Abstract
Retinal degenerative diseases, such as retinitis pigmentosa and Leber congenital amaurosis, are a leading cause of untreatable blindness with substantive impact on the quality of life of affected individuals and their families. Mouse mutants with retinal dystrophies have provided a valuable resource to discover human disease genes and helped uncover pathways critical for photoreceptor function. Here we show that the rd11 mouse mutant and its allelic strain, B6-JR2845, exhibit rapid photoreceptor dysfunction, followed by degeneration of both rods and cones. Using linkage analysis, we mapped the rd11 locus to mouse chromosome 13. We then identified a one-nucleotide insertion (c.420–421insG) in exon 3 of the Lpcat1 gene. Subsequent screening of this gene in the B6-JR2845 strain revealed a seven-nucleotide deletion (c.14–20delGCCGCGG) in exon 1. Both sequence changes are predicted to result in a frame-shift, leading to premature truncation of the lysophosphatidylcholine acyltransferase-1 (LPCAT1) protein. LPCAT1 (also called AYTL2) is a phospholipid biosynthesis/remodeling enzyme that facilitates the conversion of palmitoyl-lysophosphatidylcholine to dipalmitoylphosphatidylcholine (DPPC). The analysis of retinal lipids from rd11 and B6-JR2845 mice showed substantially reduced DPPC levels compared with C57BL/6J control mice, suggesting a causal link to photoreceptor dysfunction. A follow-up screening of LPCAT1 in retinitis pigmentosa and Leber congenital amaurosis patients did not reveal any obvious disease-causing mutations. Previously, LPCAT1 has been suggested to be critical for the production of lung surfactant phospholipids and biosynthesis of platelet-activating factor in noninflammatory remodeling pathway. Our studies add another dimension to an essential role for LPCAT1 in retinal photoreceptor homeostasis.
Keywords: gene discovery, lipid enzyme, phospholipid remodeling, retinal degeneration, visual dysfunction
Retinitis pigmentosa (RP) constitutes a group of common inherited retinal dystrophies with clinical manifestations, including nyctalopia (night blindness) and loss of peripheral vision. One of the early hallmarks of RP is photoreceptor dysfunction that is followed by the death of rod and then cone photoreceptors. To date, over 40 genes have been associated with inherited forms of RP (Retnet: www.sph.uth.tmc.edu/retnet/); these genes exhibit distinct patterns of expression (e.g., rod-specific or widely expressed) and encode proteins of diverse biological functions (1–3).
Animal models of retinal degeneration are a valuable resource in elucidation of genes for human retinal diseases, including RP and Leber congenital amaurosis (LCA) (4, 5). One of the first mouse retinal degeneration lines examined, rd1, was defective in Pde6b, encoding β-subunit of cGMP phosphodiesterase and was determined to have both an insertion and a point mutation (6, 7). The rd7 mouse line resulted from the loss of function of orphan nuclear receptor NR2E3 (8, 9), and rd16 mice carried an in-frame deletion in the broadly expressed Cep290 (10). Cross-species mutation discovery rapidly occurred in each case once the initial disease causing change (in either mice or humans) was identified (11–13). The study of proteins encoded by these genes is leading to a better understanding of the visual transduction pathway, transcription, and ciliogenesis, respectively.
In our ongoing effort to uncover genes critical for retinal function or disease, we examined the rd11 and B6-JR2845 strains of mice that were reported to exhibit retinal degeneration (4). Here we describe the detailed retinal phenotype of these mouse mutants, followed by mapping of the disease locus, and identification of mutations in the Lpcat1 gene that encodes a phospholipid remodeling enzyme, lysophosphatidylcholine (LPC) acyltransferase (AT). To assess the physiological impact of Lpcat1 mutations, we have examined membrane association of retinal pigment epithelium 65-kDa protein (RPE65), measured retinyl ester content, and evaluated dipalmitoylphosphatidylcholine (DPPC), the downstream product of LPCAT1 enzyme, in rd11 and B6-JR2845 retina. We also report the screening of LPCAT1 in patients with human retinopathy.
Results
Retinal Phenotype of the rd11 and B6-JR2845 Mutant Mice.
