Abstract
The advent of highly active antiretroviral therapy in the treatment of HIV disease has substantially extended the lifespan of individuals infected with HIV resulting in a growing population of older HIV-infected individuals. The efficacy and safety of antiretroviral agents in the population are important concerns. There have been relatively few studies assessing antiretroviral pharmacokinetics in older patients. Thirty-seven subjects aged 18–30 years and 40 subjects aged 45–79 years, naive to antiretroviral therapy, received lopinavir/ritonavir (400/100) bid, emtricitibine 200 mg qd, and stavudine 40 mg bid. Trough lopinavir concentrations were available for 44 subjects, collected at 24, 36, and 96 weeks. At week 24, older age was associated with higher lopinavir trough concentrations, and a trend was observed toward older age being associated with higher lopinavir trough concentrations when all time points were evaluated. In the young cohort, among subjects with two or more measurements, there was a trend toward increasing intrasubject trough lopinavir concentrations over time. Using a nonlinear, mixed-effects population pharmacokinetic model, age was negatively associated with lopinavir clearance after adjusting for adherence. Adherence was assessed by patient self-reports; older patients missed fewer doses than younger patients (p = 0.02). No difference in grade 3–4 toxicities was observed between the two age group. Older patients have higher trough lopinavir concentrations and likely decreased lopinavir clearance. Age-related changes in the pharmacokinetics of antiretroviral drugs may be of increasing importance as the HIV-infected population ages and as older individuals comprise an increasing proportion of new diagnoses.
Introduction
The advent of highly-active antiretroviral therapy (HAART) in the treatment of HIV disease has had a dramatic impact on reducing mortality from AIDS.1 HIV is now viewed as a chronically manageable disease, resulting in an increased prevalence of older individuals with the infection. As an example, McDavid et al. recently reported increased infection rates in women aged 50 years and older.2 The 2004 cumulative number of AIDS cases occurring in individuals greater than 50 years old at the time of diagnosis is estimated to be 114,951 individuals, about 12% of the total.3 Data for 2006 indicate that 28% of 35,314 new diagnoses involved individuals greater than 45 years of age.4 Because many individuals with HIV infection are undiagnosed, there is additional concern for older individuals who may be at higher risk for progression to AIDS.5–8
A generalized age-related decline in immune function is well recognized9,10 and features specific changes in T-lymphocyte immunoregulation.11 In HIV infection, younger patients have higher CD4+ T-lymphocytes compared to older patients infected for the same duration.12 Patients who are older at seroconversion and at initiation of HAART experience faster clinical disease progression than those who are younger.13,14 Individuals who seroconvert at an older age appear to have higher HIV RNA concentrations.15 Age-related changes in pharmacokinetics have not been well studied in this population.
Factors that contribute to altered pharmacokinetics with aging include potential increases in the bioavailability of highly extracted drugs, decreases in hepatic blood flow and liver size, and decreases in creatinine clearance and renal tubular organic acid transport.16 Nevertheless, there are few data available on the effect of aging on antiretroviral pharmacokinetics. Plasma concentrations of HIV protease inhibitors determine virologic and immunologic responses as well as toxicities. Some studies suggest that risk of antiretroviral side effects such as lipodystrophy and severe transaminase elevation are increased in patients greater than 50 years old.17,18 Understanding the effect of aging on antiretroviral pharmacokinetics is important for maximizing therapeutic effects and minimizing toxicities of HAART. The risks of metabolic toxicities from HAART are important additional concerns in older patients who may have preexisting conditions, such as diabetes, that could be exacerbated by antiretrovirals.19,20
AIDS Clinical Trials Group (ACTG) Protocol 5015 was a phase II, open-label, two-step multicenter, prospective, cross-sectional comparison and longitudinal study of two age-differentiated cohorts to determine potential mechanisms that might contribute to accelerated HIV disease progression associated with aging. A major secondary objective of the study was to assess the impact of age on the pharmacokinetics of lopinavir (LPV).
We report here an analysis of LPV concentrations at weeks 24, 36, and 96 by age group. We further tested for a within-subject trend (increasing or decreasing trough LPV concentrations over time) within each age group, and tested for age-related differences in LPV clearance. In addition, the impact of age on the development of drug toxicities was evaluated.
Materials and Methods
Informed consent approved by local Institutional Review Boards was obtained for all subjects. The primary study analysis has been previously reported.21 Briefly, the study population included eligible HIV-infected men and women who were at least 18 years of age and were either naive to or had less than 14 days of prior antiretroviral therapy. All subjects had a screening CD4+ T cell count of less than 600 cells/mm3 and an HIV-1 RNA determination >2000 RNA copies/ml at screening. In all, 90 subjects, 45 subjects per age cohort, were assigned to either Group A or B according to age: Group A: age ≥18 years and ≤30 years; Group B: age ≥45 years.
