Abstract
The total synthesis of the structurally unique secondary metabolite sporolide B (1b, Figure 1) is described. The total synthesis of 1b was developed on the basis of preliminary studies that revealed the reactivity of an appropriate o-quinone as a diene system toward a number of indene derivatives as dienophiles, first in intermolecular and thence intramolecular settings. Thus, substrates were devised (37, Scheme 5; 75, Scheme 11) that underwent exquisite intramolecular [4+2] cycloaddition reactions under thermal conditions to provide primitive sporolide-type structures that were subsequently elaborated to sporolide model system 4 (Figure 1), 9-epi-sporolide B (5, Figure 1), and sporolide B (1b). The requisite indene o-quinone precursor (75, Scheme 10) was synthesized through a rutheniumcatalyzed [2+2+2] cycloaddition reaction between a propargylic alcohol (42, Figure 8) and a chloroacetylenic cyclopentenyne (64, Scheme 9) followed by elaboration and silver-promoted oxidation of the resulting chloroindene derivative. In addition to the total synthesis of 1b, this work demonstrated, for the first time, the power of the intramolecular hetero [4+2] cycloaddition reaction in the total synthesis of complex molecules and the application of the ruthenium-catalyzed [2+2+2] cycloaddition reaction to highly substituted indene systems possessing a chlorine residue on the aromatic nucleus.
Keywords: indene [4+2] cycloaddition, natural products, o-quinone [4+2] cycloaddition, ruthenium-catalyzed [2+2+2] cycloaddition, total synthesis
Introduction
The marine actinomycete Salinospora tropica is the producer of salinosporamide A,1 a potent inhibitor of the 20S proteasome that elicited considerable interest as a lead compound for cancer chemotherapy.2 In addition to this natural product, the Fenical group has recently disclosed the isolation, from the same species, of sporolides A (1a, Figure 1) and B (1b, Figure 1).3 Despite their striking molecular architectures, the latter natural products exhibited no biological activities for which they were tested. Their conspicuous indanoid structural motif, however, gave rise to the intriguing hypothesis4 that they are derived from the 9-membered enediyne 2 (Figure 1), which was named presporolide,5 through a Bergman cycloaromatization.6 As shown in Figure 1, the conversion of the initially generated benzenoid diradical (3) from the Bergman cycloaromatization of 2 required a hitherto unknown reaction, namely the sequential trapping of the diradical with a chloride anion (ClΘ) and a proton (H⊕). This mode of reactivity was later demonstrated by Perrin and O'Connor through experimentation that included supporting kinetic studies.7 Thus, it does appear that Salinospora tropica produces, in addition to salinosporamide A, a second structurally distinct cytotoxic agent, presporolide 2, whose rapid rearrangement and subsequent reactions lead to the inactive, but revealing, sporolides A (1a) and B (1b).
Figure 1.
Molecular structures of sporolide A (1a), sporolide B (1b), presporolide (2), benzenoid diradical 3, sporolide model system 4, and 9-epi-sporolide B (5).
Fascinated by the unprecedented molecular structures of the sporolides, and the interesting hypothesis regarding their origins, we contemplated their total synthesis, an endeavor that we felt could lead to new synthetic strategies and technologies and may also serve as a prelude to a possible approach toward the more challenging structure of presporolide (2) by virtue of its expected strain and lability. In previous communications, we disclosed preliminary results leading to the construction of a sporolide model system (4, Figure 1)8 and sporolide B (1b).9 In this article, we provide a full account of our investigation that led to the total synthesis of sporolide B (1b) and its diastereoisomer, 9-epi-sporolide B (5, Figure 1).
Results and Discussion
Initial Retrosynthetic Considerations and Model Studies
Sporolides A (1a) and B (1b) share the same molecular structure except for the location of the chlorine atom on the benzenoid nucleus. Of their 24 carbons, 22 are either oxygenated or sp2 hybridized, forming a web of unusual structural motifs, including a highly substituted indane system, a [1,4]-dioxane ring, an epoxy cyclohexenone hemiacetal, and a 13-membered macrolide moiety.3a Considering the unprecedented and complex molecular architectures of the sporolides, the discovery and development of new synthetic strategies and technologies were essential prerequisites for a serious attempt of their total synthesis. Figure 2 outlines, in retrosynthetic format, two plausible approaches to sporolide B (1b). Based on the postulated biosynthesis of the sporolides (I + II → 1b),4b the first approach (Path a, Figure 2) was considered risky, despite the rather conventional steps required for its implementation, due to the expected lability of some of the obligatory intermediates. Therefore, the second approach based on the rarely employed [4+2] cycloaddition reaction of o-quinones10 (III + IV → 1b, Path b, Figure 2) became our preferred choice for initial exploration.
Figure 2.
Plausible synthetic approaches to sporolide B: (Path a) biomimetic approach, (Path b) o-quinone [4+2] cycloaddition approach.
In contemplating the o-quinone-based strategy toward sporolide B (1b), the two approaches shown retrosynthetically in Figure 3 (both relying on the same bond disconnections) came to mind. Path a requires an intermolecular o-quinone [4+2] cycloaddition with an indene partner followed by a macrolactonization of seco acid V to forge the main ring skeleton of the target molecule. Path b reverses these two key steps and requires substrate VI as an intermediate postulated to undergo an intramolecular [4+2] cycloaddition11 involving its o-quinone and indene structural motifs.
Figure 3.
Retrosynthetic analysis of sporolide B (1b) and sporolide model systems 4′ and 4.
In order to test the viability of these two synthetic strategies (paths a and b, Figure 3a), we designed model systems 4 and 4′ and pursued their synthesis from o-quinone derivative or surrogate (6 or 8, respectively, Figure 3b) and hydroxy indene fragments (7 or 9, respectively, Figure 3b). Our first task toward this goal was to prepare o-quinone 6 and study its reactivity toward suitable indene fragments. Scheme 1 summarizes the synthesis of this o-quinone starting with 3-methyl sesamol (10).12a Thus, activation of phenol 10 with MeMgBr,12b followed by addition of glyoxalate 11, led to phenolic hydroxy ester 12 in 98% yield. Reaction of the latter compound with BnBr in the presence of Cs2CO3 and NaI resulted in selective formation of hydroxy benzyl ether 13 (92% yield), which was methylated (TMSCHN2, HBF4)13 to afford fully protected derivative 14. Cleavage of the rather robust dioxolane ring within 14 was achieved through sequential treatment with Pb(OAc)4 (benzene, Δ)14 and AcOH, furnishing diphenol 15 in 95% overall yield. Interestingly, while 15 proved inert toward DDQ oxidation at ambient temperature and led to the corresponding p-quinone in the presence of DMP or CAN, it reacted smoothly with Ag2O to afford the desired o-quinone 6 in 94% yield.
Scheme 1.
Construction of o-Quinone 6a
a Reagents and conditions: a) 10, MeMgBr (3.0 M in Et2O, 1.2 equiv), THF, 0 °C, 0.5 h; then 11 (50% in toluene, 1.5 equiv), THF, 0 °C, 0.5 h, 98%; b) BnBr (3.0 equiv), Cs2CO3 (2.0 equiv), NaI (1.0 equiv), DMF, 0 → 25 °C, 3 h, 92%; c) TMSCHN2 (2.0 M in hexanes, 2.0 equiv), HBF4 (49% in H2O, 1.0 equiv), CH2Cl2, 0 → 25 °C, 2 h, 81%; d) Pb(OAc)4 (1.5 equiv), benzene, 80 °C, 16 h; e) AcOH:THF:H2O (10:5:1), 25 °C, 6 h, 95% for the two steps; f) Ag2O (2.5 equiv), CH2Cl2, 25 °C, 15 min, 94%. Bn = benzyl, TMS = trimethylsilyl.
