Abstract
Objective
This study determined the effect of subclinical mastitis (SCM) on infant breastmilk intake.
Design
Participants (60 Ghanaian lactating mothers and their infants) were from periurban communities in the Manya Krobo district of Ghana in 2006–2007. Bilateral breastmilk samples were obtained once between months 3 and 6 postpartum and tested for SCM using the California mastitis test (CMT) and the sodium/potassium (Na/K) ratio. Infants' 12-hour breastmilk intake was assessed by test weighing. CMT scoring for SCM diagnosis was scaled as ≥1 = positive (n = 37) and <1 = negative (n = 23). SCM diagnosis was confirmed as a Na/K ratio of >1.0 (n = 14).
Results
Breastmilk intake was nonsignificantly lower among infants whose mothers had elevated Na/K ratios of >1.0 (−65.1 g; 95% confidence interval −141.3 g, 11.1 g). Infants whose mothers were positive for SCM with both CMT and Na/K ratio criteria had significantly lower breastmilk intake (−88.9 g; 95% confidence interval −171.1 g, −6.9 g) compared to those whose mothers tested either negative with both tests or positive on only one. Infant weight (p < 0.01) and frequency of feeding (p = 0.01) were independently associated with breastmilk intake. However, the effect of SCM on breastmilk intake disappeared when infant weight and feeding frequency were included in a multiple linear regression model.
Conclusions
The results of this study did not show an effect of SCM on breastmilk intake among 3–6-month-old infants. A larger sample size with a longitudinal design will be needed in future studies.
Introduction
There is compelling evidence that breastfeeding is associated with optimal infant growth and protection from diarrhea, respiratory infections, and other illnesses.1–3 However, some lactating women experience breastfeeding difficulties that may lead to early cessation of breastfeeding or introduction of complementary feeding before the recommended 6 months.4–6 Subclinical mastitis (SCM) is an inflammatory condition of the lactating breast that is thought to be caused by milk stasis or infections and has been associated with elevated risk of lactation failure5,7 and poor infant weight gain.5,8,9
SCM is common among lactating women and particularly during the early postpartum period.8–11 Although SCM is most prevalent during early lactation, SCM rates of 12–23% at 14 weeks postpartum have previously been reported.8,10 We have recently reported SCM prevalence of 45.3% among periurban dwelling Ghanaian women.12 SCM is typically diagnosed as either elevated breastmilk sodium (16 mmol/L)5,13 or sodium/potassium ratio (Na/K ratio >1.0).8,10,14
It is common knowledge in the dairy industry that cows diagnosed with SCM produce less milk.15 It is further known that calves fail to grow optimally when they are nursed by a cow with chronic mammary inflammation.15,16 Kitchen17 has reported a wide range of compositional and volumetric changes in dairy milk during mammary inflammation. In human lactation, however, little is known about the effects of SCM on breastmilk composition. The mechanism by which SCM affects weight gain among breastfeeding infants also remains unknown.
SCM commonly occurs in one breast.11,18 Milk output of the unaffected breast is capable of compensating for the affected breast without noticeable changes in milk intake. Conner19 reported a case study in which an infant accepted milk from one breast with reluctance and nursed normally from the other. Milk from the rejected breast was reported to have a salty taste. Later analyses showed that the sodium content of the affected breast was 103 mmol/L compared to a typical breastmilk sodium concentration of <10 mmol/L in fully lactating women.20 The above case suggests that breastmilk compositional changes may affect how infants breastfeed; however, reverse causality may also explain the relationship where the infant feeding pattern affects breastmilk composition.20
The objective of this study was to describe the relationship between mammary inflammation and infant breastmilk intake. We hypothesized that SCM is associated with a reduction in infant milk intake.