All subjects received an open-label study treatment regimen of lopinavir/ritonavir 400 mg/100 mg bid, emtricitabine 200 mg qd, and stavudine 40 mg bid (30 mg bid for weight <60 kg) for up to 192 weeks. The soft-gel capsule formulation of lopinavir/ritonavir was the only one available at the time of the study. Participants were instructed to take their medications with food. The use of concomitant medications known to influence the pharmacokinetics of LPV or ritonavir was not permitted. Toxicities were assessed using the Division of AIDS Adverse Event Assessment Scale.22
Lopinavir assay
Plasma concentrations of LPV were determined using a validated high-performance liquid chromatography (HPLC) assay.23 The internal standard (IS), A-86093.0, was supplied by Abbott Laboratories (Abbott Park, IL). The mobile phase was 0.1% trifluoroacetic acid, acetonitrile, and methanol (53:42:5). Analytes were separated isocratically followed by a step gradient wash at 30°C using a reverse-phase Beckman C18 column and were detected at 220 nm (IS and LPV). Calibration standards ranged from 100 to 15,000 ng/ml for LPV. For all assays, quality control samples were interspersed between unknown samples. Mean correlation coefficients for calibration curves were >0.998 ± 0.001. The precision and accuracy for all assays were high, with coefficients of variation (CV) of <13% intraday and <8% interday. During the conduct of this study, the analytical laboratory participated in an external quality control program for measurement of antiretroviral drug concentrations sponsored by the Pharmacology Committee of the Adult AIDS Clinical Trials Group (AACTG).
Statistical methods
Trough plasma lopinavir concentrations
Trough samples, defined as those drawn from 10 to 14 h (inclusive) after the previous LPV dose, were reported and analyzed. Comparisons of LPV trough concentrations at a given week (or of changes from a specified early to a specified later week) between the two age groups and comparisons of scores (reflecting a within-subject monotonic trend over time) were made with a two-sided Wilcoxon rank sum test at the 5% level of significance. Overall comparison of LPV trough concentrations between the two age groups, treating evaluations at different weeks as repeated measures, was performed with a two-sided, 5% level nonparametric method.24,25 The association between trough concentration at a given week and age in years, after adjusting for adherence, was evaluated with linear regression models. Adherence was scored to reflect the 5 day dose history prior to the sample collection, with the weight for each dose rising exponentially from the earliest to the latest to reflect the impact of a missed dose on trough plasma concentration.
Intraindividual trends in plasma lopinavir concentrations over time
To determine if individual subjects displayed any trend toward increasing or decreasing trough concentrations over the three sampling intervals, a monotonic trend analysis was performed. Intraindividual monotonic trends over time were analyzed with a nonparametric method (details are available on request).
Lopinavir clearance
The association between LPV clearance and age was evaluated using all week 24, 36, or 96 LPV concentrations from specimens for which the time between the previous LPV dose and the blood draw was reported, even if it was not between 10 and 14 h. We first created a data set reflecting a plausible and approximate complete LPV dose history from study entry through the last observed LPV concentration evaluation for each subject. The data were then fit to one-compartment26 nonlinear mixed effects models using the Splus NLME function (S-PLUS Version 6.2.1 for Sun SPARC, SunOS 5.8, 32-bit: Insightful Corp 2003). LPV concentration was a function of dose history and three pharmacokinetic parameters: apparent volume of distribution, absorption rate constant, and clearance. This was done in two ways.
First, we explored a fixed effect for the natural logarithm of clearance (ln[ClLOP]) modeled as a linear function of age as a continuous variable, and as a random effect for the ln[ClLOP] intercept, with the natural logarithm of apparent volume (ln[VLOP]) and the natural logarithm of the absorption rate constant (ln[KLOP]) set to 8.77 and 0.21, respectively, for all subjects; and a starting value of 1.75 was used for the ln[ClLOP] intercept and zero for its age coefficient. Second, we investigated a model with a fixed effect for both ln[ClLOP] and ln[VLOP ], each modeled as a linear function of the candidate covariates, age and baseline weight, that resulted from a step-down procedure; this model included a random effect for the ln[ClLOP] intercept, held the natural logarithm of the absorption rate constant set to 0.210863 for all subjects, and used starting values for the ln[ClLOP] and ln[VLOP] intercept of 1.755919 and 8.772888, respectively, and zero for the covariate coefficients.
These starting values were obtained by iterative fits of a nonlinear26 least squares model to our data, starting with initial estimates of ln[KLOP], ln[ClLOP)], and ln[VLOP] based on the half-life of LPV taken with food as 9.12 h27 and oral clearance (CL/F) for LPV of 5.98 liters/h.28 These values were very similar to the oral clearance for LPV in the presence of ritonavir of 5.73 liters/h reported by Crommentuyn et al., 29 with the constant rate of elimination estimated as ln(2)/9.12 h = 0.076/h. The volume of distribution was then estimated as the ratio of the clearance to the rate of elimination, 5.98/0.076 = 78.68 liters, and with the constant rate of absorption for LPV estimated as the value reported for ritonavir by Kappelhoff et al. as 0.871/h,30 which was very similar to the value of 0.85/h published later by Moltó et al.31 for LPV. All results for LPV concentrations below the lower limit of quantification (100 ng/ml) were imputed to be 100 ng/ml (3 of the 44 subjects with trough plasma concentrations), but when imputed to be 50 ng/ml, the modeling results were almost the same and not reported.
Results
Of the 92 subjects enrolled into A5015, one subject was enrolled inadvertently and another subject never started study treatment before being lost to follow-up. Of the remaining 90 subjects, 77 had at least one LPV concentration reported from a specimen with the time from the previous dose to the specimen blood draw also reported. The 77 subjects in this study consisted of 40 in the old age group (11 female, 29 male) and 37 in the young age group (9 female, 28 male) with ages ranging from 18 to 79 (1st, 2nd, and 3rd quartiles of 26, 45, and 50.5, respectively) years. The racial/ethnic breakdown was 24 white not Hispanic, 32 black not Hispanic, 19 Hispanic, 1 Asian Pacific Islander, and 1 American Indian/Alaskan native.
