Abstract
Background
Transfusion of bacterially contaminated blood can result in sepsis and will constitute a substantial health burden to the patient.
Objective
To assess the level of transfusion related sepsis and the bacterial types responsible for the contamination at the Tamale Teaching Hospital in Ghana.
Method
We sampled 80 refrigerated donor blood at the blood bank and cultured them for bacteria. The antimicrobial sensitivities of the isolates were also determined.
Results
14 blood bags representing 17.5% grew isolates of various bacteria. Ten (10) of the 14 isolates were Gram positive cocci representing 71.42% making it the commonest contaminant. 50% of the gram positive cocci were identified to be coagulase negative staphylococci and 21.42% were Staphylococcus aureus. There were 14.28% isolates which were Gram positive rods, and were identified to be Corynebacterium diphtheroids. There were two isolates which were Gram negative rods; one was identified as Escherichia coli and the other one Klebsiella pneumoniae. Sensitivity among the organisms were varied; as all the 14 (100%) of the organisms isolated were sensitive to amikacin, only 14.28% of the coagulase negative staphylococci were sensitive to co-trimoxazole, 28.5% were sensitive to ampicillin, 42.8% were sensitive to cefuroxime and 71.4% were sensitive to ciprofloxacin. Sensitivity to gentamicin was observed to be 85.7% and 28.5% were sensitive to Tetracycline. Only the 10 Gram positive cocci were tested against erythromycin and Cloxacillin; where 70.00% were sensitive to cloxacillin and 90% were sensitive to erythromycin.
Conclusion
All the Staphylococcus aureus isolated were resistant to both ampicillin and cotrimoxazole. Potential dangers and consequences of transfusing multidrug resistance bacteria have been discussed.
Keywords: Donor blood, Bacteraemia, Tamale Teaching Hospital, Ghana
Running Title: Bacterial contamination of donor blood in Tamale
Introduction
Blood transfusion services are required to provide blood and blood components which are safe1, 2 in a cost effective way for transfusion into patients who require the blood products3, 4. However, blood transfusion is a potential source of infection by a variety of transmissible agents.2, 5, 6 Since the human immunodeficiency virus contamination of blood supply meant for transfusion has been recognized in the 1980s, rigorous screening of blood before it is supplied to recipients has been instituted and accepted politically worldwide.1, 4, 7 Yet, human error occurs during the complex processes involved in blood transfusion. Such processes as ordering of blood, blood sample processing in the laboratory, collection and transportation of the blood and administration of the blood to the patient, cause the blood to become contaminated with infectious agents other than those screened for6, 8. The screening processes do not detect all the infectious agents in the blood as a result of the low sensitivity of the tests1 or as other reports9–11 indicate as a result of not testing for the infectious agent at all. In some cases donors donate in the window phase of the infection when the numbers are too low for detection2. Most often the blood gets contaminated with bacteria at the time of bleeding of donors.1, 2, 12 The blood is then screened for agents other than the bacterial contaminant and then stored and used at a later date. The storage period may serve as incubation period for the low numbers to proliferate before it is transfused1 with the resultant reduced blood pressure, shock and collapse.13
Transfusion related transmission of human immunodeficiency virus and Hepatitis B virus and Treponema sp has steadily decreased due to the rigorous screening efforts by transfusion services, but the risk of transmission of bacteria has remained high.1, 12 Bacteria which commonly contaminate blood are able to multiply in refrigerated blood to high concentrations to initiate infection in the transfused patient especially blood that is stored for a long time in excess of 32 days.14
Medical literature during the past years is replete with case studies of apparent sepsis predominantly due to bacteria from normal skin flora.15 Many authors7, 16, 17 report skin organisms such as Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus sp. and Corynebacterium sp as common blood contaminants. Bacillus sp and Gram negative organisms such as Yersinia enterocolitica, Pseudomonas fluorescens, Pseudomonas putida Escherichia coli, Enterobacter aerogenes, Serratia liquefaciens, Campylobacter jejuni, Enterobacter sp, Flavobacterium and Salmonella sp are other bacterial isolates reported from donor blood.12, 18, 19
Immunosuppressed patients and older individuals with poor nutritional status are the most susceptible population, but healthy individuals can have a rapidly fatal outcome when transfused with a large load of bacteria alone or with the endotoxins.2, 7
Thus, due to the success with viral pathogens, bacterial contamination now has the dubious distinction of being the most common infectious risk from transfusion and has become a matter of increasing concern and attention.1, 18
Mismatched blood cause transfusion reactions, but where the blood is properly cross-matched the cause of the transfusion reactions are not known.2, 20 Bacterial contamination has been shown to be the cause of transfusion reactions in some cases.2, 20 Stored bloods at the Tamale Teaching hospital sometimes show signs of bacterial contamination, as revealed by plasma turbidity and fibrin clots. The aim of this work is to establish whether or not bacterial contamination occurs in stored donor blood meant for transfusion at the Tamale Teaching Hospital. It is also our aim to determine the bacterial species commonly responsible for the contamination, including their antimicrobial profiles and to suggest ways of reducing the contamination so as to minimize transfusing blood contaminated with bacteria.