Subjects and Methods
Study area
Data for this study were collected in the Manya Krobo district of Ghana between July 2006 and February 2007. The Manya Krobo district is located in the eastern region of Ghana and has a population of about 157,000.21 Sixty percent of households in the district live in rural communities. The major occupations of the district population include crop farming, fishing, and trading. A relatively high prevalence rate of human immunodeficiency virus of about 5% has been observed in Manya Krobo,22 compared to the national rate of 2.7 % in the year 2007.23 Exclusive breastfeeding among 3–6-month-old infants was observed to be about 83% in the year 2007 in the Manya Krobo district.24
Participants
A total of 72 mother–infant pairs were recruited from seven child welfare clinics in the Manya Krobo district. Women were included in the study if they satisfied the following criteria: at least 18 years old, singleton birth, less than 3 months postpartum, and intending to breastfeed beyond 3 months.
Data collection procedures
After informed consent was obtained from the mother, the field worker scheduled a home visit for a date when the infant would be between 3 and 6 months old.
Measurement of breastmilk intake
A pair of field workers spent 12 consecutive hours in the home of each of the participants to measure infant breastmilk intake using test weighing procedures. Test weighing involved the recording of the weight of the infant just before and then immediately after the infant received breastmilk.25 Clothing or diapers worn by the infant were not changed between weighings. Test weighing measurements were recorded to the nearest 0.5 g using the Sartorius EA15DCE-I digital scale (The Sartorious Group, Göttingen, Germany). The scale was calibrated weekly using standard weight blocks.
Maternal and infant anthropometry
Anthropometric measurements that were recorded during the home visit included infant weight, length, head circumference, and mid-upper arm circumference as well as maternal height and weight. Infant weight was measured without any clothing. All weight measurements were recorded to the nearest 0.1 kg using the Tanita BWB800S digital floor scale (Tanita Corp., Tokyo, Japan). Infant weights were measured by first having the mother stand on the scale without the infant, taring the scale, and then recording the infant's weight while the child was being held by the mother standing on the scale. Head circumference and mid-upper arm circumference were measured to the nearest 0.1 cm using a nonstretchable tape measure (Chasmors Ltd., London, UK). Both infant length and maternal height were measured to the nearest 0.1 cm using the Shorr infant/child/adult height/length measuring board (Shorr Productions, Olney, MD). Each anthropometric measurement was performed in duplicate by two field personnel who were trained and regularly standardized to perform maternal and infant anthropometric measurements using standard methods.26
Morbidity and infant feeding
During the home visit, maternal and infant 7-day health histories were recorded. Mothers were asked to recall the occurrence and frequency of any disease symptoms for both the mother (including fever, breast pain, engorgement, and nipple lesions) and the infant (including diarrhea, cough, and fever) that were experienced over the last week. Any treatment for these symptoms and the source of treatment was also recorded.
A 7-day food frequency questionnaire was used to record all dietary intake of the infant, including breastmilk and other liquid and solid foods. Mothers were asked to recall all foods or drinks taken by the infant and the frequency of intake of these items. Infant feeding patterns were determined from the food frequency data. Exclusive breastfeeding was defined as intake of breastmilk only by the infant as reported by the mother in the last 7 days.
Breastmilk collection and handling
Breastmilk samples were obtained one day prior to the scheduled 12-hour home visit. A sample of approximately 5 mL of breastmilk expressed manually from each breast was obtained from each mother for analysis of sodium and potassium concentrations. Before expressing breastmilk, the women washed both hands thoroughly with disinfectant liquid hand soap and running water, rinsed with deionized water, and dried their hands with clean paper towels. The first drops of expressed milk were discarded, after which the nipple and surrounding areola were cleaned with cotton gauze soaked with 70% ethyl alcohol. Milk expressed thereafter was collected in 60-mL plastic vials with snap-on caps. Upon completion of expression from one side, hand rinsing and breast surface cleaning were repeated as described above before milk was expressed from the second breast.