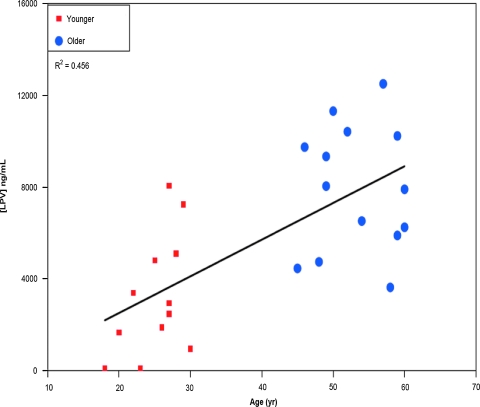
Only results from specimens drawn from 10 to 14 h (inclusive) after the previous LPV dose were included in the LPV trough concentration analyses, restricting the number of subjects in that analysis to n = 44, among whom the median number of hours between the previous LPV dose and the “trough” specimen draw combining LPV evaluations at all time points was 12.2 h for the young cohort and 13.3 h for the old cohort. To determine if there was an age–cohort effect on LPV trough concentrations, a nonparametric repeated measures test was performed that included all subjects with any evaluations (n = 44; 22 young, 22 old). LPV trough concentrations in the younger cohort were significantly lower compared to the older one, with median values at weeks 24, 36, and 96 of, respectively, 2700, 3472, and 5029 ng/ml in the young group and 7973, 5763, and 6686 ng/ml in the old group (p = 0.0410, two-sided, 99% CI = 0.0361, 0.0464; Fig. 1, week 24 only). When controlled for the adherence score (calculated as described in Materials and Methods), the effect of age on trough plasma lopinavir concentration remained significant at week 24 (p = 0.0001), but not at weeks 36 or 96 (p = 0.1229 and 0.3032, respectively). When gender was added to the above analysis, a significant positive association was observed between age and LPV trough concentration at week 24 (estimated slope of 163 ng/ml per year increase in age, 95% CI = 89–238, p = 0.0002; r2 = 0.50; Fig. 1), but this association was not seen at weeks 36 or 96 (p = 0.1638 and p = 0.3299 for weeks 36 and 96, respectively).
FIG. 1.
Lopinavir trough con-centrations at week 24 by age group. Lopinavir trough concentrations were fitted in a linear regression model as described in Materials and Methods. At 24 weeks, the median trough plasma lopinavir concentration was 2700 ng/ml in the younger group compared to 7973 ng/ml in the older group (p = 0.0001 when controlled for adherence score). The model predicted an estimated increase in week 24 trough plasma lopinavir concentration of 163 ng/ml per year increase in age (95% CI = 89–238, p = 0.0002, R2 = 0.50). (Color image can be found at www.liebertonline.com/aid).
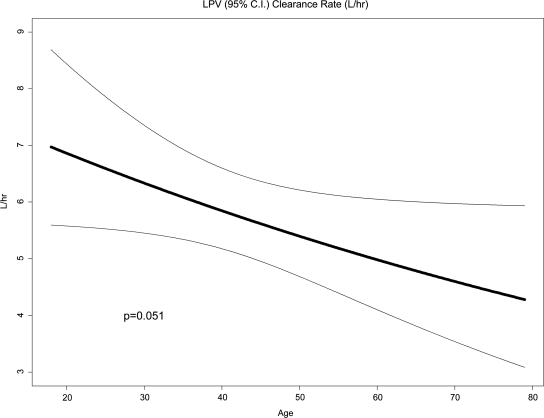
In the younger age cohort, there was marginal or statistically significant evidence of a within-subject trend of increasing trough concentrations over time (depending on whether the analysis was based on subjects with two or more evaluations or only subjects with all three evaluations). Specifically, in the young age group, the two-sided test based on all subjects who had at least two evaluations found only marginal evidence of an increasing trend over time (p = 0.06, 99% CI = 0.051, 0.063). When the same test in the young age group was based only on subjects with all three evaluations, the evidence was in the same direction but stronger (p = 0.018, 99% CI = 0.015, 0.022). There was no statistically significant or even marginal evidence of a within-subject trend over time in the older age cohort. There was no statistically significant evidence that changes over time (from week 24 to 36, week 36 to 96, or week 24 to 96) differed between the two age cohorts regardless of which subsets of subjects were used for the analysis. Fitting the first of the two nonlinear mixed-effects population pharmacokinetic models (described in Materials and Methods) of LPV concentration to all 77 subjects and all concentrations from specimens whose time of blood draw relative to the previous dose was reported, adjusting for adherence with a plausible entire dose history (see Materials and Methods) and setting the fixed effect for ln[ClLOP] as a linear function of age (in years), age was marginally negatively associated with lopinavir clearance, with a slope for ln[ClLOP] versus years of age of −0.008 (95% CI: −0.016, 0.000) ln(liters/h)/year (p = 0.051, Fig. 2). This model predicted a population mean LPV clearance of 6.86 liters/h for a 20-year-old person versus only 4.24 liters/h for an 80-year-old person. When gender was added to this model, age remained marginally statistically significant (p = 0.051, coefficient on age = −0.008) but gender was not significant. When race/ethnicity was added to the model (without gender), race was not significant but age remained significant (p = 0.032, coefficient on age = −0.009). The second of the two nonlinear mixed-effects population pharmacokinetic models described in Materials and Methods (the result of a step-down procedure) retained age and baseline weight in the submodel for ln[VLOP], and neither age nor weight in the submodel for Ln[ClLOP]. In this model, age was positively associated with ln[VLOP] [p = 0.0501, coefficient = 0.087 (SE = 0.044)] and weight was negatively associated [p = 0.0018, coefficient = −0.03778 (SE = 0.011794)].