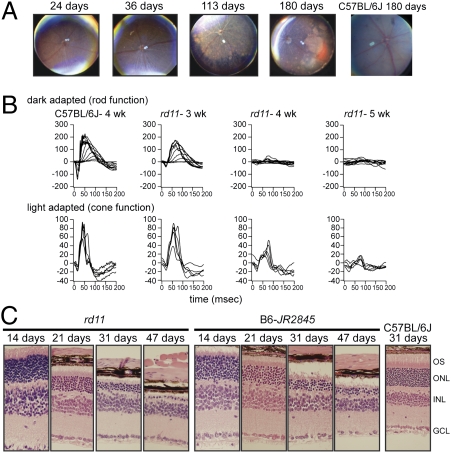
The rd11 and B6-JR2845 strains of mice were identified at The Jackson Laboratory in a screen for naturally occurring mouse models of retinal disease (4). The two strains are genetically allelic by complementation analysis and do not show any other obvious morphological or functional phenotype. Retinal fundus examination revealed a similar phenotype between both strains. Retinal vessels became attenuated by postnatal (P) day 36 with retinal degeneration obvious by P113 (Fig. 1A). Electroretinograms (ERGs) of rd11 mice presented with a reduced rod photoreceptor (dark-adapted) a-wave, which flattened out at 4 wk of age. Cone photoreceptor (light-adapted) response was similar to WT at 3 wk, but was reduced substantially at 4 and 5 wk. These results suggest that rod photoreceptors are affected before cones (Fig. 1B), consistent with a retinitis pigmentosa-like phenotype. A similar fundus and ERG profile was observed with B6-JR2845 mice (Fig. S1).
Fig. 1.
Photoreceptor degeneration and loss of ERG signal was observed in rd11 and B6-JR2845 mice. (A) Fundus image of rd11 mice at 24, 36, 113, and 180 d postnatal. Attenuated blood vessels were observed by P36. Large white patches of degeneration are seen by P133. A similar finding was observed in B6-JR2845 mice (Fig. S1). (B) ERG examination of C57BL/6J (B6) WT at 4 wk of age and rd11 mice at 3, 4, and 5 wk. Dark-adapted ERG showed a reduced a-wave in rd11 mice at 3 wk of age, with a flattening of signal by 4 wk. Light-adapted ERG was closer to WT at 3 wk of age but showed a substantial reduction of intensity at 4 and 5 wk of age. B6-JR2845 mice revealed a similar pattern (Fig. S1). (C) H&E staining of rd11, B6-JR2845, and B6 retinas. A substantial loss of photoreceptor nuclei was observed by P31, with a loss to one row of nuclei at P47 in rd11 mice. B6-JR2845 photoreceptor nuclei were also obviously reduced by P31, although more nuclei were present at P47 than in rd11 mice.
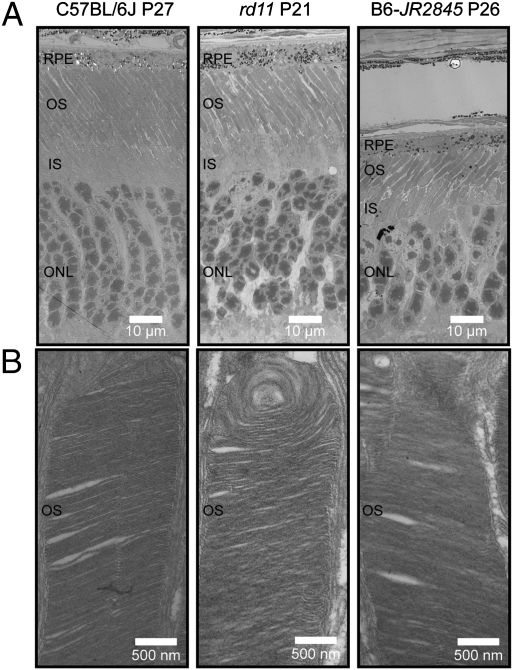
Histologically, photoreceptors appeared to develop normally in both strains of mice. At P21, no substantial degeneration was observed in rd11 mice. However, rapid photoreceptor degeneration was seen after P21. By P31, only four to five rows of photoreceptor nuclei were present instead of the usual 10 to 12 rows. At P47, only a single row of nuclei remained (Fig. 1C). B6-JR2845 mice demonstrated a similar pattern of degeneration; however, more than one row of nuclei was still present by P47 (Fig. 1C). Transmission electron microscopy (TEM) revealed outer segments in retinal photoreceptors of both strains (Fig. 2). However, the photoreceptor nuclei of rd11 and B6-JR2845 did not appear to be as ordered as B6 control mice. B6-JR2845 retinas showed thinning of the outer nuclear layer at P26, confirming a rapid degeneration in these mice (Fig. 2).
Fig. 2.
Photoreceptor disk formation is present in rd11 and B6-JR2845 mice. Retinas from C57BL/6J (B6), rd11, and B6-JR2845 mice were examined by TEM. (A) Nuclei and rod photoreceptor structure appeared to be less densely arranged in mutant mice than in WT (B6). (B) Photoreceptor outer segments were made in both rd11 and B6-JR2845 mice, suggesting that the mutation observed did not have an effect on the generation of this structure.
Linkage Analysis and Candidate Gene Screen.
