contribution of images for this review article.
Footnotes
Further, the authors have been supported by grants from the NIH (DE12998 and DE16523), as well as by a fellowship to CNS from the Department of Education.
References
- Anseth KS, Bowman CN, Brannon-Peppas L. (1996). Mechanical properties of hydrogels and their experimental determination. Biomaterials 17:1647-1657. [DOI] [PubMed] [Google Scholar]
- Asanbaeva A, Masuda K, Thonar E, Klisch S, Sah R. (2007). Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum 56:188-198. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. (2004). Mesenchymal stem cells: clinical applications and biological characterization [review]. Int J Biochem Cell Biol 36:568-584. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton R, Liu B, Murphy JM. (2001a). Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268:189-200. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. (2001b). The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun 289:519-524. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Anseth KS. (2005). The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 26:5209-5220. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Nuttelman CR, Collins SD, Anseth KS. (2006). Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials 27:6102-6110. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Schwartz MJ, Durney AR, Anseth KS. (2008). Small functional groups for the controlled differentiation of human mesenchymal stem cells encapsulated in poly(ethylene glycol) hydrogels. Nat Mater 7:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. (1997). Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64:278-294. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. (2001). The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials 22:619-626. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Nuttelman CR, Anseth KS. (1999). The effects of crosslinking density on cartilage formation in photocrosslinkable hydrogels. Biomed Sci Instrum 35:309-314. [PubMed] [Google Scholar]
- Bryant SJ, Nuttelman CR, Anseth KS. (2000). Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Polym Ed 11:439-457. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Durand KL, Anseth KS. (2003). Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A 67:1430-1436. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Arthur JA, Anseth KS. (2005). Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater 1:243-252. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Anseth KS. (2002). Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23:4315-4323. [DOI] [PubMed] [Google Scholar]
- Canal T, Peppas NA. (1989). Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res 23:1183-1193. [DOI] [PubMed] [Google Scholar]
- Caplan AI. (1991). Mesenchymal stem cells. J Orthop Res 9:641-650. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, et al. (2001). Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res 57:394-403. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. (2002). Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol 20:245-256. [DOI] [PubMed] [Google Scholar]
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. (2008). Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 11:343-353. [PubMed] [Google Scholar]
- Cohen NP, Foster RJ, Mow VC. (1998). Composition and dynamics of articular cartilage: structure, function and maintaining healthy state. J Orthop Sports Phys Ther 28:203-215. [DOI] [PubMed] [Google Scholar]
- Cook AD, Hrkach JS, Gao NN, Johnson IM, Pajvani UB, Langer R, et al. (1997). Characterization and development of RGD-peptide-modified poly(lactic acid-co-lysine) as an interactive, resorbable biomaterial. J Biomed Mater Res 35:513-523. [DOI] [PubMed] [Google Scholar]
- Cook JL, Williams N, Kreeger JM, Peacock JT, Tomlinson JL. (2003). Biocompatibility of three-dimensional chondrocyte grafts in large tibial defects of rabbits. Am J Vet Res 64:12-20. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Schiller PC, Ricardi C, Roos BA, Howard GA. (1999). Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115-1122. [DOI] [PubMed] [Google Scholar]
- Dadsetan M, Hefferan TE, Szatkowski JP, Mishra PK, Macura SI, Lu L, et al. (2008). Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials 29:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli E, Salvade A, Mimo P, Vigano M, Morrone M, Papagna R, et al. (2007). Mesenchymal stem cells cultured on a collagen scaffold: in vitro osteogenic differentiation. Arch Oral Biol 52:64-73. [DOI] [PubMed] [Google Scholar]
- Drumheller PD, Hubbell JA. (1994). Polymer networks with grafted cell adhesion peptides for highly biospecific cell adhesive substrates. Anal Biochem 222:380-388. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126:677-689. [DOI] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Mu A, Littrell R, Richardson LB, Wright JN, et al. (2007). Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25:1241-1251. [DOI] [PubMed] [Google Scholar]