FIG. 2.
Estimated lopinavir mean clearance rate by age. This figure displays the relationship between the mean lopinavir oral clearance (with the 95% confidence interval) and age (n = 77). Clearance was calculated as described in Materials and Methods.
It might be expected that LPV clearance would be predictive of virologic response, including rates of virologic rebound, as older patients have been reported to have higher rates of rebound. However, in a Cox proportional hazards model, there was no association between the time to virologic rebound and lopinavir clearance (p = 0.67, based on the clearance estimates form the random effects model with age but not volume as a fixed effect). All subjects in this study received other antiretroviral agents that may have contributed to the virologic response in addition to LPV.
Patient adherence was assessed by self-report, where patients were asked to report the number of missed doses over the previous 4 days, every 12 weeks. By this survey, older patients had significantly greater adherence than younger patients (p = 0.025). Younger patients were more likely to have missed an LPV dose in the previous 4 days (3558 missed doses out of 40,195, 8.85%) than older patients (1895 missed doses out of 39,782, 4.76%). This finding takes on added significance in light of the observation that older subjects had a higher median number of prescribed drugs (non-HIV) than younger subjects (4.0 vs. 2.0).
A total of 28 of the 90 subjects reported toxicities of grade 3 or higher that were either possibly, probably, or definitely related to the study regimen. Of these 28 subjects, 13 were from the younger cohort and 15 were from the older cohort. The estimated odds ratio of a subject experiencing a drug-related toxicity of grade 3 or higher in the older cohort as opposed to the younger cohort was 1.2 (95% CI = 0.46, 3.32; p = 0.82). Of the 90 study subjects, 54 (60%) had grade 3 or higher toxicities regardless of relation to the study regimen. Of these 54 subjects, 23 were from the younger cohort and 31 from the older cohort. The estimated odds ratio of an older subject experiencing a grade 3 or greater event versus a younger subject was 2.1 (95% CI = 0.83, 5.49; p = 0.13). We observed no age-related differences in the incidence of grade 3 or 4 toxicities. We further observed no correlation between LPV clearance and maximum grade toxicity for endocrine/metabolic, hepatic, renal, and gastrointestinal toxicities (determined by Spearman's rank and Jonckheere-Terpstra trend tests for LPV clearance and maximum grade toxicity).
Discussion
The safe use of drugs necessitates a thorough understanding of their pharmacokinetic behavior. Older individuals have long been recognized as being more susceptible to adverse drug reactions than younger subjects. In one study, elderly individuals had a 70% higher rate of hospital admissions for adverse drug reactions than younger adults, and were more likely to be receiving multiple medications.32 Van der Hooft et al.33 observed that the frequency of hospitalizations for adverse drug reactions was related to older age. HIV protease inhibitors require stable plasma concentrations to suppress viral replication and to prevent acquisition of antiretroviral drug resistance mutations. The balancing act between insuring efficacy and minimizing toxicity may become more of a challenge as older individuals become the fastest growing demographic in the United States. As HIV disease increasingly becomes a disease of older people, an understanding of the effect of aging on antiretroviral pharmacokinetics is important for predicting virologic and immunologic outcomes in this population. Age-related decrements in renal function, medical comorbidities, and the increased number of concurrent medications in older patients can potentially affect antiretroviral drug disposition.
Our study found a positive correlation between older age and LPV trough concentration that was significant at week 24. Younger subjects tended to have lower trough concentrations of LPV, with evidence for the difference being strongest at week 24. The fact that younger subjects tended to have their trough blood drawn slightly sooner after their previous LPV dose than the older subjects could have been a source of bias. However, assuming that an earlier trough blood draw would cause LPV concentrations to be higher than if taken from a later blood draw, the bias in this study, if present, would be toward the conclusion that young subjects had higher concentrations of LPV, opposite to our findings.
In our study, we observed an effect of age on LPV pharmacokinetics independent of gender or other demographic variables. In contrast, van der Leur et al.34 in a multivariate regression analysis found that body mass index was inversely associated with lopinavir plasma concentration, but there was no effect of age. Similarly, Guillemi et al.35 found no differences in trough plasma LPV concentrations in patients greater than 60 years old receiving LPV compared to patients less than 35 years old. However, in neither of these studies was a repeated measures design employed, collecting multiple samples from each patient over a broad span of time as we did. Zhou et al.36 identified age as the primary covariate (including race, body weight, and gender) influencing indinavir pharmacokinetics, where older subjects displayed a larger volume of distribution and an increase in indinavir half-life. Nevertheless, they found no effect of aging on indinavir trough plasma concentrations or AUC8h, suggesting that the decline in clearance with age might balance the effect of Vd. They further observed an age-associated decrease in clearance in a univariate analysis. We also evaluated body weight as a covariate influencing LPV pharmacokinetics and found weight negatively associated with volume. Similarly, Bouillon-Pichault et al.37 found that body weight was significantly associated with the probability of achieving adequate LPV exposure. They also found that differences in body weight accounted for much of the variability in LPV clearance, an observation that may help explain the marked variability in protease inhibitor plasma concentrations reported by other investigators.38,39 Their study had a number of differences from ours, including a larger sample size, broader age range, use of different LPV doses, a significantly higher proportion of women, and use of drugs in the combination known to affect LPV pharmacokinetics (i.e., NNRTIs).