About 2 mL of the milk sample were tested for SCM immediately after collection using the California mastitis test (CMT).27 The CMT is widely used by the dairy industry as an inexpensive and rapid “cow side” screening test of SCM. Dorosko et al.28 recently reported the use of the CMT in SCM diagnosis among human immunodeficiency virus-infected Zambian women. We used the CMT as a preliminary test for SCM. Maternal SCM status was subsequently confirmed using the Na/K ratio. The CMT involved mixing about 2 mL of milk with an equal amount of the CMT reagent in a test paddle and swirling the paddle in a counterclockwise fashion. Thickening or gelatinization of the mixture after about 10 seconds indicates SCM.27 The severity of SCM inflammation was categorized as the extent of gel formation, which was recorded on a scale of 1 to 3, with 3 representing the thickest gel formation. No gel formation was scored as 0. All CMT results were read by the same researcher (R.A.).
The remaining milk sample was kept on ice in a sealed container and transported to temporary storage in the field laboratory at −18°C. Subsequently, these samples were transported on ice to the University of Ghana (Accra) for storage in a freezer at −32°C until analyzed for sodium and potassium content.
Sodium and potassium analyses were carried out using the Easylyte ion-selective electrode analyzer (Medica Corp., Bedford, MA). Samples were first thawed to room temperature (25°C) and then homogenized before 0.1 mL of sample was aspirated into the ion-selective electrode analyzer for analyses. In addition to internal saline standards used by the Medica Easylyte analyzer, analytical quality was monitored by simultaneous analyses of milk with a saline standard with known electrolyte concentration.
Statistical analyses
Descriptive statistics including arithmetic means and standard deviations were computed for continuous maternal and infant variables. Categorical data were summarized into frequencies. Na/K ratios were computed from sodium and potassium data and categorized into SCM as follows: Na/K ratio ≤1.0 indicates no SCM, and Na/K ratio >1.0 indicates SCM. A CMT score of ≥1 was considered a positive diagnosis for SCM. Group differences for categorical variables were tested using Pearson's χ2 statistic. One-way analysis of variance was performed to test the difference in infant milk intake between SCM groups as well as the bivariate associations with other maternal and infant factors. Multiple linear regression was used to model the infant and maternal factors that predict 12-hour milk intakes. In both the analysis of variance and multiple linear regression analyses, the combined diagnostic criterion (a positive diagnosis with both Na/K ratio and CMT) was used to define maternal SCM status.
Results
Sodium and potassium data and CMT scores were available for 67 and 65 mothers, respectively. Five mothers had sodium and potassium data but did not have SCM scores, whereas seven others had sodium and potassium data but no CMT scores. Sixty out of the 72 mother–infant pairs had complete information and were included in this analysis. About 62% of mothers (n = 37) tested positive for SCM in one or both breasts using CMT ≥ 1. However, using Na/K ratio >1.0 as the threshold, 27% of women (n = 14) tested positive for SCM in one or both breasts. There were no cases of overt breast morbidities except two women in the SCM-negative group who reported breast pain.
Table 1 compares the characteristics of participants based on their Na/K-defined SCM category. Women who tested positive for SCM were younger (p = 0.02) and were likely to be primiparous (p = 0.01). There were no other significant differences in maternal characteristics between the SCM groups. Weight and head circumference were lower, but group differences did not reach statistical significance (p = 0.05). Length was significantly lower (p = 0.04) among infants whose mothers tested positive for SCM as compared to infants of mothers with no SCM.
Table 1.
Characteristics of Ghanaian Lactating Women and Their Infants by SCM Status
| |
SCM statusa |
||
|---|---|---|---|
| Characteristic | Negative (n = 46) | Positive (n = 14) | p valueb |
| Maternal | |||
| Age (years) | 26.8 (6.0) | 23.7 (4.7) | 0.02 |
| Education (years) | 7.6 (3.8) | 7.5 (2.8) | 0.23 |
| Body mass index (kg/m2) | 24.0 (3.1) | 25.6 (5.0) | 0.14 |
| Primipara | 16 (33) | 8 (73) | 0.01 |
| Ill in last 7 days | 11 (22) | 1 (9) | 0.32 |
| Fever in last 7 days | 2 (4) | 1 (9) | 0.49 |
| Infant | |||
| Age (months) | 3.2 (0.1) | 3.2 (0.1) | 0.46 |
| Weight (kg) | 6.2 (0.9) | 5.8 (0.6) | 0.05 |
| Length (cm) | 60.5 (2.1) | 59.5 (1.8) | 0.04 |
| Head circumference (cm) | 40.1 (1.2) | 39.6 (0.9) | 0.05 |
| Mid-upper-arm circumference (cm) | 13.6 (1.2) | 13.3 (0.6) | 0.19 |
| Exclusively breastfed | 43 (88) | 8 (73) | 0.21 |
| Male | 21 (43) | 6 (54) | 0.48 |
Data are mean (SD) or sample size (%).