Materials and methods
This study was carried out at the blood bank of the Tamale Teaching Hospital which is situated in the Northern region of Ghana between February to May 2007. This hospital doubles as the Northern Regional Hospital and also serves as the Teaching Hospital for the School of Medicine and Health Sciences, University for Development studies, Tamale.
Specimen
Authorities at the Tamale Teaching Hospital, led by the Hospital's ‘blood organizer’ solicit blood for transfusion from the general public in mass blood donation campaign. Large quantities of blood are donated to the blood bank. Such donated blood for transfusion formed the study cohort. Donor bloods that tested positive for transfusion transmissible infections (normally HIV, HBV, HCV, and Syphilis) were not included in this study. Expired donor blood and blood that showed signs of bacterial contamination, as revealed by plasma turbidity and fibrin clots were also not included.
Sample collection and testing
Eighty blood bags containing freshly donated blood, and stored in the blood bank refrigerator at the Tamale Teaching Hospital laboratory were randomly selected. These were made up of 56 voluntary donors and 24 replacement donors. The cords to the main bags were folded into four parts. The quarter closest to the bag was chosen as a site for puncture. The puncture site was swabbed with 70% alcohol to disinfect and allowed to dry, 3 mls of blood was drawn using sterile syringe and needle and delivered into 15 mls of Brain-Heart Infusion broth contained in a sterile bottle. These sample broths were incubated at 37°C for up to 7 days. After overnight incubation these broths were sub-cultured, using a sterile loop, onto Blood agar, Chocolate agar and MacConkey agar plates every day for 3 consecutive days. The blood and the MacConkey agar plates were incubated aerobically and the chocolate agar was incubated in a candle jar for anaerobic growing.
After overnight incubation the plates were inspected for bacterial growth. The bacteria were identified by their colonial morphology, Gram reaction, biochemical tests and sugar fermentation tests. The isolates were further identified thoroughly by the Mini API 20 NE identification systems, (bioMeriuex Marcy—l'Etoile, France).
Susceptibility testing
The susceptibility of the isolates to antimicrobial agents was examined by an agar diffusion method on Muller-Hinton agar (Oxoid Ltd, Basingstoke, UK) using antibiotics impregnated paper discs, (Abtek Biologicals, UK), containing the following antibiotics with concentrations as shown below: Ampicillin 10mg, Gentamicin 10µg, Cotrimoxazole 25µg, Cefuroxime 30µg, Penicillin 1.5IU, Tetracycline 30µg, Erythromycin 5µg, Cloxacillin 1.5IU Ceftriaxone 30µg and Chloramphenicol 30µg. In this study methicillin resistance was not tested for amongst the staphylococcus species indentified. Using a sterile straight wire a pure colony of the test isolate was inoculated into peptone water and the turbidity adjusted to the equivalence of 0.5 McFarland opacity standards. A sterile swab was then used to seed the Muller-Hinton Agar to give a confluent bacterial growth. Using a pair of sterile forceps the antimicrobial discs were placed on the dry agar surface as appropriate.