Since the mode of [4+2] cycloaddition reactions of o-quinones with dienophiles depends on the nature of their substituents and is often unpredictable,10 an initial investigation of the reactivity of o-quinone 6 toward indenes was deemed important. Scheme 2 summarizes a number of illuminating observations made in this model study. Thus, reaction of 6 with indene (16) in toluene at 110 °C proceeded smoothly, but afforded 1,2-cyclohexadione system 17 as a mixture with its facial diastereoisomer 17′ (ca. 1.5:1 dr), products of the undesired reactivity mode for the o-quinone that involved its all-carbon diene system. The precise structure of this cycloadduct was evident from its NMR spectroscopic analysis, including the nOe correlation between the methyl group and its adjacent proton as indicated on its structure (see 17, Scheme 2). The exquisite regioselectivity of this cycloaddition reaction may be explained by assuming polarization10e of the two reactants as indicated on transition state 6,16-TS (see Scheme 2) and the relatively low steric demands associated with the olefinic bond of indene 16. This leads to the engagement of the dienophile with the apparently more reactive, all-carbon diene system of o-quinone 6. This reasoning led us to believe that by increasing the steric demands10f of the indene dienophile through fusion of the additional cyclopentane ring as required for our target molecule, we may be able to reverse the reactivity mode of the o-quinone component from the all-carbon diene to the 1,2-dicarbonyl moiety. Indeed, indene derivative 1815 reacted with o-quinone 6 under the same conditions as above to afford, presumably through transition state 6,18-TS (see Scheme 2), 1,4-dioxane derivative 19 (ca. 1:1 mixture with its facial diastereoisomer 19′) exclusively and in 57% yield. Needless to say that this was exciting news, but before we describe the next phase of the campaign we should note that indene derivative 20 (Scheme 2) reacted with o-quinone 6 in yet another exquisite mode. Specifically, this [4+2] cycloaddition reaction led to carbocyclic system 21 as the major product in high yield (80%, ca. 10:1 mixture with its facial diastereoisomer 21′), presumably through transition state 6,20-TS as shown in Scheme 2. In this case, the regioselectivity exhibited by the o-quinone moiety of 6 toward indene (i.e. 16) is preserved, whereas that of the dienophile is now reversed, apparently due to the presence of the polarizing carbonyl group within the indene partner. The higher reactivity of the latter component may explain its ability to overcome its steric demands and reach the more reactive, but more sterically shielded, all-carbon diene system of o-quinone 6. The structure of the observed major product 21, preferentially crystallized from EtOAc/hexanes (m.p. 210–212 °C, acetone/hexanes), was unambiguously proven through X-ray crystallographic analysis16 (see ORTEP representation, Figure 4).
Scheme 2.
Intermolecular [4+2] Cycloaddition Reaction Between Indene Derivatives and o-Quinone 6a
a Reagents and conditions: indene derivatives 16, 18, 20 (1.0 equiv), o-quinone 6 (1.2 equiv), toluene, 115 °C, 4 h, 17, 17′ (82%, ca. 1.5:1 dr), 19, 19′ (57%, ca. 1:1 dr), 21, 21′ (80%, ca. 10:1 dr).
Figure 4.
ORTEP of 21 derived from X-ray crystallographic analysis (non hydrogen atoms are shown as 30% ellipsoids).
These studies suggested that hydroxy indene derivative 7 (Scheme 3) might be an appropriate partner, indeed, for o-quinone 6 in our first strategy toward sporolide model system 4′. Its construction from benzoic acid derivative 2217 is summarized in Scheme 3. Thus, reduction of the carboxylic acid moiety of 22 with borane (80% yield), followed by protection of the resulting alcohol (dihydropyran, TsOH·H2O, 95% yield) furnished THP ether 23. This benzyl bromide derivative (23) was then used to alkylate cyclopentanone (24, LDA) to afford aryl iodide 25 (65% yield), whose intramolecular Nozaki–Hiyama–Kishi reaction (CrCl2, NiCl2)18 led to tricyclic tertiary alcohol 26 in 76% yield. Exposure of the latter compound to TsOH·OH caused both elimination of water and cleavage of the THP ether to afford the desired hydroxy indene 7 in 82% yield. The other required indene derivative (9) for model system 4 (path b, see Figure 3b), was formed from 7 by one carbon homologation in 76% overall yield through the standard four-step sequence summarized in Scheme 3.
Scheme 3.
Construction of Indene Derivatives 7 and 9a
a Reagents and conditions: a) BH3·THF (1.0 M in THF, 2.0 equiv), THF, 25 °C, 2 h, 80%; b) DHP (1.2 equiv), TsOH·H2O (0.1 equiv), CH2Cl2, 0 °C, 0.5 h, 95%; c) 24 (1.3 equiv), LDA (1.3 equiv), THF:HMPA (5:1), −78 → 0 °C, 1 h; then 23 (1.0 equiv), −78 → 25 °C, 2 h, 65%; d) CrCl2 (4.0 equiv), NiCl2 (0.02 equiv), DMF, 100 °C, 16 h, 76%; e) TsOH·H2O (0.1 equiv), benzene, 80 °C, 0.5 h; then MeOH, 25 °C, 0.5 h, 82%; f) DMP (1.2 equiv), NaHCO3 (3.0 equiv), CH2Cl2, 25 °C, 0.5 h, 95%; g) MeOCH2PPh3Br (1.5 equiv), KHMDS (0.5 M in toluene, 1.5 equiv), THF, 0 → 25 °C, 2 h, 96%; h) HCl (aq., 2.0 N, 4.0 equiv), THF, 60 °C, 3 h, 88%; i) NaBH4 (1.1 equiv), THF:MeOH (5:1), 0 °C, 0.5 h, 95%. DHP = 3,4-dihydro-2H-pyran, DMP = Dess–Martin periodinane, HMPA = hexamethylphosphoramide, KHMDS = potassium hexamethyldisilazane, LDA = lithium diisopropylamide, Ts = p-toluenesulfonyl.
With both required fragments now available, we proceeded to explore the first approach to the designed sporolide model system 4′ involving, in that order, the [4+2] cycloaddition and macrolactonization reactions, as outlined in Scheme 4. Thus, heating hydroxyl indene derivative 7 with o-quinone 6 in toluene at 115 °C led, as expected, and presumably through transition state 6,7-TS, to 1,4-dioxa cycloadduct 27, together with its facial diastereoisomer (27′), in 62% combined yield (ca. 1:1 dr). This mixture was taken forward through the rest of the sequence in order to test the key macrolactonization step. Oxidation of 27+27′ (DMP, 88% yield), followed by olefination of the resulting aldehyde (28+28′, CH2=PPh3), furnished styrene derivative 29 (together with its facial diastereoisomer 29′), whose asymmetric dihydroxylation (AD-mix β)19 afforded diols 30+30′ (93% yield). Finally, saponification of the ethyl ester moiety within 30+30′ (LiOH) furnished seco acids 31+31′ (98% yield). All attempts to macrolactonize 31+31′ through either carboxyl20 or hydroxyl activation21 (see legend, Scheme 4) failed to produce any detectable amounts of the desired model system 32 (or its facial diastereoisomer 32′), presumably due to overwhelming strain within the expected transition state for the macrolactonization reaction. These failures led us to the second, and more daring, appoach that involving the risky intramolecular [4+2] cycloaddition as the means to forge the sporolide framework.
Scheme 4.