SCM status: positive was defined as Na/K ratio >1.0; negative was defined as Na/K ratio ≤1.0.
Group differences in continuous and categorical characteristics were tested with Student's t test and Pearson's χ2, respectively. Fisher's exact test was used for three factors: ill in last 7 days, fever in last 7 days, and exclusively breastfed.
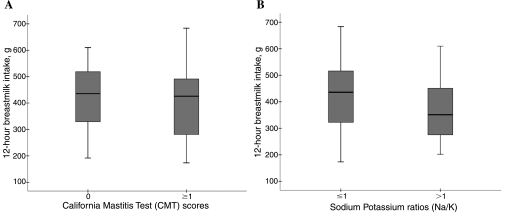
Average breastmilk intake for all infants during the 12-hour observation was 407.7 ± 127.2 g, with a range of 173.5 g to 683.5 g. Figure 1 displays the distribution of breastmilk intake for the SCM groups as diagnosed by either CMT or Na/K ratio. There was no difference in breastmilk intake between infants whose mothers were diagnosed with a positive CMT score and those whose mothers had a negative CMT score (403.9 ± 130.7 g vs. 425.6 ± 121.8 g, respectively; p = 0.52). Using the Na/K ratio for SCM diagnosis, however, milk mean intake was lower but not significantly different (−65.1 g; 95% confidence interval −141.3, 11.1) among infants whose mothers had an Na/K ratio >1.0 compared to infants whose mothers had a non-elevated milk Na/K ratio.
FIG. 1.
12-hour breastmilk intake of Ghanaian infants by test for maternal SCM. (A) Intake compared between CMT score groups: CMT = 0 (n = 37) or ≥1 (n = 23). The 25th and 75th percentiles are demarcated by the box, the median is represented by the dark horizontal line, and the whiskers represent 1.5 multiplied by the interquartile range. There were no significant differences between groups for both tests. (B) Intake compared between Na/K ratio groups: Na/K ratio ≤1.0 (n = 46) or >1 (n = 14). The 25th and 75th percentiles are demarcated by the box, the median is represented by the dark horizontal line, and the whiskers represent 1.5 multiplied by the interquartile range. There were no significant differences between groups for both tests.
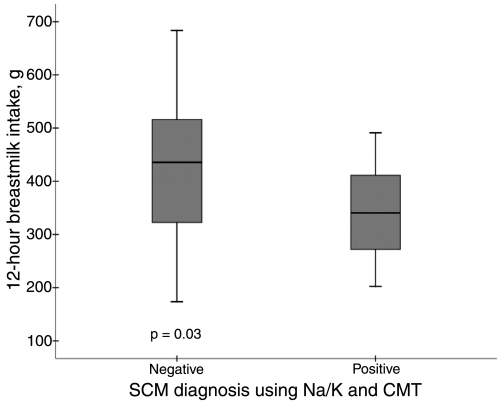
Eighteen percent (n = 11) of mothers tested positive for SCM with both CMT and Na/K ratio. Infants of these women (Fig. 2) consumed significantly less breastmilk (−88.9 g; 95% confidence interval −171.1, −6.9) during the 12-hour observation period compared to those whose mothers were diagnosed as either negative on both SCM tests or positive for only one of the tests.
FIG. 2.
12-hour breastmilk intake of Ghanaian infants by maternal SCM diagnosis as SCM negative (n = 49) or SCM positive (n = 11). SCM positive includes mothers who had both CMT score ≥1 and Na/K ratio >1.0 in at least one breast; SCM negative includes all other mothers. The 25th and 75th percentiles are demarcated by the box, the median is represented by the dark horizontal line, and the whiskers represent 1.5 multiplied by the interquartile range.