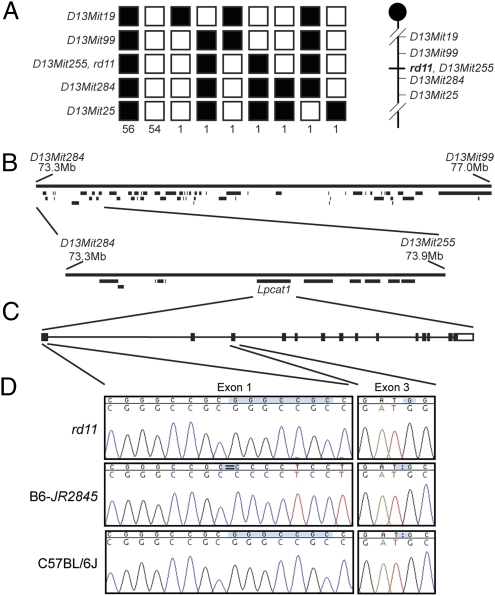
To localize the genetic defect, we performed linkage analysis of rd11 mice and identified a locus on mouse chromosome 13 between markers D13Mit99 and D13Mit284 (Fig. 3A). This critical genomic region corresponds to 3.7 Mb with 62 potential candidate genes (Fig. 3B) and is equivalent to human chromosome 7q35 or 5p15. Analysis of additional mice narrowed the genetic locus to flanking markers D13Mit284 and D13Mit255 within an interval of 0.6 Mb harboring 11 genes. Because of established importance of calcium and high concentration of lipids in photoreceptors (14–16), we selected Lpcat1 as a candidate gene because of its putative Ca2+ binding and AT properties. Sequencing of Lpcat1 in rd11 mice revealed a homozygous one-nucleotide insertion in exon 3 (c.420–421insG) (Fig. 3D). The WT mouse LPCAT1 protein consists of 534 amino acids; however, the frame-shift in rd11 mice is predicted to result in an abnormal protein after amino acid 140 and a stop after residue 178. Examination of B6-JR2845 DNA showed a homozygous deletion of seven nucleotides in exon 1 (c.14–20delGCCGCGG) (Fig. 3D), which is predicted to result in a frame-shift after codon 8 and a stop codon after 21. These two DNA mutations were not observed in nine WT mouse strains (Fig. S2).
Fig. 3.
Mutations causing rd11 and B6-JR2845 phenotype are located within Lpcat1. (A) Haplotypes of rd11 mice. Fifty-six homozygous and 54 heterozygous rd11 mouse genotypes are shown as black and white blocks, respectively, over five markers. Individual mice with recombinations are as shown. The initial analysis suggested breakpoints at markers D13Mit284 and D13Mit99. (B) Physical map of the genetic region between D13Mit284 and D13Mit99. Further haplotyping narrowed the genetic interval between markers D13Mit284 and D13Mit255. Within this region were 11 candidate transcripts, including Lpcat1. (C) Intron-exon structure for the Lpcat1 gene. Coding sequences are shown as filled boxes and noncoding regions are unfilled. (D) Sequence analysis of Lpcat1 gene in rd11, B6-JR2845 and C57BL/6J (B6) mice. The rd11 strain contained an insertion of a G residue in exon 3. B6-JR2845 mice harbored a seven base-pair deletion in a GC-rich repeat region in exon 1. Neither mutation was observed in B6 mice (current figure) or in eight other control strains (Fig. S2).
Lpcat1 Expression Analysis.
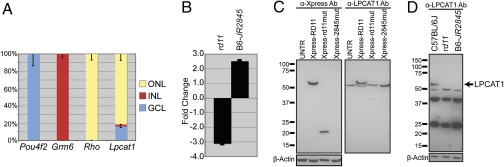
RNA analysis of a human multiple tissue Northern blot identified LPCAT1 RNA in all tissues examined (heart, brain, liver, skeletal muscle, kidney, and pancreas) with maximum expression in placenta and lung (Fig. S3). Lpcat1 transcripts were detectable by RT-PCR in GFP-tagged flow-sorted rod photoreceptors and in mouse retina from embryonic day 12 through 4 mo. (Fig. S3). Quantitative RT-PCR (qRT-PCR) of laser capture microdissected nuclei from retinal outer, inner, and ganglion cell layers suggested broad expression of Lpcat1 in mouse retina (Fig. 4A).
Fig. 4.