Synthesis of Dihydroxy Acid 31 and Failed Attempts to Form Macrocycle 32 Through Macrolactonizationa
a Reagents and conditions: a) 6 (1.0 equiv), 7 (1.2 equiv), toluene, 115 °C, 3 h, 62% [27+27′ (not shown), ca. 1:1 dr]; b) DMP (1.5 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 0.5 h, 88% [28+28′ (not shown), ca. 1:1 dr]; c) MePPh3Br (4.5 equiv), KHMDS (0.5 M in toluene, 3.0 equiv), THF, −78 → 25 °C, 0.5 h; then 28+28′ (ca. 1:1 dr, 1.0 equiv), THF, −78 → 25 °C, 0.5 h, 91% [29+29′ (not shown), ca. 1:1 dr]; d) AD-mix β (1.4 g per 1.0 mmol 29+29′), t-BuOH:H2O (1:1), 0 °C, 4 h, 93% [30+30′ (not shown), ca. 1:1 dr]; e) LiOH (30 equiv), dioxane:H2O (5:1), 80 °C, 6 h, 98% [31+31′ (not shown), ca. 1:1 dr]; f) carboxylic acid activation methods (Corey– 22 Yamaguchi,23 cyanuric chloride,24 Mukaiyama salt,25 Shiina26); alcohol activation method (Mitsunobu21).
Scheme 5 summarizes the intramolecular o-quinone [4+2] cycloaddition approach to sporolide model system 4 from fragments 15 and 9. Thus, catechol 15 was protected as orthoester 33 [CH(OEt)3, TsOH·H2O, 96% yield], which was saponified (LiOH, 99% yield) to afford carboxylic acid 34. Coupling of the latter intermediate with hydroxy indene 9 (see Scheme 3 for preparation) through DCC/4-DMAP esterification led to ester 35 (84% yield), whose exposure to TsOH·H2O afforded catechol ester 36 in 98% yield. Pleasantly, the latter compound served as a precursor, first to o-quinone 37, and thence to the desired, doubly cyclized cycloadduct 38a. Thus, heating the deep-red solution of 36 in toluene at 120 °C (oil bath) in the presence of Ag2O afforded compound 38a, presumbly through o-quinone 37, in 60% isolated yield. Indeed, the latter intermediate was prepared from 36 by oxidation with Ag2O at room temperature (99% yield) and, when heated in toluene at 120 °C, furnished compound 38a in 50% yield. This product was obtained as a single diastereoisomer [out of the four possible diastereoisomers (38a–d) shown in Figure 5]. Its structure was consistent with its NMR spectral data, including the 13C NMR chemical shifts for the two newly formed tertiary dioxane carbons (δ = 101.5 and 98.3 ppm, 150 MHz, CDCl3). An X-ray crystallographic analysis27 of the debenzylated derivative (39, m.p. 233–234 °C, acetone/hexanes) of 38a provided unambiguous proof of its structure as shown (see ORTEP representation, Figure 6). This stereochemical outcome can be attributed to a combination of electronic and steric effects as shown in Figure 5. Electron donation from the benzyl ether oxygen toward the C6′ carbonyl oxygen results in polarization of the o-quinone system of substrate 37. The partial negative charge (δ−) on this oxygen atom matches the partial positive charge (δ+) on the C10 indene olefinic carbon, thereby providing guidance for the regiochemical outcome of the ensuing [4+2] cycloaddition. The other factors controlling the outcome of this reaction include steric (1,3-allylic strain) and feasibility of bond formation (ability of the two polarized ends to reach each other to form a bond). As depicted in Figure 5, from the four possible transition states (38a–d-TS) only 38a-TS leading to desired product 38a is favored by both electronic matching and sterics. Indeed, in this case the OMe group (front) resides opposite the OBn (back) and the Oδ− is well positioned beneath the Cδ+ for bonding interaction and ring formation. Transition state 38b-TS suffers from 1,3-allylic strain (OBn group resides on the same side as OMe group, both back). Transition state 38c-TS suffers from both 1,3-allylic strain (both OBn and OMe in front) and a mismatch of the positions of the partial charges (δ− front, δ+ back). Transition state 38d-TS suffers from a mismatch of the positions of the partial charges (δ+ front, δ− back). In view of these considerations, the fact that only product 38a is formed in this intramolecular [4+2] cycloaddition is no surprise.
Scheme 5.
Synthesis of Sporolide Model System 4a
a Reagents and conditions: a) CH(OEt)3 (2.0 equiv), TsOH·H2O (0.2 equiv), 4 Å MS (80 mg per mmol of 15), benzene, 80 °C, 16 h, 96%; b) LiOH (20 equiv), dioxane:H2O (5:1), 80 °C, 3 h, 99%; c) 34 (1.25 equiv), 9 (1.0 equiv), DCC (1.3 equiv), 4-DMAP (0.2 equiv), CH2Cl2, 25 °C, 3 h, 84 %; d) TsOH·H2O (0.05 equiv), MeOH, 25 °C, 24 h, 98%; e) one-step procedure: Ag2O (2.0 equiv), toluene (c = 0.005 M solution), 120 °C, 1 h, 60%; two-step procedure: (i) Ag2O (2.0 equiv), CH2Cl2, 25 °C, 5 min, 99%; (ii) toluene (c = 0.005 M solution), 120 °C, 3 h, 50%; f) H2 (balloon), Pd(OH)2/C (20% wt), EtOH, 25 °C, 14 h, 85%; g) PIFA (1.05 equiv), PMBOH (10.0 equiv), K2CO3 (5.0 equiv), MeCN, 0 → 25 °C, 5 min, 87%; h) DDQ (1.5 equiv), CH2Cl2:H2O (10:1), 25 °C, 15 h, 96%; i) t-BuOOH (5.0 equiv), DBU (0.2 equiv), CH2Cl2, 40 °C, 24 h, 60%. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DCC = N,N′-dicyclohexylcarbodiimide, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, 4-DMAP = 4-dimethylaminopyridine, PIFA = phenyliodine bis(trifluoroacetate), PMB = p-methoxybenzyl.
Figure 5.
Four possible diastereoisomers of macrolide 38 (a–d) and rationale for the exclusive formation of the only isomer (i.e. 38a).
Figure 6.
ORTEP of compound 39 derived from X-ray crystallographic analysis (atoms are shown as 30% ellipsoids).
Having secured key intermediate 38a, its further elaboration, requiring dearomatization and appropriate functionalization, was undertaken (Scheme 5). Thus, hydrogenolysis of the benzyl ether within 38a [H2, Pd(OH)2/C] furnished phenol 39 (85% yield), whose exposure to PIFA28 in the presence of PMBOH and K2CO3 led to p-hemiquinone 40 in 87% yield. Treatment of the latter intermediate with DDQ gave p-hydroxy hemiquinol 41 (96% yield), which underwent selective epoxidation (t-BuOOH, DBU, 40 °C)29 under controlled conditions to afford the targeted sporolide model system 4 in 60% yield. The regio- and stereocontrol observed in the latter reaction was ensured, not only by the directing effect of the hemi-acetal moiety, but also by the shielding effects imposed upon the top face of the olefinic bond by the macrocyclic structural motif of the substrate (41). That all structural motifs were present within 4 (m.p. 218–219 °C, acetone/hexanes) in their proper stereochemical arrangements was proven through an X-ray crystallographic analysis30 (see ORTEP representation, Figure 7).
Figure 7.
ORTEP of sporolide model system 4 derived from X-ray crystallographic analysis (atoms are shown as 30% ellipsoids).
Unsuccessful Forays Toward Sporolide B
The successful model study culminating in the synthesis of sporolide model system 4 set the foundation for the next phase of the program toward the total synthesis of 1b, the sporolide chosen as our synthetic target molecule. The two approaches considered toward sporolide B (1b) are shown retrosynthetically in Figure 8. Path a was based on the obvious disconnection of the ester bond within advanced precursor VII obtained through disassembly of the 1,4-dioxane ring of sporolide B (1b), as depicted. This analysis led to key building blocks carboxylic acid VIII and hydroxy chloroindene IX as the requisite fragments for the projected synthetic strategy.
Figure 8.
Retrosynthetic analysis [paths a (top) and b (bottom)] of sporolide B (1b). P = protecting group.