The plates were incubated aerobically at 37°C and read after 18–24 hours of incubation. Zones of inhibition surrounding the discs were measured using a ruler and their various diameters compared with that of standard interpretative charts provided by the manufacturer. The organisms were reported as “Sensitive” or “Resistant”.
This method is consistent with the procedure described by the Clinical and Laboratory Standards Institute (CLS I). Quality control was routinely performed using Escherichia coli ATCC 25922, and Pseudomonas aeruginosa, TCC 27853 and Staphylococcus aureus ATCC 29213.
Results
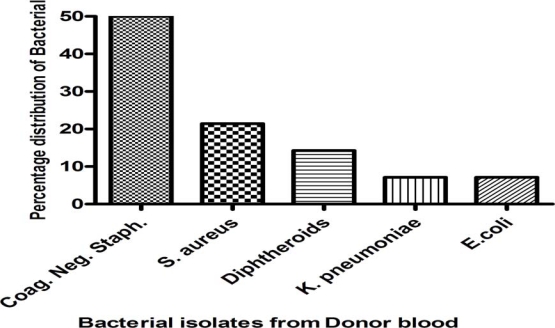
From the 80 refrigerated donor bloods, 14 blood bags representing 17.5% showed isolates of various bacteria (Fig. 1). Ten of the isolates were Gram positive cocci representing 71.42% making it the commonest isolate. 7(50%) of the gram positive cocci were identified to be coagulase negative staphylococci and 3 (21.42%) were Staphylococcus aureus. There were 2(14.28%) isolates, which were Gram positive rods, and were identified as Corynebacterium diphtheroids. There were two isolates which were Gram negative rods; one was identified as Escherichia coli and the other one Klebsiella pneumoniae
Figure 1.
Percentage distribution of bacterial isolates from donor blood at the Tamale Teaching Hospital
The organisms showed variable susceptibility to the antibiotics tested as shown in Table 1. All the 14 (100%) of the organisms isolated were sensitive to amikacin, but only 14.2% of the coagulase negative Staphylococci were sensitive to co-trimoxazole, 28.5% were sensitive to tetracycline. Cefuroxime, gentamicin and ciprofloxacin registered higher sensitivities with values of 85.7% and 71.4% respectively. Only the Gram positive isolates were tested against erythromycin and cloxacillin; where coagulase negative staphylococci isolated had sensitivities of 42.8% and 57.1% respectively None of all the Staphylococcus aureus isolates were sensitive to both ampicillin and cotrimoxazole; but were all sensitive (100%) to amikacin, erythromycin, gentamicin and penicillin and also recorded moderate sensitivity (66.6%) against cefuroxime and cloxacillin. S. aureus sensitivity against ciprofloxacin and tetracycline were 33.3%.
Table 1.
The percentage sensitivity pattern of the various organisms isolated, CNS = Coagulase negative staph.
| Antibiotics | Gram positive cocci | Gram positive rod | Gram negative rod | ||
| CNS | S. aureus | Diphtheroids | E. coli | Klebsiella spp | |
| AMP | 57.1 | 0 | 0 | 0 | 0 |
| AK | 100 | 100 | 100 | 100 | 100 |
| COT | 14.2 | 0 | 0 | 100 | 0 |
| CPR | 71.4 | 33.3 | 100 | 100 | 100 |
| CRX | 42.8 | 66.6 | 100 | 100 | 100 |
| CXC | 57.1 | 66.6 | 0 | 0 | 0 |
| ERY | 42.8 | 100 | 50 | 0 | 0 |
| GEN | 85.7 | 100 | 100 | 100 | 100 |
| PEN | 57.1 | 100 | 100 | 0 | 0 |
| TET | 28.5 | 33.3 | 0 | 0 | 0 |
AMP-Ampicillin; AK-Amikacin; COT-Cotrimoxazole; CPR-Ciprofloxacin; CRX-Cefuroxime; CXC-Cloxacillin; ERY-Erythromycin; GEN-Gentamicin PEN-Penicillin; TET-Tetracycline
Discussions
This study has established that bacteraemia of stored blood at the Tamale Regional Hospital is about 17.5%. This is considered very high compared to reports from other countries.2, 15, 21 Sepsis associated with transfusion of bacterially contaminated blood is generally regarded as a very rare occurrence.3, 4, 8 Similar works7 reported low levels in Taiwan but other scientists have reported levels as high as 10%18, 22, 23 which are lower than values obtained in this study.