Our observations may help explain other age-related differences observed in patients on HAART. Studies have suggested that virologic response to HAART is greater in older patients than in younger patients, but the immunologic response (recovery of CD4+ cells) is blunted. Although some investigators observed no differences across age groups in virologic suppression in HAART-treated patients,38 a number of researchers have observed better virologic responses including a higher proportion of virologically suppressed patients,40,41 a shorter time to becoming suppressed,42 greater virologic suppression,43 and greater durability of viral suppression40 in older adult patients. Interestingly, we observed no age-related differences in the occurrence of grade 3–4 toxicities. This is a potentially important finding supporting the safety of lopinavir/ritonavir in older patients. Improved medication adherence in older patients, as observed in our study, is consistent with the results reported by others.41 Better adherence among older patients could contribute to higher plasma concentrations and greater virologic responses, although Goodkin et al.44 observed better virologic responses in older patients independent of the effect of medication adherence.
In contrast to the enhanced virologic responses seen in older patients, several studies have found the recovery of CD4+ lymphocytes in response to HAART to be blunted in older patients.45 These effects include a lower absolute CD4+ lymphocyte count increase in response to HAART46 and slower rates of CD4+ lymphocyte recovery,47–49 a decreased proportion of naive CD4+ cells in untreated individuals, and diminished naive CD4+ cell restoration,50–53 although some investigators report no age-related changes in these parameters.38,49
Several factors could explain age-related changes in LPV pharmacokinetics. LPV is mainly a cytochrome (CYP) 3A4 substrate, and changes in the expression and activity of the subclass have been reported at various stages from infancy to adulthood.54,55 However, decreases in CYP3A activity in elderly individuals have not been consistently demonstrated.56,57 This may reflect the biological importance of CYP3A and the large capacity for CYP3A metabolism in the liver. In some studies of phenotyping using CYP3A4 probes, gender differences in metabolism are observed, which persist at older ages.56–59 However, in population studies of calcium channel blocker pharmacokinetics, drugs that are also CYP3A substrates, observed gender differences showed no effect of age.60–62 Schwartz57 has suggested that coadministered medications may play a more important role than age or gender in older individuals because they are more likely to be on multiple drugs.
Coadministration of LPV with ritonavir, a highly potent CYP3A inhibitor, may increase the sensitivity of LPV as a probe for age-related changes in metabolism of CYP3A substrates. Combining LPV with ritonavir results in a 13-fold increase in steady-state LPV concentrations,63 and CYP3A4 is wholly responsible for the metabolism of LPV. Because of the profound effect of ritonavir in boosting LPV concentrations, even a modest increase in ritonavir concentrations could translate into a significant pharmacokinetic effect. Unfortunately, plasma sample volumes were not adequate to assay for ritonavir concentrations in our study.
Changes in liver size and liver blood flow with age seem to be well supported in the medical literature.16 Between young adulthood and old age, liver size decreases by 24–35% and liver blood flow decreases by 35%,64–66 effects that can result in diminished clearance of drugs with a high first-pass metabolic extraction, such as LPV and ritonavir.62
Recognizing that HIV protease inhibitors are substrates of MRPs and MDR-1/p-glycoprotein, important studies examining age-related changes in transporter expression come from research in oncology. For example, Plasschaert et al.67 reported higher activity and expression of p-glycoprotein in older patients with T cell acute lymphoblastic leukemia. Ritonavir is both a p-glycoprotein inhibitor and substrate.68 The effects of drug transporters on pharmacokinetics are difficult to predict as changes in transporter function on drug absorption compared with drug elimination could produce opposite effects on plasma concentrations.
The binding of protease inhibitors to plasma proteins may also be an important interaction that modulates the disposition of these drugs. Some HIV protease inhibitors are highly bound to orosomucoid or α1-acid glycoprotein (AAG), and the concentration of this serum protein influences free concentrations of these antiretrovirals and their pharmacologic effects.69,70 Plasma concentrations of AAG were strongly associated with indinavir concentrations but less so with ritonavir concentrations.71 Concentration-dependent binding of lopinavir to orosomucoid appears to occur in vivo, an interaction that influences the level of unbound drug and may be important in lopinavir pharmacokinetics.72 Concentrations of AAG have been reported to be affected by age and disease states,73–75 but were not measured in this study.
Our studies point to a decrease in the clearance of LPV as a likely contributor to the increased trough concentrations seen in older subjects. Clearance was calculated using data from all 77 of these subjects. In older patients, hepatic drug clearance may be reduced by up to 30% with aging, and renal elimination decreased by up to 50%.76 Hilmer77 identified reduced hepatic and renal clearance as the most significant changes influencing pharmacokinetics with normal aging, and suggested that changes in oral bioavailability in aging result from reduced first-pass hepatic metabolism for high extraction drugs, such as LPV and ritonavir. She suggests that changes in volume of distribution are smaller than changes in clearance and contribute less significantly to other pharmacokinetic parameters. The association between age and trough LPV concentration was significant only at 24 weeks. A trend toward higher plasma concentrations in older individuals was also observed at weeks 48 and 96, but failed to reach statistical significance most likely because there were fewer data points at these times. Even though older subjects had a higher median number of nonantiretroviral medications than younger subjects, it is not likely that drug interactions explains the differences in trough concentration, as drugs known to interact with lopinavir/ritonavir were not allowed in the study.