Although a synthesis of enantiopure carboxylic acid VIII (P = Bn) could be secured (see below), repeated attempts to construct hydroxy chloroindene IX through conventional methods were met with difficulties, presumably due to the sensitive and crowded nature of this molecule. These failures let us to consider the alternative strategy (path b) shown in Figure 8 as a possible avenue to reach the desired precursor VII. In this scenario, the chlorobenzenoid nucleus in VII was disassembled through a metal-catalyzed [2+2+2] cycloaddition31 that led to propargylic alcohol 42 and chloroacetylene enyne 43 as the desired building blocks for sporolide B (1b). These building blocks were then traced back to even simpler starting materials through the disconnections indicated on their structures (see Figure 8).
The hydroxy ester fragment 42 was synthesized through coupling and elaboration of carboxylic acid 48 and acetylenic diol 49, starting with benzaldehyde derivative 4432 as summarized in Scheme 6. Thus, methylenation of aldehyde 44 (Ph3P=CH2, 98% yield) followed by asymmetric dihydroxylation (AD-mix β)19 of the resulting styrene (45) furnished 1,2-diol 46 in 96% yield and 98% ee (chiral HPLC). Conversion of the latter compound to methoxy primary alcohol 47 required temporary silylation of the primary alcohol (TBSCl, 99% yield), methylation of the secondary alcohol (t-BuOK, MeI, 95% yield), and removal of the TBS group (TBAF, 99% yield). Generation of the corresponding aldehyde from primary alcohol 47 (DMP, 78% yield) followed by Pinnick oxidation (96% yield, 98% ee, chiral HPLC) then led to carboxylic acid 48. This intermediate proved rather sensitive to racemization even on standing, and, therefore, was freshly prepared and coupled with acetylenic diol 4933 in the presence of EDCI and 4-DMAP to afford hydroxy ester 50 in 73% yield. At this point, it was necessary to partially oxidize the methylene protecting moiety within 50 in order to facilitate its pending rupture under mild conditions at a later stage. This was accomplished through the action of Pb(OAc)4 in benzene14 at 75 °C, which afforded acetoxy derivative 42 in 89% yield.
Scheme 6.
Construction of Propargylic Alcohol 42a
a Reagents and conditions: a) MePPh3Br (1.5 equiv), KHMDS (1.0 M in toluene, 1.2 equiv), THF, −78 → 0 °C, 0.5 h; then 44, THF, −78 → 0 °C, 0.5 h, 98%; b) AD-mix β (1.4 g per mmol 45), t-BuOH:H2O (1:1), 25 °C, 8 h, 96%, 98% ee by chiral HPLC; c) TBSCl (1.5 equiv), Et3N (1.5 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 25 °C, 8 h, 99%; d) t-BuOK (3.0 equiv), MeI (4.0 equiv), MeCN, 0 → 25 °C, 16 h, 95%; e) n-Bu4NF (1.0 M in THF, 1.5 equiv), THF, 25 °C, 16 h, 99%; f) DMP (1.5 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 1 h, 78%; g) NaClO2 (4.5 equiv), NaH2PO4·2H2O (3.0 equiv), 2-methyl-2-butene (2.5 equiv), t-BuOH:H2O (1:1), 25 °C, 0.5 h, 96%; h) 49 (1.3 equiv), EDCI (1.2 equiv), 4-DMAP (0.2 equiv), CH2Cl2, 25 °C, 3 h, 73%; i) Pb(OAc)4 (1.5 equiv), benzene, 75 °C, 1 h, 89%. EDCI = 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride.
The readily available iodocyclopentenone 5134 served as an excellent precursor to the desired chloroacetylenic enyne fragment 43 as shown in Scheme 7. Thus, Luche reduction (NaBH4, CeCl3·7H2O)35 of 51 followed by benzylation (BnBr, NaH, n-Bu4NI) of the resulting secondary alcohol furnished vinyl iodide 52 in 95% overall yield. Carboxymethylation36 of the latter compound [CO, Pd(PPh3)2Cl2, MeOH] afforded methyl ester 53 in 95% yield. A sequence of four steps was then employed to convert ester 53 to enone 54 [(i) DIBAL-H, 95% yield; (ii) DHP, TsOH·H2O; (iii) TBAF, (iv) DMP, 83% overall yield for the three steps]. Iodination (I2, 80% yield) of this enone (54) afforded iodoenone 55, whose CBS reduction [(S)-CBS-Me]37 and TBS protection of the so-obtained alcohol (ca. 10:1 dr) led to the intended TBS ether 56 in 97% overall yield for the two steps. Incidently, reduction of iodoenone 55 under standard Luche reduction conditions35 led, as expected on steric grounds, to the corresponding 1,4-syn diol. Sonogashira coupling38 of vinyl iodide 56 with TMS acetylene (79% yield) then furnished, after THP cleavage (Et2AlCl) and DMP oxidation (73% yield for the two steps), aldehyde 57. This aldehyde was added to lithio chloroacetylene, generated in situ from cis-1,2-dichloroethylene and MeLi,39 to give propargylic alcohol 58 (ca. 7:1 dr, chelation-controlled, see path a in 57-TS, Scheme 7) in 79% yield. Pure 58 was obtained at this stage by chromatographic removal of all other minor diastereoisomers obtained in the CBS reduction (55 → 56) and the lithio chloroacetylene addition (57 → 58). Finally, the TMS group was removed from intermediate 58 with K2CO3 in MeOH to afford compound 59, whose hydroxy group was acetylated, leading to the targeted fragment chloroacetylenic enyne 43 in 93% yield for the two steps.
Scheme 7.
Construction of Chloroacetylenic Enyne 43a
a Reagents and conditions: a) NaBH4 (1.2 equiv), CeCl3·7H2O (1.2 equiv), MeOH, −78 °C, 1 h; b) NaH (60% in mineral oil, 1.5 equiv), THF, 0 °C, 0.5 h; then BnBr (1.5 equiv), n-Bu4NI (0.2 equiv), THF, 0 → 25 °C, 16 h, 95% for the two steps (ca. 10:1 dr); c) Pd(PPh3)2Cl2 (0.05 equiv), Et3N (5.0 equiv), CO (balloon), MeOH, 70 °C, 3 h, 95%; d) DIBAL-H (1.0 M in toluene, 2.5 equiv), toluene, −78 → −10 °C, 1 h, 95%; e) DHP (1.5 equiv), TsOH·H2O (0.1 equiv), CH2Cl2, 0 °C, 0.5 h; f) n-Bu4NF (1.0 M in THF, 1.5 equiv), THF, 25 °C, 3 h; g) DMP (1.2 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 0.5 h, 83% for the three steps; h) I2 (3.0 equiv), CH2Cl2:pyridine (1:1), 25 °C, 15 h, 80%; i) (S)-CBS-Me (1.0 M in toluene, 0.1 equiv), BH3·THF (1.0 M in THF, 1.3 equiv), THF, −30 °C, 2 h, 99%; j) TBSCl (2.0 equiv), imidazole (2.0 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 25 °C, 16 h, 97%; k) TMS-acetylene (1.5 equiv), Pd(PPh3)2Cl2 (0.02 equiv), CuI (0.04 equiv), Et2NH, 25 °C, 16 h, 79%; l) Et2AlCl (1.8 M in toluene, 2.0 equiv), CH2Cl2, −25 → 25 °C, 3 h; m) DMP (1.5 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 0.5 h, 73% for the two steps; n) cis-1,2-dichloroethylene (4.5 equiv), MeLi (1.6 M in Et2O, 3.0 equiv), Et2O, 0 °C, 0.5 h; then 57, Et2O, 0 °C, 15 min, 79% of pure 58 (ca. 7:1 dr, all the undesired diastereoisomers were chromatographically removed at this stage); o) K2CO3 (1.5 equiv), MeOH, 25 °C, 1 h, 99%; p) Ac2O (1.5 equiv), Et3N (2.0 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 0 °C, 0.5 h, 93%. (S)-CBS-Me = (S)-(−)-2-methyl-CBS-oxazaborolidine.