In multiple linear regression analysis, factors that predicted total grams of infant milk intake included infant weight in kilograms (b = 68.6, SE = 16.8; p < 0.01) and total number of breastfeeds during the 12-hour observation period (b = 18.4, SEM = 6.9; p = 0.01) but not maternal SCM status (b = −52.6, SEM = 35.9; p = 0.15). Other factors that were included in the model but that did not explain additional variance in milk intake were maternal age and body mass index as well as infant sex, length, head circumference, and arm circumference.
Discussion
The objective of this study was to determine whether maternal SCM status was associated with infant milk intake. Our results showed that infants whose mothers were diagnosed with SCM using the two diagnostic criteria (CMT score ≥1 and Na/K ratio >1.0) consumed significantly less milk (p = 0.034). The observed association, however, disappeared when the model accounted for infant weight and breastfeeding frequency. Part of the effect of SCM on intake may be captured by these two factors. Both low feeding frequency and poor infant nutritional status could contribute to suboptimal breastfeeding practice, thereby increasing the risk of SCM. In addition, the small number of SCM cases (n = 11) limited the power of the regression analysis to detect an effect.
To our knowledge, this is the first study to investigate the relationship between SCM and infant intake of breastmilk beyond early lactation. Manganaro et al.29 have recently reported an inverse relationship between breastmilk sodium and infant milk intake during the first week postpartum. Their results were consistent with findings in dairy cattle in which SCM is known to reduce milk output and permanently impair lactational performance.17,30 The outcome of the current study suggests that for children beyond early infancy (children older than 3 months), maternal SCM may not influence breastmilk intake.
SCM typically occurs unilaterally, and therefore it is possible for milk output from a healthy breast to compensate for the adverse effect of SCM on an affected breast.9 As reported by Connor,19 infants may be capable of differentiating between normal breastmilk and that with elevated sodium and thus exhibit a preference for the latter. An ideal design for a study of the effect of SCM on lactational performance, therefore, would be to measure milk secretion from one breast independently of the other. The test weighing methodology that was used in the present research would impose considerable interference on the “on-demand” feeding relationship between mother and infant because it would require a new weighing each time the child changed breasts during a feeding session.
An elevated Na/K ratio was more prevalent among younger and primiparous mothers in this study. A study in Zambia has also reported that primiparous mothers had significantly higher Na/K ratios from week 1 through 16 postpartum.11 These findings demonstrate a need to focus interventions on supporting young and first-time mothers to maintain optimal breast health during lactation.
In this study, we measured infant milk intake using test weighing as a proxy for milk output because it is a simple procedure and its effect on the maternal–infant feeding relationship is only minimal.25 Also, in this study insensitive water loss was not estimated. Arthur et al.25 have demonstrated significant underestimation of breastmilk intake using an infant test weighing without consideration for insensitive water loss. The comparison of breastmilk intake between groups in this study is, however, not affected by not controlling for insensitive water loss.
The CMT was used in this study as a screening test during recruitment because it is a simple and inexpensive diagnostic procedure that gives immediate results.27 Dorosko et al.28 had reported that CMT could serve as a screening tool for SCM based on the high correlation with somatic cell counts. We have observed in our studies in this community that the CMT overestimates the prevalence of SCM.24 The Na/K ratio, which is commonly used to diagnose SCM,8,10 was therefore used to confirm CMT scores. Either CMT or Na/K ratio alone failed to demonstrate significant differences in breastmilk intake between infants based on their mothers' SCM status. The ability of the combined SCM diagnostic criteria to distinguish breastmilk intake differences may be related to the ability of the combined diagnoses to detect more severe mammary inflammation.