Expression of Lpcat1 in WT and mutant retina. (A) Quantitative RT-PCR analysis was performed with Lpcat1 from laser-capture microdissected cells collected from glangion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). Samples were normalized to Hprt. For each gene tested, ΔCt qRT-PCR values were summed and displayed in graphical format. POU class 4 homeobox 2 (Pou4f2), glutamate receptor, metabotropic 6 (Grm6), and Rhodopsin, (Rho) were used as controls for GCL, INL, and ONL, respectively. Lpcat1 was present in all nuclear layers. Error bars show SD of the mean. (B) Quantitative RT-PCR of retinal RNA from C57BL/6J, rd11, and B6-JR2845 mice to test for nonsense-mediated decay. Lpcat1 expression fold changes of −3.1 and 2.5 were observed in rd11 and B6-JR2845 mice, respectively, compared with C57BL/6J control. (C) Western blot of HEK 293T cells transfected with Xpress-tagged WT or mutant Lpcat1 cDNA. UNTR, untransfected. Lpcat1 cDNA containing the rd11 mutation generated a truncated protein product and no protein was observed with the B6-JR2845 mutation. The same immunoblot incubated with anti-LPCAT1 antibody demonstrated an endogenous band at the expected size in all lanes with a higher band corresponding to the Xpress-tagged LPCAT1 protein in the Xpress-RD11 transfected lane. (D) Immunoblot of retinas from C57BL/6J (adult), rd11 (P22), and B6-JR2845 (P21) mice. Anti-LPCAT1 antibody showed cross-reactivity to multiple proteins in addition to one at the expected size (59 kDa). The 59-kDa band was observed in WT mice, but was not present in the rd11 or B6-JR2845 lanes. β-Actin was used as a protein loading control (C and D).
Effect of Mutations on Lpcat1 RNA and Protein.
Premature stop codon in transcripts can lead to nonsense-mediated decay (17). RT-PCR analysis showed a 3-fold decrease in the expression of Lpcat1 in rd11 retina compared with B6 control mice (Fig. 4B), consistent with nonsense-mediated decay. On the other hand, a 2.5-fold increase was detected in retinal Lpcat1 transcripts from B6-JR2845 mice. Interestingly, the mutation in B6-JR2845 mice is within a GC-rich region, and a deletion can therefore enhance Lpcat1 transcription by altering secondary structure.
To examine the effect of mutations on LPCAT1 protein, we transfected WT and mutant Lpcat1 cDNA in pcDNA4c vector in HEK293T cells. Immunoblot analysis of transfected cell extracts using Xpress antibody showed a WT LPCAT1 protein of ∼64 kDa. The rd11 mutation produced a truncated protein around 20 kDa, whereas no protein was detected with B6-JR2845 mutation (Fig. 4C). The anti-LPCAT1 antibody detected endogenous LPCAT1 protein in all lanes. As expected, this protein was smaller than Xpress-tagged LPCAT1 obtained with the WT expression construct (Fig. 4C). The anti-LPCAT1 antibody identifies LPCAT1 protein in immunoblots of WT mouse retina but not of rd11 and B6-JR2845 mutants (Fig. 4D).
Lipid Analysis.
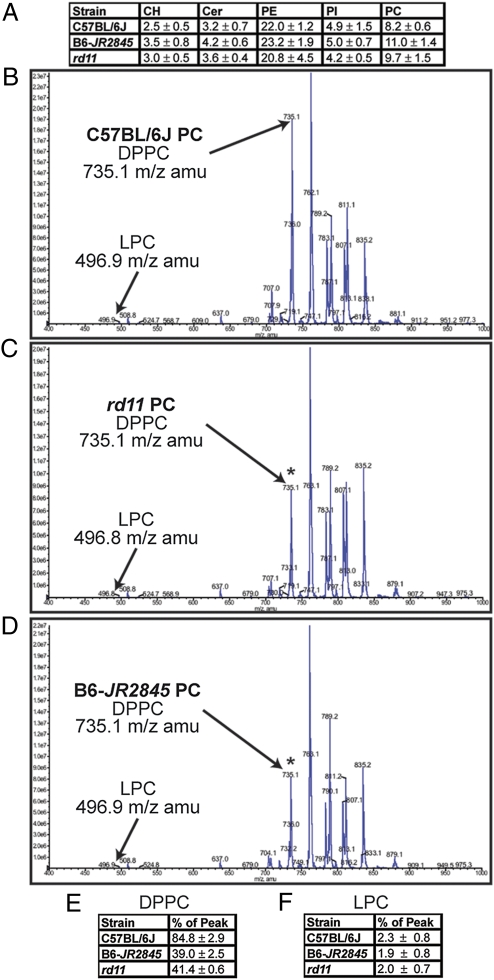
As LPCAT1 enzyme converts LPC to DPPC, we predicted that loss of Lpcat1 should affect retinal lipid composition. Hence, HPLC and MS analysis was used to profile lipid extracts from pooled sets of retina from B6, rd11, and B6-JR2845 mice. HPLC weight-for-weight analysis of lipid headgroups did not reveal a substantive difference between the strains (Fig. 5A). Additional experiments showed that within the phosphatidylcholine (PC) head group, the ratio of DPPC to the peak lipid class (C34:1) was reduced from an average of 84% in B6 mice to 38 to 41% in rd11 or B6-JR2845 mice (n = 4 litters for each strain) (Figs. 5 B–E). A concomitant increase in another PC lipid subclass including LPC was not observed (Figs. 5 B–D). These data suggest that loss of Lpcat1 has a detrimental effect on DPPC production in the retina.
Fig. 5.