With both fragments acetylenic ester 42 and chloroacetylenic enyne 43 in hand, their [2+2+2] cycloaddition to form the desired indene structural motif became the next task. As shown in Scheme 8, it was found that reaction of these compounds in the presence of catalytic amounts of Cp*RuCl(COD)40 at ambient temperature furnished a single regioisomer (77% yield) whose structure was determined to be that depicted as 60 (for a mechanistic rationale for this outcome, see below). Acetylation of the lone hydroxyl group within the latter intermediate (Ac2O, 4-DMAP, Et3N, 81% yield), followed by treatment with aq. HF caused desilylation and liberation of the catechol moiety (50% yield, unoptimized) to afford diphenolic hydroxy diacetate 61, in which the C-11 stereocenter had apparently epimerized (ca. 1:1 dr). This mixture was oxidized with Ag2O to furnish o-quinone 62 in 89% yield (mixture of C-11 diastereoisomers, ca. 1:1 dr). Attempts to induce the desired intramolecular [4+2] cycloaddition of substrate 62 (heating in toluene up to 120 °C), however, failed, leading to either no reaction or decomposition, with no detectable amounts of the expected cycloadduct 63. The steric clash encountered by the approaching o-quinone moiety with the OBn group (see transition state 62-TS, Scheme 8) is apparently prohibitive.
Scheme 8.
Synthesis of o-Quinone 62 and Failed Attempt to Induce Its Intramolecular [4+2] Cycloaddition Reactiona
a Reagents and conditions: a) 42 (1.0 equiv), 43 (1.0 equiv), Cp*RuCl(COD) (0.10 equiv), DCE, 25 °C, 0.5 h, 77%; b) Ac2O (2.0 equiv), Et3N (2.0 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 0 °C, 0.5 h, 81%; c) HF (48% aqueous solution, excess), MeCN, 25 °C, 0.5 h; then MeOH (excess), 25 °C, 3 h, 50%; d) Ag2O (2.5 equiv), CH2Cl2, 25 °C, 0.5 h, 89%. COD = 1,5-cyclooctadiene, Cp* = pentamethylcyclopentadienyl, DCE = 1,2-dichloroethane.
Total Synthesis of 9-epi-Sporolide B
Having failed to induce the intramolecular [4+2] cycloaddition reaction with the o-quinone-indene substrate possessing the desired C-9 configuration for sporolide B, we resorted to a modified strategy involving a substrate with the C-9 inverted stereochemistry in order to remove the steric barrier to the intended [4+2] cycloaddition imposed by this substituent. The new strategy required chloroacetylene 64 (Scheme 9) as a key building block. Its synthesis from the readily available iodoenone 6541 is summarized in Scheme 9.
Scheme 9.
Synthesis of Chloro Enediyne 64a
a Reagents and conditions: a) NaBH4 (1.2 equiv), CeCl3·7H2O (1.2 equiv), MeOH, −78 °C, 1 h, 99%, ca. 10:1 dr; b) TBSCl (2.0 equiv), imidazole (2.0 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 25 °C, 3 h, 95%; c) TMS-acetylene (1.2 equiv), Pd(PPh3)2Cl2 (0.02 equiv), CuI (0.04 equiv), Et2NH, 25 °C, 16 h, 98%; d) Et2AlCl (1.8 M in toluene, 2.0 equiv), CH2Cl2, −25 → 25 °C, 2 h, 99%; e) DMP (1.2 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 1 h, 79%; f) cis-1,2-dichloroethylene (4.5 equiv), MeLi (1.6 M in Et2O, 3.0 equiv), Et2O, 0 °C, 0.5 h; then 68, Et2O, 0 °C, 10 min, ca. 5:1 dr; g) DMP (1.5 equiv), NaHCO3 (5.0 equiv), 25 °C, 0.5 h, 93% for the two steps; h) DIBAL-H (1.0 M in toluene, 1.5 equiv), toluene, −78 °C, 0.5 h, 81% of pure 70 (ca. 7:1 dr, all the undesired diastereoisomers were chromatographically removed at this stage); i) K2CO3 (1.5 equiv), MeOH, 25 °C, 1 h, 99%; j) Ac2O (1.5 equiv), Et3N (1.5 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 0 °C, 0.5 h, 98%.
Thus, Luche reduction35 of iodoenone 65 furnished hydroxy compound 66 (ca. 10:1 dr), which was converted to its TBS ether 67 (TBSCl) in 94% overall yield. Sonogashira coupling of 67 with TMS acetylene [Pd(PPh3)2Cl2 cat., CuCl cat., Et2NH, 98% yield], followed by THP removal (Et2AlCl, 99% yield) and DMP oxidation (79% yield), led to aldehyde 68. Addition of the latter substrate to the lithio derivative of chloroacetylene, generated from 1,2-cis-dichloroethylene, resulted in the formation of propargylic alcohol 69 as the major product (ca. 5:1 dr, chelation-controlled, see transition state 68-TS, Scheme 9) possessing the undesired 11(R) configuration. Attempts to invert the C-11 stereocenter through the Mitsunobu reaction failed, prompting us to adopt an oxidation (DMP)-reduction (DIBALH) protocol, which led to the 11(S) propargylic alcohol 70 in a ca. 7:1 dr (steric effect, see depiction 69a, Scheme 9) and 95% combined yield. Chromatographic purification at this stage led to pure isomer 70 (81% yield), from which the TMS group was removed (K2CO3, MeOH, 99% yield) to afford hydroxy acetylene 71, whose acetylation under standard conditions furnished the coveted chloroacetylenic enyne fragment 64 (98% yield).
As previously (see Scheme 8 above), the [2+2+2] fusion of acetylenic fragments 42 and 64 under the influence of Cp*RuCl(COD) cat.40 was expected to proceed regioselectively toward the desired meta-substituted chlorobenzyl alcohol system 72 (see Scheme 10). This expectation was based on the established mechanism of the reaction (see Figure 9)40 which dictated preference, due to steric control, for only one orientation (out of the four possible) for the approach of the propargylic component (i.e. 42) to the initially formed metallocycle (see transition state XI, Figure 9). As seen from inspection of the alternative transition state XI′ (Figure 9), this orientation of the two reacting species suffers from severe steric clash between the bulky R group and the ruthenium and its ligand entourage and, therefore, does not provide a productive pathway. Similarly, the other two orientations (not shown) of the propargylic component on the side of the metallocycle chlorine residue are disfavored due to steric repulsion, either from the ruthenium moiety or the chlorine residue, and are, therefore, nonproductive. Indeed, exposure of a mixture of chloroacetylenic enyne 64 (1.1 equiv) and propargylic alcohol ester 42 (1.0 equiv) to Cp*RuCl(COD) cat. in 1,2-dichloroethane at room temperature for 0.5 h led to chloroindene 72 in 87% yield. The ortho-substituted product 72′, that would have been formed through transition states XI′ and XII′ (Figure 9), was not observed. The impressively short reaction time of this process was attributed to a possible coordination of the free hydroxyl group onto the ruthenium nucleus that may help in the initial anchoring of the hydroxy acetylene onto the metal (see XI, Figure 9). Supporting this notion was the observation that the corresponding acetyl-protected propargylic substrate (not shown) reacted with chloroacetylenic enyne 64 in the presence of Cp*RuCl(COD) cat. under the same conditions significantly slower (ca. 10-fold) and with eroded regioselectivity (ca. 10:1). Erosion of regioselectivity (meta:ortho regioisomers, ca. 20:1 → 10:1) was also observed in the rhodium-catalyzed reaction (Wilkinson's catalyst) of 42 and 64 which, however, proceeded equally well to afford the desired chloroindene 71 (85% combined yield). Proceeding toward the targeted o-quinone 75 (Scheme 10), the exclusively obtained hydroxy chloroindene product 72 was acetylated (Ac2O, 4-DMAP, Et3N, 92% yield), and the resulting product (73) was treated, initially with aq. HF in MeCN and then with MeOH, to cleave both the silyl ether and the acetoxy acetal moiety, furnishing hydroxy catechol 74 in 74% yield. The latter compound was then smoothly oxidized with Ag2O to afford the desired o-quinone 75 in 94% yield.