We have demonstrated modest age-related differences in the concentrations of LPV. Although these are unlikely to affect LPV efficacy or toxicity, given its broad therapeutic index, more attention should be paid to age-related changes in concentrations of other drugs used in this patient population as the epidemic matures and new classes of antiretroviral drugs become available. Recent unpublished studies have found increased concentrations of darunavir78 and LPV79 in older subjects. Future studies should consider the effects of aging on concentrations of other antiretrovirals, given the potential impact on long-term efficacy and safety.
Acknowledgments
Special thanks to the ACTE 5015 protocol team and the study participants. This work was supported by the AIDS Clinical Trials Group (ACTG) funded by the National Institute of Allergy and Infectious Diseases, including U01 AI68636, AI069465, and AI068634.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Murphy EL. Collier AC. Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 2.McDavid K. Li J. Lee LM. Racial and ethnic disparities in HIV diagnoses for women in the United States. J Acquir Immune Defic Syndr. 2006;42(1):101–107. doi: 10.1097/01.qai.0000199353.11479.08. [DOI] [PubMed] [Google Scholar]
- 3.HIV/AIDS Surveillance Report. Centers for Disease Control and Prevention; Atlanta, GA: 2004. HIV infection and AIDS in the United States. [Google Scholar]
- 4.New Estimates of U.S. HIV Prevalence. Centers for Disease Control and Prevention; Atlanta, GA: 2006. Oct, 2008. [Google Scholar]
- 5.Hall HI. McDavid K. Ling Q. Sloggett A. Determinants of progression to AIDS or death after HIV diagnosis, United States, 1996 to 2001. Ann Epidemiol. 2006;16(11):824–833. doi: 10.1016/j.annepidem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Porter K. Babiker A. Bhaskaran K, et al. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362(9392):1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 7.Egger M. May M. Chêne G. Phillips AN, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. Erratum in Lancet 2002;360(9340):1178. [DOI] [PubMed] [Google Scholar]
- 8.Kitahata MM. Gange SJ. Abraham AG, et al. Effect of Early versus deferred antiretroviral therapy for HIV on Survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruver AL. Hudson LL. Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarazona R. Solana R. Ouyang Q. Pawelec G. Basic biology and clinical impact of immunosenescence. Exp Gerontol. 2002;37(2–3):183–189. doi: 10.1016/s0531-5565(01)00182-6. [DOI] [PubMed] [Google Scholar]
- 11.van Baarle D. Tsegaye A. Miedema F. Akbar A. Significance of senescence for virus-specific memory T cell responses: Rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97(1):19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Geskus RB. Meyer L. Hubert JB. Schuitemaker H. Causal pathways of the effects of age and the CCR5-Delta32, CCR2-64I, and SDF-1 3'A alleles on AIDS development. J Acquir Immune Defic Syndr. 2005;39(3):321–326. doi: 10.1097/01.qai.0000142017.25897.06. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet F. Thiébaut R. Chêne G, et al. Determinants of clinical progression in antiretroviral-naive HIV-infected patients starting highly active antiretroviral therapy. Aquitaine Cohort, France, 1996–2002. HIV Med. 2005;6(3):198–205. doi: 10.1111/j.1468-1293.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Chow KY. Ang LW. Verghesse I, et al. Measurable predictive factors for progression to AIDS among HIV-infected patients in Singapore. Ann Acad Med Singapore. 2005;34(1):84–89. [PubMed] [Google Scholar]
- 15.Touloumi G. Pantazis N. Babiker AG. Differences in HIV RNA concentrations before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS. 2004;18(12):1697–1705. doi: 10.1097/01.aids.0000131395.14339.f5. [DOI] [PubMed] [Google Scholar]
- 16.Cusack BJ. Pharmacokinetics in older persons. Am J Geriatr Pharmacother. 2004;2(4):274–302. doi: 10.1016/j.amjopharm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Nunez M. Lana R. Mendoza JL. Martin-Carbonero L. Soriano V. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27(5):426–431. doi: 10.1097/00126334-200108150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk A. Breuer JP. Kremer G, et al. Risk factors for the HIV-associated lipodystrophy syndrome in a cross-sectional single-centre study. Eur J Med Res. 2000;5(10):443–448. [PubMed] [Google Scholar]
- 19.Luther VP. Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23(3):567–583. doi: 10.1016/j.cger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Meraviglia P. Schiavini M. Castagna A. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: Evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5(5):334–343. doi: 10.1111/j.1468-1293.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalayjian RC. Spritzler J. Pu M, et al. Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J Infect Dis. 2005;192(9):1577–1587. doi: 10.1086/466527. [DOI] [PubMed] [Google Scholar]
- 22.http://rcc.tech-res.com/DAIDS%20RCC%20FORMS/ToxicityTables_HVTN_18sep02_updated.pdf http://rcc.tech-res.com/DAIDS%20RCC%20FORMS/ToxicityTables_HVTN_18sep02_updated.pdf
- 23.Lamotte C. Peytavin G. Farinotti R. Determination of nelfinavir, a potent HIV protease inhibitor, and its active metabolite M8 in human plasma by high-performance liquid chromatography with photodiode-array detection. J Chromatogr B. 1999;735:159–170. doi: 10.1016/s0378-4347(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 24.Wei LJ. Johnson WE. Combining dependent tests with incomplete repeated measurements. Biometrika. 1985;72(2):359–364. [Google Scholar]
- 25.Xu X. Tian I. Wei LJ. Combining dependent tests for linkage or association across multiple phenotypic traits. Biostatistics. 2003;4(2):223–229. doi: 10.1093/biostatistics/4.2.223. [DOI] [PubMed] [Google Scholar]
- 26.Davidian M. Giltinan DM. Nonlinear Models for Repeated Measurement Data. Chapman & Hall; New York: 1995. Section 9.3. [Google Scholar]
- 27.Bertz R, et al. Steady-state pharmacokinetics of Kaletra (lopinavir/ritonavir 400/100 mg BID) in HIV-infected subjects when taken with food. 2nd International Workshop on Clinical Pharmacology of HIV Therapy; Apr 2–4;2001 ; The Netherlands. [Google Scholar]
- 28.Abbott Laboratories. Ref: 03-5395-R11. Revised: November, 2004.