- Freeman DM, Bergman G, Glover G. (1997). Short TEMR microscopy: accurate measurement and zonal differentiation of normal hyaline cartilage. Magn Reson Med 38:72-81. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. (2003). Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage 11:55-64. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Vega MD, Boettiger D. (1999). Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell 10:785-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Rameshwar P. (2007). MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. Proc Natl Acad Sci USA 104:15484-15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habraken WJ, Wolke JG, Mikos AG, Jansen JA. (2006). Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed 17:1057-1074. [DOI] [PubMed] [Google Scholar]
- Hern DL, Hubbell JA. (1998). Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue engineering. J Biomed Mater Res 39:266-276. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. (2007). Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol 212:281-284. [DOI] [PubMed] [Google Scholar]
- Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, et al. (2004). Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 320:914-919. [DOI] [PubMed] [Google Scholar]
- Jansen JA, Vehof JW, Ruhe PQ, Kuboki Y, Takita H, Mikos AG, et al. (2005). Growth factor-loaded scaffolds for bone engineering. J Control Release 101:127-136. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AL, Goldberg VM, Yeo JU. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265-272. [DOI] [PubMed] [Google Scholar]
- Jones DG, Peterson L. (2006). Autologous chondrocyte implantation. J Bone Joint Surg Am 88:2501-2520. [DOI] [PubMed] [Google Scholar]
- Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. (2004). Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res A 71:528-537. [DOI] [PubMed] [Google Scholar]
- Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Kurovesis S, Perez SA, et al. (2005). Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv 65:321-329. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Prendergast PJ. (2005). Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J Biomech 38:1413-1422. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim HW, Suh H. (2003). Sustained release of ascorbate-2-phosphate and dexamethasone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem cells. Biomaterials 24:4671-4679. [DOI] [PubMed] [Google Scholar]
- Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. (2006). Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174:677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, et al. (2007). Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 25:689-696. [DOI] [PubMed] [Google Scholar]
- Lee CR, Grodzinsky AJ, Spector M. (2001). The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials 22:3145-3154. [DOI] [PubMed] [Google Scholar]
- Li WJ, Tuan RS. (2005). Polymeric scaffolds for cartilage tissue engineering. Macromol Symp 227:65-75. [Google Scholar]
- Longobardi L, Torello M, Buckaway C, O’Rear L, Horton WA, Hwa V, et al. (2003). A novel insulin-like growth factor (IGF)-independent role for IGF binding protein-3 in mesenchymal chondroprogenitor cell apoptosis. Endocrinology 144:1695-1702. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Weber FE, Schmoekel HG, Schanse JC, Kohler T, Müller R, et al. (2003). Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 21:513-518. [DOI] [PubMed] [Google Scholar]
- Mackay A, Beck S, Murphy JM, Barry FP, Chichester CO, Pittenger MF. (1998). Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4:415-428. [DOI] [PubMed] [Google Scholar]
- Maegawa N, Kawamura K, Hirose M, Yajima H, Takakura Y, Ohgushi H. (2007). Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2). J Tissue Eng Regen Med 1:306-313. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Yuan X, Tuan RS. (2006). Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14:179-189. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. (2007). Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 28:5280-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LA, Reid AJ, Campbell VA, Prendergast PJ. (2008). Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen-GAG scaffold: experimental and computational analysis. Ann Biomed Eng 36:185-194. [DOI] [PubMed] [Google Scholar]
- Moioli EK, Clark PA, Sumner DR, Mao JJ. (2008). Autologous stem cell regeneration in craniosynostosis. Bone 42:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. (2002). Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 46:704-713. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Tripodi MC, Anseth KS. (2005). Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 24:208-218. [DOI] [PubMed] [Google Scholar]