Mutations in Lpcat1 result in reduced retinal levels of DPPC. (A) HPLC analysis showing percent weight for weight. Retinal lipids from four litters of each strain were extracted and subjected to HPLC analysis. Averages of CH, Cer, PE, PI, and PC lipid groups are shown as a percent weight for weight (wt/wt) with SE. No substantive difference in the PC lipid group was observed among C57BL/6J (B6), B6-JR2845 or rd11 mice. CH, cholesterol; Cer, ceramide, PE, phosphatidylethanolamine, PI, phosphatidylinositol, PC, phosphstidylcholine. (B–D) Retinal lipid extracts from B6 (B), rd11 (C), and B6-JR2845 (D) mice were examined by HPLC and MS. Within the phosphatidylcholine headgroup class, the m/z of 735.1, corresponding to DPPC, was substantially reduced relative to the peak lipid m/z observed in both rd11 and B6-JR2845. The observed loss was significant when compared with B6 (t test, P < 0.001). (E) DPPC content expressed as a percentage (%) of peak lipid. PC lipids from retinal extracts were subjected to MS/MS analysis. The peak corresponding to DPPC was compared with the peak lipid class in the PC headgroup and expressed as a percentage of peak. The data presented is an average of four sets of extracts from each strain with SE shown. A student's t test showed a significant difference between B6 and B6-JR2845 (P = 7.63 × 10−5), and between B6 and rd11 (P = 5.54 × 10−4), but not between B6-JR2845 and rd11 (P = 0.223).
As mutant mice exhibited lower retinal levels of DPPC, we hypothesized that adding DPPC to their diet may have a beneficial effect. Breeding cages were therefore supplemented with 2 g/kg DPPC in mouse chow, and the resulting pups were examined. Retinal histology of adult rd11 or B6-JR2845 mice supplemented with DPPC chow did not demonstrate any significant change from mutants fed with unsupplemented chow (Fig. S4). We therefore checked if dietary supplementation of DPPC affects retinal lipid composition. No change in DPPC levels was observed in supplemented P22 rd11 mouse retina compared with unsupplemented rd11 mutants (n = 3) (Fig. S5).
As LPCAT1 may have a role in the noninflammatory pathway, we decided to measure PAF C16 and lyso-platelet activating factor (Lyso-PAF) C16. We observed a lower amount of both lipids in mutant mice than WT (n = 3 for each strain) (Table S1). Notably, some measurements of Lyso-PAF were below our threshold of detection and were calculated as being absent from the respective sample. This result may lead to a lower estimate of Lyso-PAF in our analysis. Although lower amounts of PAF are consistent with the loss of LPCAT1 enzyme, it is unclear why PAF and Lyso-PAF are both reduced in amount in mutant mice.
Human Mutation Screen of LPCAT1.
Based on the retina-specific phenotype of Lpcat1 mouse mutants, we were encouraged to perform a mutation screen of exons and boundary regions of LPCAT1 in patients with RP or LCA (Table S2). A multicenter study of 233 patients failed to identify putative disease-causing sequence changes in the LPCAT1 gene. Eight intronic variations, distant from splice acceptor and splice donor sites, were observed. Several silent coding alterations (p.L53L, p.A133A, p.P287P, p.K426K, p.T455T, p.L459L) and common coding alterations (p.L77P, p.T125A, p.M427T) were detected. Five patients with different retinal dystrophy phenotypes showed nonsynonymous amino acid substitutions (p.R114W, p.H266Y, p.A480G, p.A485T, and p.S522L); however, as a second sequence change was not observed in these patients, it is difficult to assign causality or significance.
Discussion
Lipids have numerous biological roles in membrane bilayers, endocytosis, signaling, and neuroprotection (18–21). Retinal photoreceptors contain a modified primary cilia structure (called the “outer segment”) with highly specialized membrane discs that harbor opsin molecules and other proteins required for phototransduction, the first step in the process of vision. Photoreceptor outer segment discs are apically displaced daily following a circadian rhythm and phagocytosed by the retinal pigment epithelium (RPE) (22). The lipid environment of discs plays a critical role in rhodopsin activity and signaling (23, 24). Of the PC in disk lipids, 16:0 (palmitate) is second highest in amount, after 22:6 (docosahexaenoic acid, DHA) PC (16). Interestingly, PC and cholesterol disk lipid composition change over time. Cholesterol and 16:0 PC are present at high levels in the younger, more basal discs, and 22:6 PC is greater in older, more apical discs (15, 16). Pioneering observations of PC lipid distribution have recently been confirmed through newer methods (25). Interestingly, DHA from plasma is reduced in amount in patients with RP (26) and leads to reduced retinal function in animals when removed from their diet (27). Supplementation with DHA has been observed to be beneficial in some studies of RP in mouse and man (28, 29). However, the potentially positive impact of DHA might not be applicable across all forms of RP (30). Additionally, 22:6 is a substrate for neuroprotectin D1, an antiapoptotic molecule believed to be important for photoreceptor survival (31). Although the importance of DHA in the retina is established, the relationship between loss of DPPC and photoreceptor cell death has not been elucidated. Here we report the discovery of Lpcat1 as a retinal disease gene and show that changes in DPPC levels in the retina are associated with photoreceptor dysfunction and degeneration.