Scheme 10.
Synthesis of o-Quinone 75a
a Reagents and conditions: a) 42 (1.0 equiv), 64 (1.1 equiv), Cp*RuCl(COD) (0.07 equiv), DCE, 25 °C, 0.5 h, 87%; b) Ac2O (2.0 equiv), Et3N (2.0 equiv), 4-DMAP (0.1 equiv), CH2Cl2, 0 °C, 0.5 h, 92%; c) HF (48% aqueous solution, excess), MeCN, 25 °C, 0.5 h; then MeOH (excess), 25 °C, 3 h, 74%; d) Ag2O (2.5 equiv), CH2Cl2, 25 °C, 0.5 h, 94%.
Figure 9.
Mechanistic rationale for the exclusive regioselectivity of the ruthenium-catalyzed [2+2+2] cycloaddition reaction of acetylenic components 42 and 64 to afford indene 72.
With o-quinone 75 in hand, we were now ready to attempt the much anticipated intramolecular [4+2] cycloaddition reaction (which had failed previously with the correct C-9 sporolide stereochemistry as discussed above, see Scheme 8). This time, and much to our delight, heating 75 at 110 °C in toluene for 1.5 h resulted in the formation of the desired cycloadduct 76 as a single isomer and in 40% yield (based on 50% consumed starting material, Scheme 11). Repeated attempts to improve the yield of this reaction (e.g. prolonged or shortened reaction times, lower or higher temperatures, Lewis acid catalysts, microwaves) proved fruitless, apparently due to the sensitivity of both the substrate (75) and the product (76). Due to the robustness of the sequence leading up to o-quinone 75, however, sufficient quantities of sporolide scaffold 76 could be secured for further elaboration. The impressive exclusivity by which cycloadduct 76 is formed from 75 can be explained, as in the case of model system 4 (see above, Scheme 5 and Figure 5), upon inspection (manual molecular models) of postulated transition states [75-TS and 75-TS′ (generated by 180 ° rotation around C2′-C3′ bond), see Scheme 11] that reveals the difficulties with 75-TS′ (inhibitive 1,3-allylic strain) and the impossibility of the other two diastereofacial arrangements (not shown).
Scheme 11.
Intramolecular Hetero [4+2]-Cycloaddition Reaction of o-Quinone 75a
a Conditions: a) toluene, 110 °C, 1.5 h, 40% (based on 50% recovered starting material).
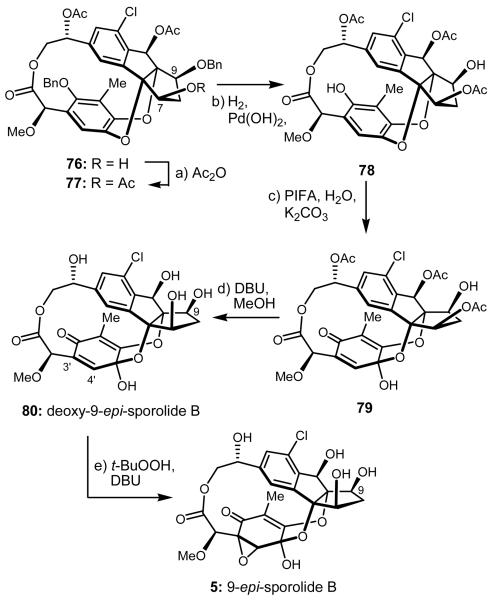
At this stage and with sporolide B scaffold 76 in hand, we opted to explore the pending sequence to 9-epi-sporolide B (5, see Scheme 12) not only to obtain a new sporolide entity, but also as a means to scout the chemistry ahead in preparation for the final drive toward our ultimate target, sporolide B (1b) (which required inversion of configuration at C-9). Only five steps were needed to reach 9-epi-sporolide B (5) from intermediate 76. Thus, and as shown in Scheme 12, hydroxy compound 76 was acetylated (Ac2O, 4-DMAP, Et3N) to afford triacetate 77 (92% yield), from which both benzyl groups were removed through hydrogenolysis [H2, Pd(OH)2/C, 92% yield], leading to hydroxy phenol 78. The phenol moiety of the latter compound was dearomatized by reaction with PIFA in MeCN in the presence of H2O and K2CO3 at 0 °C to afford p-hemiquinol 79 in a regio- and stereoselective manner (66% yield). All three acetates were then removed from 79 through the action of DBU in CH2Cl2:MeOH (3:1) at 40 °C, leading to 3′,4′-deoxy-9-epi-sporolide B (80) in 77% yield. Finally, the remaining oxygen atom was successfully installed onto the latter intermediate regio- and stereoselectively, as expected on steric grounds, through the use of t-BuOOH and DBU in CH2Cl2 at 40 °C, furnishing 9-epi-sporolide B (5) in 72% yield.
Scheme 12.
Synthesis of 9-epi-Sporolide B (5)a
a Reagents and conditions: a) Ac2O (2.0 equiv), Et3N (2.0 equiv), 4-DMAP (0.10 equiv), CH2Cl2, 0 °C, 0.5 h, 92%; b) H2 (balloon), Pd(OH)2 (10% on carbon, 2.0 equiv), EtOAc, 25 °C, 4 h, 92%; c) PIFA (2.0 equiv), H2O (2.0 equiv), K2CO3 (5.0 equiv), MeCN, 0 °C, 0.5 h, 66%; d) DBU (10.0 equiv), CH2Cl2:MeOH (3:1), 40 °C, 4 h, 77 %; e) t-BuOOH (10 equiv), DBU (5.0 equiv), CH2Cl2, 40 °C, 3 h, 72%.
Total Synthesis of Sporolide B
Armed with the confidence gained through the successful synthesis of 9-epi-sporolide B (5), we turned our attention toward the final stage of the total synthesis of sporolide B itself (1b, Scheme 13). This task required inversion of the C-9(S) configuration within 76, which was intentionally and temporarily introduced as such for the sole purpose of facilitating the otherwise unattainable intramolecular o-quinone [4+2] cycloaddition reaction. To this end, the hydroxyl group of 76 was ephemerally protected with a TES group (TESOTf, 95% yield), and the benzyl groups were cleaved by hydrogenolysis [H2, Pd(OH)2/C, 92% yield] to afford hydroxy phenol 82 through bis-benzyl ether TES derivative 81 (see Scheme 13). Oxidative dearomatization of phenolic substrate 82 with PIFA in the presence of PMBOH furnished p-hemiquinone 83 (75% yield). Attempts to employ SN2 displacement reactions (e.g. Mitsunobu reaction21 or mesylate displacement42) as a means to achieve the desired inversion at C-9 failed, forcing us to adopt an oxidation–reduction protocol with the thought to exploit the 1,3-directing power of the C-7 β-hydroxyl group within a hydroxy carbonyl substrate. Thus, oxidation of 83 with DMP produced ketone 84 in 90% yield. Desilylation of the latter compound (aq. HF, 85% yield) yielded β-hydroxy ketone 85, whose reduction with Me4NBH(OAc)343 provided inverted [9(R)] diol 86 in 85% yield as a single diastereoisomer, apparently through the expected intramolecularly-assisted hydride delivery from the β-face of the molecule. Cleavage of the PMB group from 86 with DDQ (70% yield), followed by methanolysis of the acetate groups from the resulting hemiacetal diacetate (DBU, MeOH, 40 °C), afforded 3′,4′-deoxysporolide B (87, 78% yield), whose intended regio- and stereoselective epoxidation was achieved through the use of t-BuOOH-DBU to afford sporolide B (1b) in 63% yield. The spectral data of synthetic 1b were in accordance with those reported for the natural product.44
Scheme 13.