- 29.Crommentuyn KLM. Kappelhoff BS. Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-infected patients. Br J Clin Pharm. 2005;60(4):378–389. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapellhoff BS, et al. Development and validation of a population pharmacokinetic model for ritonavir used as a booster or as an antiviral agent in HIV-1-infected patients. Br J Clin Pharmacol. 2004;59:174–182. doi: 10.1111/j.1365-2125.2004.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moltó J. Barbanoj MJ. Miranda C. Blanco A. Santos JR. Negredo E. Costa J. Domingo P. Clotet B. Valle M. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet. 2008;47(10):681–692. doi: 10.2165/00003088-200847100-00005. [DOI] [PubMed] [Google Scholar]
- 32.Kongkaew C. Noyce PR. Ashcroft DM. Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–1025. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 33.van der Hooft CS. Sturkenboom MC. van Grootheest K, et al. Adverse drug reaction-related hospitalisations: A nationwide study in The Netherlands. Drug Saf. 2006;29(2):161–168. doi: 10.2165/00002018-200629020-00006. [DOI] [PubMed] [Google Scholar]
- 34.van der Leur MR. Burger DM. la Porte CJ. Koopmans PP. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. 2006;28(5):650–653. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 35.Guillemi SA, et al. Int Cong Drug Therapy HIV; Glasgow, UK: 2004. Lopinavir trough concentration remains consistent for adult patients regardless of age. #P292. [Google Scholar]
- 36.Zhou XJ. Havlir DV. Richman DD, et al. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14(18):2869–2876. doi: 10.1097/00002030-200012220-00008. [DOI] [PubMed] [Google Scholar]
- 37.Buillon-Pichault M. Jullien V. Piketty C, et al. A population analysis of weight-based differences in lopinavir pharmacokinetics and possible consequences for protease inhibitor-naïve and experienced patients. Antiviral Ther. 2009;14(7):923–929. doi: 10.3851/IMP1414. [DOI] [PubMed] [Google Scholar]
- 38.Smith DE. Jeganathan S. Ray J. Atazanavir plasma concentrations vary significantly between patients and correlate with increased serum bilirubin concentrations. HIV Clin Trials. 2006;7(1):34–38. doi: 10.1310/0KX0-H9VH-99EE-5D0L. [DOI] [PubMed] [Google Scholar]
- 39.Nettles RE. Kieffer TL. Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42(8):1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 40.Patterson K. Napravnik S. Eron J, et al. Effects of age and sex on immunological and virological responses to initial highly active antiretroviral therapy. HIV Med. 2007;8(6):406–410. doi: 10.1111/j.1468-1293.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 41.Silverberg MJ. Leyden W. Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167(7):684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 42.Kilaru KR. Kumar A. Sippy N, et al. Immunological and virological responses to highly active antiretroviral therapy in a non-clinical trial setting in a developing Caribbean country. HIV Med. 2006;7(2):99–104. doi: 10.1111/j.1468-1293.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 43.Bosch RJ. Bennett K. Collier AC, et al. Pretreatment factors associated with 3-year (144-week) virologic and immunologic responses to potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(3):268–277. doi: 10.1097/QAI.0b013e31802c7e20. [DOI] [PubMed] [Google Scholar]
- 44.Goodkin K. Shapshak P. Asthana D, et al. Older age and plasma viral load in HIV-1 infection. AIDS. 2004;18(Suppl 1):S87–S98. [PubMed] [Google Scholar]
- 45.Wutoh AK. Elekwachi O. Clarke-Tasker V. Daftary M. Powell NJ. Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S106–S114. doi: 10.1097/00126334-200306012-00007. [DOI] [PubMed] [Google Scholar]
- 46.Kalayjian RC. Spritzler J. Pu M, et al. Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J Infect Dis. 2005;192(9):1577–1587. doi: 10.1086/466527. [DOI] [PubMed] [Google Scholar]
- 47.Moore RD. Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 48.Nogueras M. Navarro G. Antón E, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grabar S. Kousignian I. Sobel A, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18(15):2029–2038. doi: 10.1097/00002030-200410210-00007. [DOI] [PubMed] [Google Scholar]
- 50.Wolbers M. Battegay M. Hirschel B, et al. CD4+ T-cell count increase in HIV-1-infected patients with suppressed viral load within 1 year after start of antiretroviral therapy. Antivir Ther. 2007;12(6):889–897. [PubMed] [Google Scholar]
- 51.Connick E. Lederman MM. Kotzin BL, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181(1):358–363. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 52.Viard JP. Mocroft A. Chiesi A, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: Evidence from the EuroSIDA study. EuroSIDA Study Group. J Infect Dis. 2001;183(8):1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 53.Smith CJ. Sabin CA. Youle MS, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190(10):1860–1868. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 54.de Wildt SN. Kearns GL. Leeder JS. van den Anker JN. Cytochrome P450 3A: Ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 55.Fakhoury M. Litalien C. Medard Y. Cave H. Ezzahir N. Peuchmaur M. Jacqz-Aigrain E. Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age. Drug Metab Dispos. 2005;33(11):1603–1607. doi: 10.1124/dmd.105.005611. [DOI] [PubMed] [Google Scholar]