The Tamale Regional Hospital itself has no data on blood bank bacteraemia, transfusion related sepses or mortality records for comparison, as reported by others13, 19, 22, 24 because it is perceived that transfusion related sepsis or mortality are rare phenomena. Probably low quantity of inoculums of bacteria occurs in the blood which only results in transient bacteraemia on transfusion15 or as a result of antibiotic coverage6, 14, 25 which masks the signs and symptoms.
Proper blood donor skin disinfection has long been recognized as the surest way to reduce blood contamination.6, 18, 19, 22 Apart from skin contaminants are the problems caused by the haematogenous pathogens such as the Malaria parasites, Yersinia enterocolitica and the Salmonella sp which are carried by asymptomatic blood donors. The isolates obtained in our study were mostly skin associated organisms and indicators of faecal contamination organisms, and are often considered contaminants related to either procedure during donor bleeding22, or of taking the sample for culture.26 Bacteraemia caused by coagulase negative staphylococci, including other skin microbiota like the diphtheroids, is difficult to demonstrate as ‘true pathogen’, and therefore can be ignored in routine diagnosis. Bacteraemia caused by such bacteria can lead to deleterious consequences for the immunocompromised patients18, 19 such as the premature and newborns. We have demonstrated in our study that the isolates obtained showed varied susceptibility to the antibiotics tested. While all the isolates were sensitive to Amikacin, only 14.2% and 28.5% of the coagulase negative staphylococci isolated were sensitive to cotrimoxazole and Tetracycline respectively. With similarly increasing reports about antimicrobial resistance in Ghana,27, 28 and elsewhere29, 30, the risks of transfusing bacterially contaminated donor blood is high and transfusing blood with multidrug resistant strains of bacteria may worsen the plight of the already sick and the immunocompromised.
A possible explanation for the high resistance of donor blood isolates is associated with the ease of procuring antibiotics over the counter in Ghana.27, 28, 31
A study by the World Health Organization's Programme for appropriate Health Care Technology (ATH) has shown a correlation between the occurrence of multi-resistant bacteria and antibiotic consumption patterns. The majority of Ghanaians (especially the inhabitants in the Tamale area) are poor and live on less than one dollar a day, so they opt for the cheaper and the readily available sell-over the counter-drugs such as the ampicillin, tetracycline and the co-trimoxazole, leading to the abuse of these drugs and the concomitant development of resistance of bacteria in Ghana.