LPCAT1 was recently cloned by two different groups and implicated in several biological functions (32, 33). The LPCAT1 enzyme, lysophosphatidylcholine acyltransferase 1, preferentially transfers palmitoyl-CoA to LPC and is part of the lipid remodeling pathway (Lands’ cycle) used to generate lipid diversity after de novo synthesis (32, 33). Because of its high expression in lung, it was hypothesized that LPCAT1 generates DPPC, an important component of lung surfactant (32, 33). A recent knock-in mouse introducing the β-galactosidase gene after exon 9 of Lpcat1 had reduced AT activity compared with the WT. The reduced function of LPCAT1 with subsequent loss of DPPC was suggested to be critical for transitioning to air breathing in mice (34). In our investigations, we did not observe an obvious pathology in rd11 and B6-JR2845 mice consistent with the loss of surfactant; however, it is possible that such a phenotype may be induced under appropriate stress conditions. Additional study of rd11 and B6-JR2845 pups after birth may reveal the phenotype described above. In vitro experiments suggest the existence of compensatory mechanisms in lung epithelial cells when LPCAT1 is overexpressed (35). Whether or not such compensation occurs in rd11 and B6-JR2845 mice remains to be elucidated. As DPPC is not completely lost in retinas of rd11 and B6-JR2845 mice, it is possible that other LPCAT family members are able to partially compensate for the loss of LPCAT1.
In addition to its LPCAT function, LPCAT1 also acts as lyso-PAF AT and is thought to have a role in the noninflammatory PAF remodeling pathway (36). LPCAT1 is highly expressed in colorectal cancer cells and suggested to influence membrane fluidity, potentially playing a role in cancer cells’ progression toward metastasis (37). Recently, another study observed a decrease in Lpcat1 expression in retinas of diabetic mice (38). Our data further emphasize the importance of LPCAT1 in diverse cellular processes, with an as yet undefined new role in photoreceptor biology.
The retinal phenotypes of Lpcat1 mutant mice raise important mechanistic questions. The presence of outer segment discs in rd11 and B6-JR2845 retina suggests that LPCAT1 activity and appropriate DPPC levels are not essential for photoreceptor morphogenesis but may be critical for homeostasis and functional maintenance. Consistent with this, a recent qRT-PCR analysis shows that Lpcat1 expression is increased from P2 to P25 (39). Further investigations are required to precisely delineate the role of LPCAT1 in the retina and photoreceptors.
Photoreceptor-RPE interaction is crucial for the retinoid cycle during phototransduction and two critical proteins of this cycle are lecithin retinol AT and RPE65 (40). DPPC is involved in the formation of retinyl esters through lecithin retinol AT (41, 42). DPPC is also thought to act as a palmitoylation switch for RPE65 (43), although this hypothesis is controversial (44). We therefore tested the two hypotheses through the measurement of retinyl esters and by examining putative soluble and membrane-bound RPE65 isoforms, respectively, in whole eye and RPE of WT and mutant mice. No significant change in retinyl ester content or RPE65 localization was observed (Fig. S6), suggesting that altered DPPC levels do not have significant impact on these processes.
LPCAT1 plays a role in the noninflammatory response to oxidization and inflammation (38). The loss of LPCAT1 could lead to increased presence or slower enzymatic processing of LPC that, in turn, can lead to membrane disruption (45) and Ca2+ influx (46), either of which may then contribute to photoreceptor cell death. As we did not observe an increase in LPC amount, this is unlikely to be the cause of disease in these mice. LPCAT1 loss may also lead to slower alkyl-PC formation, part of a noninflammatory PAF inactivation pathway (38). This hypothesis would predict that increased levels of lyso-PAF would be generated, allowing for a greater inflammatory response after light damage. Inflammation and immune response play critical roles in etiology of age-related macular degeneration (47–49) and retinopathies (50). LPCAT1’s role in the noninflammatory pathway would lead to a decrease in PAF and an increase in Lyso-PAF (36); however, lower amounts of PAF and Lyso-PAF were observed in both mutant strains compared with WT mice. Further research is needed to understand the underlying biochemical mechanism of photoreceptor cell death caused by the loss of LPCAT1 function.
Because the addition of DHA to diet had a beneficial effect on retinal degeneration (28), we performed a similar experiment using DPPC supplementation. In our studies, the supplemented diet did not change retinal DPPC content or improve pathology in rd11 or B6-JR2845 mice. We suggest that a larger dietary amount of DPPC, another form of lipid, or a different intake method might be needed to rescue the Lpcat1 mutant phenotype. Alternatively, local synthesis of DPPC is required to meet photoreceptor needs.