Total Synthesis of Sporolide B (1b)a
a Reagents and conditions: a) TESOTf (1.5 equiv), Et3N (2.0 equiv), CH2Cl2, 0 °C, 0.5 h, 95%; b) H2 (balloon), Pd(OH)2 (10% on carbon, 2.0 equiv), EtOAc, 25 °C, 4 h, 92%; c) PIFA (1.5 equiv), PMBOH (10.0 equiv), K2CO3 (5.0 equiv), MeCN, 0 °C, 0.5 h, 75%; d) DMP (2.0 equiv), CH2Cl2, 25 °C, 1 h, 90%; e) HF (48% aqueous solution, excess), MeCN, 25 °C, 2 h, 85%; f) Me4NBH(OAc)3 (10.0 equiv), MeCN:AcOH (10:1), 25 °C, 2 h, 85%; g) DDQ (5.0 equiv), CH2Cl2:H2O (10:1), 25 °C, 5 h, 70%; h) DBU (10.0 equiv), CH2Cl2:MeOH (3:1), 40 °C, 4 h, 78%; i) t-BuOOH (10.0 equiv), DBU (5.0 equiv), CH2Cl2, 40 °C, 3 h, 63%. Tf = trifluoromethanesulfonyl.
Conclusion
A stereocontrolled total synthesis of sporolide B (1b) has been achieved through a designed strategy that also delivered 9-epi-sporolide B (5) and sporolide model system 4. In addition to delivering the latter compound, the undertaken model studies revealed the sensitivity of the intermolecular [4+2] cycloaddition reaction of o-quinones and indene-type dienophiles and its dependence on the substituents on both partners with regard to regioselectivity and diastereofacial selectivity. The gathered intelligence from these studies proved decisive in guiding the design of the eventually adopted synthetic strategy toward sporolide B (1b) and may prove useful in future exploration of the chemistry of o-quinones. The ultimately evolved synthetic strategy toward sporolide B involved a thermally induced intramolecular hetero [4+2] cycloaddition reaction with an appropriately designed substrate that proceeded with exquisite regio- and stereoselectivity. As a result of these investigations, this unprecedented intramolecular o-quinone [4+2] cycloaddition process may now be added to the repertoire of synthetic tools for future employment in complex molecule construction. In addition, the described chemistry demonstrated the power of the ruthenium-catalyzed [2+2+2] cycloaddition of acetylenic substrates for the construction of highly substituted indenes, including chlorinated systems that may prove useful in chemical and medicinal synthetic endeavors.
Supplementary Material
Acknowledgment
We thank Drs. D.-H. Huang, R. Chadha, and G. Siuzdak for NMR spectroscopic, X-ray crystallographic, and mass spectrometric assistance, respectively. We also gratefully acknowledge Dr. R. Hungate and Mr. R. Jensen of Amgen, Thousand Oaks, California, for the preliminary 13C NMR study of synthetic sporolide B. Financial support for this work was provided by National Institute of Health (USA) and the Skaggs Institute for Research grants, an Andrea Elizabeth Vogt Memorial Fellowship (to J. W.), and a Univeristy of Siena Scholarship (to L. B.)
Footnotes
Supporting Information Available: Experimental procedures and compound characterization. crystallographic information files (CIF) This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew. Chem., Int. Ed. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]; (b) Williams PG, Buchanan GO, Feling RH, Kauffman CA, Jensen PR, Fenical W. J. Org. Chem. 2005;70:6196–6203. doi: 10.1021/jo050511+. [DOI] [PubMed] [Google Scholar]
- 2.(a) Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao T-H, Nicholson B, Deyanat-Yazdi G, Mai B, Jensen PR, Fenical W, Neuteboom STC, Lam KS, Palladino MA, Potts BCM. J. Med. Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]; (b) Palladino MA, Neuteboom STC, Theodora C, Macherla VR, Potts BC. Patent WO 02005002572 2005
- 3.(a) Buchanan GO, Williams PG, Feling RH, Kauffman CA, Jensen PR, Fenical W. Org. Lett. 2005;7:2731–2734. doi: 10.1021/ol050901i. [DOI] [PubMed] [Google Scholar]; (b) Fenical W, Jensen PR. Nature Chem. Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]; (c) Jensen PR, Williams PG, Oh D-C, Zeigler L, Fenical W. Appl. Environ. Microbiol. 2007;73:1146–1152. doi: 10.1128/AEM.01891-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Oh D-C, Williams PG, Kauffman CA, Jensen PR, Fenical W. Org. Lett. 2006;8:1021–1024. doi: 10.1021/ol052686b. [DOI] [PubMed] [Google Scholar]; (b) McGlinchey RP, Nett M, Moore BS. J. Am. Chem. Soc. 2008;130:2406–2407. doi: 10.1021/ja710488m. [DOI] [PubMed] [Google Scholar]; (c) Nett M, Moore BS. Pure Appl. Chem. 2009;81:1075–1084. [Google Scholar]
- 5.Udwary DW, Zeigler L, Asolkar RN, Singan V, Lapidus A, Fenical W, Jensen PR, Moore BS. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10376–10381. doi: 10.1073/pnas.0700962104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RR, Bergman RG. J. Am. Chem. Soc. 1972;94:660. For selected reviews on naturally occurring enediynes exhibiting Bergman cycloaromatization properties, see: Nicolaou KC, Dai W-M. Angew. Chem. Int. Ed. Engl. 1991;30:1387–1416. Smith AL, Nicolaou KC. J. Med. Chem. 1996;39:2103–2117. doi: 10.1021/jm9600398.
- 7.Perrin CL, Rodgers BL, O'Connor JM. J. Am. Chem. Soc. 2007;129:4795–4799. doi: 10.1021/ja070023e. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaou KC, Wang J, Tang Y. Angew. Chem., Int. Ed. 2008;47:1432–1435. doi: 10.1002/anie.200705334. [DOI] [PubMed] [Google Scholar]
- 9.(a) Nicolaou KC, Tang Y, Wang J. Angew. Chem., Int. Ed. 2009;48:3449–3453. doi: 10.1002/anie.200900264. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li P, Menche D. Angew. Chem., Int. Ed. 2009;48:5078–5080. doi: 10.1002/anie.200901894. [DOI] [PubMed] [Google Scholar]
- 10.(a) Horner L, Merz H. Justus Liebigs Ann. Chem. 1950;570:89–120. [Google Scholar]; (b) Finley KT. In: The Chemistry of Quinoid Compounds. Patai S, Rappoport Z, editors. II. John Wiley & Sons; New York: 1988. pp. 538–717. [Google Scholar]; (c) Nair V, Kumar S. Synlett. 1996:1143–1147. [Google Scholar]; (d) Kharisov BI, Mendez-Rojas MA, Garnovskii AD, Ivakhnenko EP, Ortiz-Mendez U. J. Coord. Chem. 2002;55:745–770. [Google Scholar]; (e) Aly AA, Ehrhardt S, Hopf H, Dix I, Jones PG. Eur. J. Org. Chem. 2006:335–350. [Google Scholar]; (f) Varma RL, Ganga VB, Sureshc E, Suresh CH. Tetrahedron Lett. 2006;47:917–921. [Google Scholar]
- 11.For recent examples of macrocyclization by standard cycloadditions, see: Zapf CW, Harrison BA, Drahl C, Sorensen EJ. Angew. Chem., Int. Ed. 2005;44:6533–6537. doi: 10.1002/anie.200502119. Johannes JW, Wenglowsky S, Kishi Y. Org. Lett. 2005;7:3997–4000. doi: 10.1021/ol051553n. Baran PS, Burns NZ. J. Am. Chem. Soc. 2006;128:3908–3909. doi: 10.1021/ja0602997. Snyder SA, Corey EJ. J. Am. Chem. Soc. 2006;128:740–742. doi: 10.1021/ja0576379.