Conclusion
This research has established that bacterial contamination of stored donor blood for transfusion occurs in the Tamale Teaching Hospital. Most of the contamination is due to introduction of skin microbiota into the blood bag as a result of improper skin disinfection during blood collection a procedure which we strongly recommend must be taught and supervised at all times. Knowledge of the antibiotic susceptibility patterns in our setting will allows us to be more cautious in the choice of first-line agents. Furthermore, establishment of the susceptibility pattern of locally occurring isolates by comparing zone size breakpoints with Etest, agar dilution method and as well as molecular genotyping of resistant strains will be the future direction of this pilot study. We recommend that national surveillance programmes be established to study the level of blood bank blood contamination with bacteria, so as to establish national levels of contamination. Only with continued surveillance of donor blood contamination and of susceptibility patterns and a larger sample size of isolates will provide a more substantial answer to the issue of donor blood contamination in Ghana
References
- 1.Dodd RY. Bacterial contamination and transfusion safety: Experience in the united states. Transfus Clin Biol. 2003;10:6–9. doi: 10.1016/s1246-7820(02)00277-x. [DOI] [PubMed] [Google Scholar]
- 2.Hillyer C D, Josephson C D, Blajchman MA, Vostal JG, Epstein J S, Goodman J L. Bacterial contamination of blood components: Risks, strategies, and regulation: Joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program. 2003:575–589. doi: 10.1182/asheducation-2003.1.575. [DOI] [PubMed] [Google Scholar]
- 3.Blajchman MA. Incidence and significance of the bacterial contamination of blood components. Dev Biol (Basel) 2002;108:59–67. [PubMed] [Google Scholar]
- 4.Blajchman MA, Ali AM. Bacteria in the blood supply: An overlooked issue in transfusion medicine. In: Nance SJ, editor. Blood safety: Current challenges. Bethesda, MD: AABB; 1992. pp. 213–228. [Google Scholar]
- 5.Brecher M E, Hay S, Corash L, Hsu J, Lin L. Evaluation of bacterial inactivation in prestorage pooled, leukoreduced, whole blood-derived platelet concentrates suspended in plasma prepared with photochemical treatment. Transfusion. 2007;47:1896–1901. doi: 10.1111/j.1537-2995.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 6.Brecher M E, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. doi: 10.1128/CMR.18.1.195-204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong PY, Colville VL, White V, Walker HM, Morris R A. Validation and assessment of a blood-donor arm disinfectant containing chlorhexidine and alcohol. Transfusion. 2004;44:1238–1242. doi: 10.1111/j.1537-2995.2004.03362.x. [DOI] [PubMed] [Google Scholar]
- 8.Brecher M E, Holland PV, Pineda AA, Tegtmeier G E, Yomtovian R. Growth of bacteria in inoculated platelets: Implications for bacteria detection and the extension of platelet storage. Transfusion. 2000;40:1308–1312. doi: 10.1046/j.1537-2995.2000.40111308.x. [DOI] [PubMed] [Google Scholar]
- 9.Ampofo W, Nii-Trebi N, Ansah J, Abe K, Naito H, Aidoo S, Nuvor V, Brandful J, Yamamoto N, Ofori-Adjei D, et al. Prevalence of blood-borne infectious diseases in blood donors in ghana. J Clin Microbiol. 2002;40:3523–3525. doi: 10.1128/JCM.40.9.3523-3525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armah HB, Narter-Olaga EG, Adjei A A, Asomaning K, Gyasi R K, Tettey Y. Seroprevalence of human t-cell lymphotropic virus type i among pregnant women in accra, ghana. J Med Microbiol. 2006;55:765–770. doi: 10.1099/jmm.0.46426-0. [DOI] [PubMed] [Google Scholar]
- 11.Diop S, Calattini S, Abah-Dakou J, Thiam D, Diakhate L, Gessain A. Seroprevalence and molecular epidemiology of human t-cell leukemia virus type 1 (htlv-1) and htlv-2 in blood donors from dakar, senegal. J Clin Microbiol. 2006;44:1550–1554. doi: 10.1128/JCM.44.4.1550-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner S J, Friedman L I, Dodd R Y. Transfusion-associated bacterial sepsis. Clin Microbiol Rev. 1994;7:290–302. doi: 10.1128/cmr.7.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelfriet C P, Reesink HW, Blajchman MA, Muylle L, Kjeldsen-Kragh J, Kekomaki R, Yomtovian R, Hocker P, Stiegler G, Klein H G, et al. Bacterial contamination of blood components. Vox Sang. 2000;78:59–67. doi: 10.1159/000031151. [DOI] [PubMed] [Google Scholar]