In conclusion, we have identified a unique gene for retinal dystrophy in mice and highlighted the importance of maintaining specific lipid profiles in photoreceptors. A few other human retinal dystrophy genes (ELOVL4, ABCA4, REP-1, and Prominin) are believed to be involved in lipid metabolism, transport, protein lipidation, and formation of membrane protrusions (51–56). Although we could not establish disease causality in our patient cohort, LPCAT1 remains a strong candidate gene for human retinal degeneration because of the importance of DPPC and lipid metabolism in retina. It is possible that LPCAT1 mutations have a more pleiotropic effect in humans. Hence, further LPCAT1 mutation screening in patients with both syndromic and nonsyndromic retinal degeneration is desirable. Our studies show that elucidation of LPCAT1 function and biology is expected to provide fundamental insights into diverse physiological processes and disease.
Materials and Methods
Histological and ERG Analysis of rd11 and B6-JR2845 Mice.
Animal work was performed with approval from the National Eye Institute and The Jackson Laboratory Animal Care and Use Committees. Histology and ERGs were carried out as previously described (57).
Linkage Analysis of rd11 Mice.
Genotyping of mice and linkage analysis of rd11 mice were completed as previously described (57). Genetic markers defining the critical domain were D13Mit284 and D13Mit99. The physical region corresponding these markers outlines a 3.7-Mb region containing 62 genes. Additional screening totaling 1,301 samples narrowed the disease locus to markers D14Mit255 and D13Mit284. This genetic area consists of 11 genes, including Lpcat1.
Mouse Mutation Screen.
We examined BC005662/Aytl2/Lpcat1 as a candidate gene for the rd11 and B6-JR2845 mice. Lpcat1 was selected because of its putative Ca+ binding domain and AT activity. Primers for all 14 exons are included in Table S3. Mouse strains A/J, AE/J, BALB/cJ, C3H/cJ, C57BL/6J, CAST1/EiJ, DBA/2J, MOLC/Rk, and NON/Lt were used as controls.
Transmission Electron Microscopy.
C57BL/6J (B6), rd11 and B6-JR2845 mouse retinas at ages P27, P21, and P26, respectively, were prepared similar to that previously described (58). Samples were analyzed through the University of Michigan Electron Microscopy core.
Laser Capture Microdissection.
An enucleated mouse eye was flash-frozen in 100% OCT. Then, 10-μm thick sections were placed on PALM Membrane slides nuclease-free (Zeiss), dehydrated in 70% alcohol for 2 min, and stained with Cresyl Violet staining (LCM Staining Kit, Ambion) for 30 s and dehydrated in 70 and 100% alcohol for visualization of cell nuclei. The sections were microdissected using the laser capture method [PALM Microlaser System; Laser Microdissecton and Pressure Catapulting (LMPC), Zeiss]. The cells were collected from the GCL, INL, and ONL. Cells were stored at −80 °C.
RNA-Based Analyses.
Laser-capture microdissected cellular RNA was extracted through standard methods (Qiagen), reverse-transcribed, and amplified using WT-Ovation One-Direct kit (Nugen). TaqMan Gene Expression Assays (Applied Biosystems) using Lpcat1 (Mm00461015_m1) were carried out on each amplified cDNA according to the manufacturer's protocol. POU class 4 homeobox 2 (Pou4f2, Mm00454754_s1), glutamate receptor metabotropic 6 (Grm6, Mm00841148_m1), and Rhodopsin, (Rho, Mm00520345_m1) were used as controls for the GCL, INL, and ONL, respectively. Samples were normalized to Hprt (Mm01310747_g1). PCR amplifications were performed in triplicate. RT-PCR and Northern blot analysis on WT mouse retinal RNA and on a human multiple tissue Northern blot (Clontech) were carried out as previously described (59). Primers used for RT-PCR are listed in Table S3. For the nonsense-mediated decay test, qRT-PCR was performed in triplicate on three sets of P21- to P23-aged retina cDNA samples derived from each mouse strain (B6, rd11, B6-JR2845). Lpcat1 and Hprt TaqMan Gene Expression Assays were used as described above. The comparative CT, or 2−ΔΔCt, method of analysis was performed using Hprt for normalization of expression levels.
Immunoblot Analysis.
Lpcat1 cDNA was amplified and cloned into the pcDNA4c vector (Invitrogen) and the rd11 and B6-JR2845 mutations were introduced through site-directed mutagenesis. Primers used for cloning and mutagenesis reactions are listed in Table S3. HEK293T cell transfection, harvesting and immunoblotting were performed as previously described (59). Anti-Xpress monoclonal antibody (1:4,000; Invitrogen, 46–0528), anti–β-actin (1:5,000; Sigma-Aldrich, A2228) primary antibody and anti-AYTL2 (LPCAT1) antibody (1:750; Sigma-Aldrich, HPA022268) were used on the HEK293T immunoblot.