The Na/K ratio diagnosis is typically made using a threshold of 1.0, which is considered to be equivalent to a sodium concentration of 18 mmol/L.31 This level of milk sodium is observed in breastmilk during mammary tight junction opening as well as during weaning. However, it is not known whether the fluctuations in milk electrolytes observed during onset of lactation or weaning follow the same pattern as the acute changes occurring in the milk of non-weaning lactating women.20 Improvement in the sensitivity and specificity of diagnostic tests for SCM would be useful.
The answer to our research question “does mammary inflammation reduce infant breastmilk intake?” could have useful implications for clinical decision-making. Although the study outcome is limited by a small sample size, it appears that as far as breastmilk intake is concerned, SCM may not be an important predictor among children beyond 3 months old. We are currently preparing a manuscript for publication of a longitudinal study to test the relationship between SCM and infant growth that was carried out in the same district but with a different sample of women. In this study, however, breastmilk intake data were not measured. A longitudinal study design with adequate sample size will be needed to adequately establish the relationship between infant feeding and breastmilk composition.
Conclusions
In this setting, we were unable to demonstrate a difference in breastmilk intake among infants whose mothers had SCM compared to those whose mothers did not. The small sample size may have limited our ability to show this difference. A longitudinal study involving a larger sample size is needed to adequately clarify the relationship between SCM and infant breastmilk intake. We are therefore unable, with these results, to define of the relationship between infant breastmilk intake and SCM.
Acknowledgments
Funding for this study was provided by grant HD43620 from the National Institutes of Health, Special Research Initiation Grants (SPRIG) from Iowa State University, and the Graduate College Research Fund and Julia F. Anderson International Fund, College of Family and Consumer Sciences, Iowa State University.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lauer JA. Betran AP. Barros AJ, et al. Deaths and years of life lost due to suboptimal breast-feeding among children in the developing world: A global ecological risk assessment. Public Health Nutr. 2006;9:673–685. doi: 10.1079/phn2005891. [DOI] [PubMed] [Google Scholar]
- 2.Bachrach VR. Schwarz E. Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: A meta-analysis. Arch Pediatr Adolesc Med. 2003;157:237–243. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 3.Baker D. Taylor H. Henderson J. Inequality in infant morbidity: Causes and consequences in England in the 1990s. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Epidemiol Community Health. 1998;52:451–458. doi: 10.1136/jech.52.7.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Strategy for Infant and Young Child Feeding. World Health Organization; Geneva: 2003. [Google Scholar]
- 5.Morton JA. The clinical usefulness of breast milk sodium in the assessment of lactogenesis. Pediatrics. 1994;93:802–806. [PubMed] [Google Scholar]
- 6.Segura-Millan S. Dewey KG. Perez-Escamilla R. Factors associated with perceived insufficient milk in a low-income urban population in Mexico. J Nutr. 1994;124:202–212. doi: 10.1093/jn/124.2.202. [DOI] [PubMed] [Google Scholar]
- 7.Aperia A. Broberger O. Herin P. Zetterstroem R. Salt content in human breast milk during the first three weeks after delivery. Acta Paediatr Scand. 1979;68:441–442. doi: 10.1111/j.1651-2227.1979.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomo E. Filteau SM. Tomkins AM, et al. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans R Soc Trop Med Hyg. 2003;97:212–216. doi: 10.1016/s0035-9203(03)90124-6. [DOI] [PubMed] [Google Scholar]
- 9.Filteau SM. Rice AL. Ball JJ, et al. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or β-carotene. Am J Clin Nutr. 1999;69:953–958. doi: 10.1093/ajcn/69.5.953. [DOI] [PubMed] [Google Scholar]