- 14.Bradley R M, Gander R M, Patel S K, Kaplan H S. Inhibitory effect of 0 degree c storage on the proliferation of yersinia enterocolitica in donated blood. Transfusion. 1997;37:691–695. doi: 10.1046/j.1537-2995.1997.37797369443.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldman M, Blajchman MA. Blood product-associated bacterial sepsis. Transfus Med Rev. 1991;5:73–83. doi: 10.1016/s0887-7963(91)70194-6. [DOI] [PubMed] [Google Scholar]
- 16.Werch JB, Mhawech P, Stager C E, Banez E I, Lichtiger B. Detecting bacteria in platelet concentrates by use of reagent strips. Transfusion. 2002;42:1027–1031. doi: 10.1046/j.1537-2995.2002.00157.x. [DOI] [PubMed] [Google Scholar]
- 17.Yomtovian R, Lazarus H M, Goodnough LT, Hirschler N V, Morrissey A M, Jacobs M R. A prospective microbiologic surveillance program to detect and prevent the transfusion of bacterially contaminated platelets. Transfusion. 1993;33:902–909. doi: 10.1046/j.1537-2995.1993.331194082380.x. [DOI] [PubMed] [Google Scholar]
- 18.Morel P, Herve P. Detection of bacterial contamination of platelets concentrates. International forum 6. Vox Sang. 2003;85:230–232. [Google Scholar]
- 19.Perez P, Salmi L R, Follea G, Schmit J L, de Barbeyrac B, Sudre P, Salamon R. Determinants of transfusion-associated bacterial contamination: Results of the french bacthem case-control study. Transfusion. 2001;41:862–872. doi: 10.1046/j.1537-2995.2001.41070862.x. [DOI] [PubMed] [Google Scholar]
- 20.Brecher M E, Hay S N, Rothenberg S J. Monitoring of apheresis platelet bacterial contamination with an automated liquid culture system:A university experience. Transfusion. 2003;43:974–978. doi: 10.1046/j.1537-2995.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 21.Goodnough L T, Shander A, Brecher ME. Transfusion medicine: Looking to the future. Lancet. 2003;361:161–169. doi: 10.1016/S0140-6736(03)12195-2. [DOI] [PubMed] [Google Scholar]
- 22.Morrow J F, Braine H G, Kickler T S, Ness P M, Dick J D, Fuller A K. Septic reactions to platelet transfusions. A persistent problem. JAMA. 1991;266:555–558. [PubMed] [Google Scholar]
- 23.McDonald C P, Hartley S, Orchard K, Hughes G, Brett M M, Hewitt P E, Barbara J A. Fatal clostridium perfringens sepsis from a pooled platelet transfusion. Transfus Med. 1998;8:19–22. doi: 10.1046/j.1365-3148.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 24.Ness P, Braine H, King K, Barrasso C, Kickler T, Fuller A, Blades N. Single-donor platelets reduce the risk of septic platelet transfusion reactions. Transfusion. 2001;41:857–861. doi: 10.1046/j.1537-2995.2001.41070857.x. [DOI] [PubMed] [Google Scholar]
- 25.Brecher M E, Hay S N, Rothenberg S J. Evaluation of a new generation of plastic culture bottles with an automated microbial detection system for nine common contaminating organisms found in plt components. Transfusion. 2004;44:359–363. doi: 10.1111/j.1537-2995.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez F E, Rogge K J, Tarrand J, Lichtiger B. Bacterial contamination of cellular blood components. A retrospective review at a large cancer center. Ann Clin Lab Sci. 1995;25:283–290. [PubMed] [Google Scholar]
- 27.Mills-Robertson FC, Newman M, Mensah P, Addy M E. Multiple resistant salmonella in accra. Ghana Medical Journal. 2003;37:165–169. [Google Scholar]
- 28.Adjei O. Urinary tract infections. Symposium, durban. AFRIQUE DU SUD. 2004;24:S32–S34. doi: 10.1016/j.ijantimicag.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Ozumba U C. Antimicrobial resistance problems in a university hospital. J Natl Med Assoc. 2005;97:1714–1718. [PMC free article] [PubMed] [Google Scholar]
- 30.Tonks A. Drug resistance a worldwide threat, warns a report. BMJ. 1994:1109. [Google Scholar]
- 31.Adjei O. A survey of bacterial pathogens in clinical materials and their antimicrobial susceptibility in Kumasi Ghana. East Afr Med J. 1997;74:450–454. [PubMed] [Google Scholar]