- 56.Parkinson A. Mudra DR. Johnson C. Dwyer A. Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199(3):193–209. doi: 10.1016/j.taap.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz JB. The current state of knowledge on age, sex and their interactions on clinical pharmacology. Clin Pharm Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 58.Cotreau MM. von Molte LL. Greenblatt DJ. The influence of age and sex on the clearance of CYP450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 59.Cummins C. Wu C-Y. Benet L. Sex related differences in the clearance of cytochrome P450 3A4 substrates may be caused by p-glycoprotein. Clin Pharm Ther. 2002;72:474–489. doi: 10.1067/mcp.2002.128388. [DOI] [PubMed] [Google Scholar]
- 60.Krecic-Shepard M. Park K. Barnas C. Slimko J. Kerwin D. Schwartz J. Race and sex influence clearance of nifedipine: Results of a population study. Clin Pharmacol Ther. 2000;68:130–142. doi: 10.1067/mcp.2000.108678. [DOI] [PubMed] [Google Scholar]
- 61.Kang D. Vercotta D. Schwartz J. Population analyses of sustained-release verapamil in patients: Age, race and sex effects. Clin Pharm Ther. 2003;73:31–40. doi: 10.1067/mcp.2003.21. [DOI] [PubMed] [Google Scholar]
- 62.Kang D. Vercotta D. Schwartz J. Population analyses of amlodipine in patients living in the community and in nursing homes. Clin Pharm Ther. 2006;79:114–124. doi: 10.1016/j.clpt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Cvetkovic RS. Goa KL. Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 64.Wynne H. Drug metabolism and ageing. J Br Menopause Soc. 2005;11(2):51–56. doi: 10.1258/136218005775544589. [DOI] [PubMed] [Google Scholar]
- 65.Zeeh J. The aging liver: Consequences for drug treatment in old age. Arch Gerontol Geriatr. 2001;32(3):255–263. doi: 10.1016/s0167-4943(01)00090-5. [DOI] [PubMed] [Google Scholar]
- 66.Schmucker DL. Liver function and phase I drug metabolism in the elderly: A paradox. Drugs Aging. 2001;18(11):837–851. doi: 10.2165/00002512-200118110-00005. [DOI] [PubMed] [Google Scholar]
- 67.Plasschaert SL. Vellenga E. de Bont ES, et al. High functional P-glycoprotein activity is more often present in T-cell acute lymphoblastic leukaemic cells in adults than in children. Leuk Lymphoma. 2003;44(1):85–95. doi: 10.1080/1042819021000040288. [DOI] [PubMed] [Google Scholar]
- 68.Washington CB. Duran GE. Man MC, et al. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(3):203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- 69.Schön A. del Mar Ingaramo M. Freire E. The binding of HIV-1 protease inhibitors to human serum proteins. Biophys Chem. 2003;105(2–3):221–230. doi: 10.1016/s0301-4622(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 70.Zhang XQ. Schooley RT. Gerber JG. The effect of increasing alpha1-acid glycoprotein concentration on the antiviral efficacy of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180(6):1833–1837. doi: 10.1086/315123. [DOI] [PubMed] [Google Scholar]
- 71.Jones K. Hoggard PG. Khoo S, et al. Effect of alpha1-acid glycoprotein on the intracellular accumulation of the HIV protease inhibitors saquinavir, ritonavir and indinavir in vitro. Br J Clin Pharmacol. 2001;51(1):99–110. doi: 10.1046/j.0306-5251.2001.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boffito M. Hoggard PG. Lindup WE, et al. Lopinavir protein binding in vivo through the 12-hour dosing interval. Ther Drug Monit. 2004;26(1):35–39. doi: 10.1097/00007691-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Woo J. Chan HS. Or KH. Arumanayagam M. Effect of age and disease on two drug binding proteins: Albumin and alpha-1-acid glycoprotein. Clin Biochem. 1994;27(4):289–292. doi: 10.1016/0009-9120(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 74.Mackiewicz A. Mackiewicz K. Glycoforms of serum alpha 1-acid glycoprotein as markers of inflammation and cancer. Glycoconj J. 1995;12(3):241–247. doi: 10.1007/BF00731326. [DOI] [PubMed] [Google Scholar]
- 75.Mackiewicz A. Khan MA. Górny A, et al. Glycoforms of alpha 1-acid glycoprotein in sera of human immunodeficiency virus-infected persons. Infect Dis. 1994;169(6):1360–1363. doi: 10.1093/infdis/169.6.1360. [DOI] [PubMed] [Google Scholar]
- 76.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 77.Hilmer SN. ADME-tox issues for the elderly. Expert Opin Drug Metab Toxicol. 2008;4(10):1321–1331. doi: 10.1517/17425255.4.10.1321. [DOI] [PubMed] [Google Scholar]
- 78.Fabbiani M. DiGiambenedetto S. Ragazzoni E. DeSimone A. Pharmacokinetic variability of darunavir, raltegravir in routine clinical practice. 10th International Workshop on Clinical Pharmacology of HIV Therapy; Amsterdam. 2009. #P_11. [Google Scholar]
- 79.Von Hentig N. Stephen C. Nisius G. Bickel M. Factors related to very high lopinavir plasma concentrations in an unselected outpatient cohort of HIV-infected adults. 10th International Workshop on Clinical Pharmacology of HIV Therapy; Amsterdam. 2009. #P_43. [Google Scholar]