- 12.(a) Chen X, Chen J, De Paolis M, Zhu J. J. Org. Chem. 2005;70:4397–4408. doi: 10.1021/jo050408k. [DOI] [PubMed] [Google Scholar]; (b) Casiraghi G, Cornia M, Rassu G. J. Org. Chem. 1988;53:4919–4922. [Google Scholar]
- 13.Aoyama T, Shioiri T. Tetrahedron Lett. 1990;31:5507–5508. [Google Scholar]
- 14.Hussain HH, Babic G, Durst T, Wright JS, Flueraru M, Chichirau A, Chepelev LL. J. Org. Chem. 2003;68:7023–7032. doi: 10.1021/jo0301090. [DOI] [PubMed] [Google Scholar]
- 15.Halterman RL, Zhu C. Tetrahedron Lett. 1999;40:7445–7448. [Google Scholar]
- 16.CCDC-773715 contains the supplementary crystallographic data for compoung 21. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 17.Shankar S, Vaidyanathan G, Affleck D, Welsh PC, Zalutsky MR. Bioconjugate Chem. 2003;14:331–341. doi: 10.1021/bc025636p. [DOI] [PubMed] [Google Scholar]
- 18.(a) Takai K, Kimura K, Kuroda T, Hiyama T, Nozaki H. Tetrahedron Lett. 1983;24:5281–5284. [Google Scholar]; (b) Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. VCH; Weinheim: 1996. pp. 711–730. [Google Scholar]; (c) Fürstner A. Chem. Rev. 1999;99:991–1046. doi: 10.1021/cr9703360. [DOI] [PubMed] [Google Scholar]
- 19.(a) Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong KS, Kwong HL, Morikawa K, Wang ZM. J. Org. Chem. 1992;57:2768–2771. [Google Scholar]; (b) Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 20.Parenty A, Moreau X, Campagne J-M. Chem. Rev. 2006;106:911–939. doi: 10.1021/cr0301402. [DOI] [PubMed] [Google Scholar]
- 21.(a) Kurihara T, Nakajima Y, Mitsunobu O. Tetrahedron Lett. 1976;17:2455–2458. [Google Scholar]; (b) Mitsunobu O. Synthesis. 1981:1–28. [Google Scholar]; (c) Hughes DL. Org. React. 1992;42:335–656. [Google Scholar]; (d) Hughes DL. Org. Prep. Proced. Int. 1996;28:127–164. [Google Scholar]
- 22.Corey EJ, Nicolaou KC. J. Am. Chem. Soc. 1974;96:5614–5616. [Google Scholar]
- 23.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
- 24.Venkataraman K, Wagle DR. Tetrahedron Lett. 1980;21:1893–1896. [Google Scholar]
- 25.Mikaiyama T, Usui M, Saigo K. Chem. Lett. 1976:49–50. [Google Scholar]
- 26.Shiina I, Kubota M, Ibuka R. Tetrahedron Lett. 2002;43:7535–7539. [Google Scholar]
- 27.CCDC-667552 contains the supplementary crystallographic data for compoung 39. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 28.Tamura Y, Yakura T, Haruta J, Kita Y. J. Org. Chem. 1987;52:3927–3930. [Google Scholar]
- 29.Zilbeyyaz K, Sahin E, Kilic H. Tetrahedron: Asymmetry. 2007;18:791–796. [Google Scholar]
- 30.CCDC-669253 contains the supplementary crystallographic data for compoung 4. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 31.(a) Shore NE. Chem. Rev. 1988;88:1081–1119. [Google Scholar]; (b) Saito S, Yamamoto Y. Chem. Rev. 2000;100:2901–2916. doi: 10.1021/cr990281x. [DOI] [PubMed] [Google Scholar]; (c) Varela JA, Sa C. Chem. Rev. 2003;103:3787–3802. doi: 10.1021/cr030677f. [DOI] [PubMed] [Google Scholar]; (d) Henry GD. Tetrahedron. 2004;60:6043–6061. [Google Scholar]; (e) Yamamoto Y. Curr. Org. Chem. 2005;9:503–509. [Google Scholar]; (f) Kotha S, Brahmachary E, Lahiri K. Eur. J. Org. Chem. 2005;70:4741–4761. [Google Scholar]; (g) Chopade PR, Louie J. Adv. Synth. Catal. 2006;348:2307–2327. [Google Scholar]; (h) Tanaka K. Synlett. 2007:1977–1993. [Google Scholar]; (i) Heller B, Hapke M. Chem. Soc. Rev. 2007;36:1085–1094. doi: 10.1039/b607877j. [DOI] [PubMed] [Google Scholar]; (j) Agenet N, Busine O, Slowinski F, Gandon V, Aubert C, Malacria M. Org. React. 2007;68:1–302. [Google Scholar]; (k) Shibata T, Tsuchikama K. Org. Biomol. Chem. 2008;6:1317–1323. doi: 10.1039/b720031e. [DOI] [PubMed] [Google Scholar]; (l) Galan BR, Rovis T. Angew. Chem., Int. Ed. 2009;48:2830–2834. doi: 10.1002/anie.200804651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito N, Tashiro K, Maru Y, Yamaguchi K, Kubo A. J. Chem. Soc., Perkin Trans. 1997;1:53–69. [Google Scholar]
- 33.Yadav JS, Chander MC, Joshi BV. Tetrahedron Lett. 1988;29:2737–2740. [Google Scholar]
- 34.(a) Johnson CR, Braun MP. J. Am. Chem. Soc. 1993;115:11014–11015. [Google Scholar]; (b) Curran TT, Hay DA, Koegel CP. Tetrahedron. 1997;53:1983–2004. [Google Scholar]
- 35.Luche JL. J. Am. Chem. Soc. 1978;100:2226–2227. [Google Scholar]
- 36.Pichlmaira S, de Lera Ruiza M, Basua K, Paquette LA. Tetrahedron. 2006;62:5178–5194. [Google Scholar]
- 37.Corey EJ, Helal CJ. Angew. Chem., Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 38.(a) Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;50:4467–4470. [Google Scholar]; (b) Miller MW, Johnson CR. J. Org. Chem. 1997;62:1582–1583. [Google Scholar]
- 39.Phillips D, Wickham P, Potts G, Arnold A. J. Med. Chem. 1968;11:924–928. doi: 10.1021/jm00311a600. [DOI] [PubMed] [Google Scholar]
- 40.(a) Yamamoto Y, Ogawa R, Itoh K. Chem. Commun. 2000:549–550. [Google Scholar]; (b) Yamamoto Y, Arakawa T, Ogawa R, Itoh K. J. Am. Chem. Soc. 2003;125:12143–12160. doi: 10.1021/ja0358697. [DOI] [PubMed] [Google Scholar]; (c) Oshima N, Suzuki H, Moro-oka Y. Chem. Lett. 1984;13:1161–1164. [Google Scholar]
- 41.Iodoenone 65 was prepared following the same chemical transformation steps as depicted in Scheme 7 (51 → 56), starting from the opposite enantioisomer of 51.
- 42.Torisawa Y, Okabe H, Ikegami S. Chem. Lett. 1984;13:1555–1556. [Google Scholar]
- 43.Evans DA, Chapman KT, Carreira EM. J. Am. Chem. Soc. 1988;110:3560–3578. [Google Scholar]
- 44.We thank Prof. William Fenical for a sample of natural sporolide B.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.