- 10.Willumsen JF. Filteau SM. Coutsoudis A, et al. Breast milk RNA viral load in HIV-infected South African women: Effects of subclinical mastitis and infant feeding. AIDS. 2003;17:407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kasonka L. Makasa M. Marshall T, et al. Risk factors for subclinical mastitis among HIV-infected and uninfected women in Lusaka, Zambia. Paediatr Perinat Epidemiol. 2006;20:379–391. doi: 10.1111/j.1365-3016.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 12.Aryeetey R. Marquis GS. Brakohiapa L, et al. Subclinical mastitis is common among Ghanaian women lactating 3 to 4 months postpartum. J Hum Lact. 2008;24:263–267. doi: 10.1177/0890334408316077. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD. Kumwenda N. Taha ET, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–674. doi: 10.1128/cdli.6.5.671-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores M. Filteau S. Effect of lactation counseling on subclinical mastitis among Bangladeshi women. Ann Trop Paediatr. 2002;22:85–88. doi: 10.1179/027249302125000210. [DOI] [PubMed] [Google Scholar]
- 15.Newman MA. Wilson LL. Cash EH, et al. Mastitis in beef cows and its effects on calf weight gain. J Anim Sci. 1991;69:4259–4272. doi: 10.2527/1991.69114259x. [DOI] [PubMed] [Google Scholar]
- 16.Kirkbride CA. Mastitis in beef cows. J Am Vet Med Assoc. 1977;170:1141–1142. [PubMed] [Google Scholar]
- 17.Kitchen BJ. Review of the progress of dairy science: Bovine mastitis: Milk compositional changes and related diagnostic tests. J Dairy Res. 1981;48:167–188. doi: 10.1017/s0022029900021580. [DOI] [PubMed] [Google Scholar]
- 18.Filteau SM. Leitz G. Mulokozi G, et al. Milk cytokines and subclinical breast inflammation in Tanzanian women: Effects of dietary red palm oil or sunflower oil supplementation. Immunology. 1999;97:595–600. doi: 10.1046/j.1365-2567.1999.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conner AE. Elevated levels of sodium and chloride in milk from mastitic breast. Pediatrics. 1979;63:910–911. [PubMed] [Google Scholar]
- 20.Neville MC. Allen JC. Archer PC, et al. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54:81–92. doi: 10.1093/ajcn/54.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Ghana Statistical Service. 2000 Population and Housing Census, Summary Report of Final Results. Ghana Statistical Service; Accra, Ghana: 2002. [Google Scholar]
- 22.National AIDS Control Program. Sentinel Survey Report. National AIDS Control Program; Accra, Ghana: 2004. 2005. [Google Scholar]
- 23.Atuahene K. Current trends in HIV/AIDS and related government actions in Ghana. Conference paper presented at the 2nd African Nutritional Epidemiology Conference; Legon, Accra, Ghana. Aug 18;2006 . [Google Scholar]
- 24.Aryeetey R. Iowa State University; Ames: 2007. Does subclinical mastitis predict the growth of Ghanaian infants? [Ph.D. dissertation] [Google Scholar]
- 25.Arthur PG. Hartmann PE. Smith M. Measurement of the milk intake of breast-fed infants. J Pediatr Gastroenterol Nutr. 1987;6:758–763. doi: 10.1097/00005176-198709000-00017. [DOI] [PubMed] [Google Scholar]
- 26.de Onis M. Garza C. Victora CG, et al. The WHO Multicentre Growth Reference Study: Planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl):S15–S26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 27.Shitandi A. Kihumbu G. Assessment of the California mastitis test usage in smallholder dairy herds and risk of violative antimicrobial residues. J Vet Sci. 2004;5:5–9. [PubMed] [Google Scholar]
- 28.Dorosko SM. Thea DM. Saperstein G, et al. Veterinary field test as screening tool for mastitis and HIV-1 viral load in breastmilk from HIV-infected Zambian women. Breastfeed Med. 2007;2:172–175. doi: 10.1089/bfm.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manganaro R. Marseglia L. Mami C, et al. Breast milk sodium concentration, sodium intake and weight loss in breast-feeding newborn infants. Br J Nutr. 2007;97:344–348. doi: 10.1017/S0007114507280572. [DOI] [PubMed] [Google Scholar]
- 30.Morin DE. Petersen GC. Whitmore HL, et al. Economic analysis of a mastitis monitoring and control program in four dairy herds. J Am Vet Med Assoc. 1993;202:540–548. [PubMed] [Google Scholar]
- 31.Willumsen JF. Filteau SM. Coutsoudis A, et al. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]