- Oliveira JM, Costa SA, Leonor IB, Malafaya PB, Mano JF, Reis RL. (2008). Novel hydroxyapatite/carboxymethylchitosan composite scaffolds prepared through an innovative ”autocatalytic” electroless coprecipitation route. J Biomed Mater Res A 88:470-480. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bord S, Triffitt JT. (1998). Skeletal progenitor cells and ageing human populations. Clin Sci 94:549-555. [DOI] [PubMed] [Google Scholar]
- Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. (2007). Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28:3217-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Lakes RS. (1992). Biomaterials: an introduction. New York: Springer. [Google Scholar]
- Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. (2000). Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng 2:9-29. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. (2006). Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Advanced Mater 18:1345-1360. [Google Scholar]
- Pier GB, DesJardin D, Girout M, Garner C, Bennett SE, Pekoe G, et al. (1994). Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect Immun 62:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147. [DOI] [PubMed] [Google Scholar]
- Prestwich GD, Marecak DM, Marecak JF, Vercruysse KP, Ziebell MR. (1998). Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J Control Release 53:93-103. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71-74. [DOI] [PubMed] [Google Scholar]
- Rice MA, Anseth KS. (2004). Encapsulating chondrocytes in copolymer gels: bimodal degradation kinetics influence cell phenotype and extracellular matrix development. J Biomed Mater Res A 70:560-568. [DOI] [PubMed] [Google Scholar]
- Rice MA, Sanchez-Adams J, Anseth KS. (2006). Exogenously triggered, enzymatic degradation of photopolymerized hydrogels with polycaprolactone subunits: experimental observation and modeling of mass loss behavior. Biomacromolecules 7:1968-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas CN, Anseth KS. (2008). The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29:2370-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas CN, Cole BB, Kasko AM, Anseth KS. (2007). Chondrogenicdifferentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng 13:1025-1034. [DOI] [PubMed] [Google Scholar]
- Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. (2008). Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 36:235-246. [DOI] [PubMed] [Google Scholar]
- Seliktar D, Zisch AH, Lutrolf MP, Wrana JL, Hubbell JA. (2004). MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A 68:704-716. [DOI] [PubMed] [Google Scholar]
- Sharma B, Elisseeff JH. (2004). Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng 32:148-159. [DOI] [PubMed] [Google Scholar]
- Suzawa M, Takada I, Yanagisawa J, Ohtaka F, Ogawa S, Kato S, et al. (2003). Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol 5:224-230. [DOI] [PubMed] [Google Scholar]
- Swieszkowski W, Tuan BH, Karzydlowski KJ, Hutmader DW. (2007). Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng 24:489-495. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Yamamoto M, Tabata Y. (2005). Design of an osteoinductive biodegradable cell scaffold based on controlled release technology of bone morphogenetic protein. Israel J Chem 45:465-475. [Google Scholar]
- Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, et al. (2007). Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25:2730-2738. [DOI] [PubMed] [Google Scholar]
- Toh WS, Liu H, Heng BC, Rufaihah AJ, Ye CP, Cao T. (2005). Combined effects of TGFbeta1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors 23:313-321. [DOI] [PubMed] [Google Scholar]
- Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. (2008). Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27:12-21. [DOI] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Haskins K, Anseth KS. (2007). The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28: 3004-3011. [DOI] [PubMed] [Google Scholar]
- Williams GM, Lin JW, Sah RL. (2007). Cartilage reshaping via in vitro mechanical loading. Tissue Eng 13:2903-2911. [DOI] [PubMed] [Google Scholar]
- Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. (2001). Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res 19:738-749. [DOI] [PubMed] [Google Scholar]
- Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. (2005). The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 26:5991-5998. [DOI] [PubMed] [Google Scholar]
- Yoneno K, Ohno S, Tanimoto K, Honda K, Tanaka N, Doi T, et al. (2005). Multidifferentiation potential of mesenchymal stem cells in three-dimensional collagen gel cultures. J Biomed Mater Res A 75:733-741. [DOI] [PubMed] [Google Scholar]