Retinas from adult B6, P22 rd11 and P21 B6-JR2845 mice were sonicated directly in 2× SDS buffer with protease inhibitor, and run out by SDS/PAGE. Anti-LPCAT1 antibody (1:750) was preadsorbed on an immunoblot with retinal extracts from P22 rd11, and P21 B6-JR2845 mice before being used experimentally. Mouse anti-rabbit HRP conjugated light chain specific antibody (1:4,000; Millipore, MAB201P) was used to detect the primary antibody. Mouse anti–β-actin antibody was used as a control for loading.
Retinal Lipid Extractions, HPLC, and MS Analysis.
Retinas from B6, rd11, and B6-JR2845 mice were collected at P21 to P24 and lipids were extracted using the Bligh and Dyer protocol (60). One litter of pups was pooled for each experiment and three sets of lipids were examined for each strain. Quantitative phospholipid analysis (weight % analysis) was performed by HPLC by Avanti Polar Lipids. Semiquantitative analysis of major phospholipid species although flow-infusion MS was also carried out by Avanti Polar Lipids. PAFs were separated in a Waters Corp. Symmetry 300 C-18 column (3.5 μm, 2.1 × 150 mm) using an Agilent Technologies Series 1200 HPLC equipped with a binary pump and automatic sample injector. The column was run at 0.1 mL/min and the PAFs separated using a gradient starting with 25% solvent A (20% acetonitrile, 80% water 5 mM ammonium acetate) and 75% solvent B (84% methanol, 16% methylene chloride, 1 mM ammonium acetate). The gradient increased linearly to 100% solvent B in 20 min and was maintained at 100% B until 50 min. The solvents were then changed to original conditions (25% B) for an additional 10 min to reequilibrate the column. The total run time was 60 min. Quantification of PAFs was performed by MS using a Waters/Micro mass QTOF micro equipped with an electron spray probe (ESi). The entire flow of the LC was diverted into the QTOF. The ESi probe settings were as follows: capillary voltage, 3,000 v; sample cone, 35; extraction cone, 3.0; source temp, 120 °C; desolation temp, 180 °C.
Human Mutation Screen.
We examined retinal dystrophy patients from the United States, Canada, and the United Kingdom. Human mutation screens were approved by the University of Michigan Institutional Review Board, McGill Research Ethics Board at Montreal Children’s Hospital, and Moorfields Eye Hospital Ethics Committee, respectively. Primers designed for this study are listed in Table S3. For the Michigan and United Kingdom study, LPCAT1 exon PCR products were amplified and sequenced through standard methods. The Canadian/McGill study tested a total of 50 patients (25 patients with LCA and 25 with autosomal recessive juvenile-onset RP). Patients were strongly selected as the then known seven LCA genes (GUCY2D, RPE65, AIPL1, CRX, CRB1, RPGRIP1, RDH12) were excluded by sequencing and all patients were consanguineous to improve chances of finding mutations. PCR products of each exon (including flanking introns) were mixed with equal amounts of normal control and examined by DHPLC. Trace profiles were examined by visual inspection of chromatograms by two independent observers and compared with the WT DNA fragment profile. Samples with aberrant profiles were subsequently sequenced.
Supplementary Material
Acknowledgments
We thank Jessica Chang, Mohammad Othman, and Renee Pigeon for assistance and the National Eye Institute Biological Imaging Core for equipment usage. Funding for this research was provided by the National Eye Institute Intramural program, National Institutes of Health Grant EY019943, Foundation Fighting Blindness, Research to Prevent Blindness, Canadian Institutes of Health Research, Foundation Fighting Blindness-Canada, Fonds de la Recherche en Santé Québec, Reseau de Vision, and Foundation for Retinal Research. G.E.T. was a recipient of a Fight for Sight Summer Student Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002897107/-/DCSupplemental.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Trifunovic D, et al. A high-resolution RNA expression atlas of retinitis pigmentosa genes in human and mouse retinas. Invest Ophthalmol Vis Sci. 2008;49:2330–2336. doi: 10.1167/iovs.07-1513. [DOI] [PubMed] [Google Scholar]
- 3.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125(2):151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang B, et al. Mouse models of ocular diseases. Vis Neurosci. 2005;22:587–593. doi: 10.1017/S0952523805225075. [DOI] [PubMed] [Google Scholar]
- 5.Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision Res. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowes C, et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 7.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhmedov NB, et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci USA. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Rattner A, Nathans J. Effects of L1 retrotransposon insertion on transcript processing, localization and accumulation: Lessons from the retinal degeneration 7 mouse and implications for the genomic ecology of L1 elements. Hum Mol Genet. 2006;15:2146–2156. doi: 10.1093/hmg/ddl138. [DOI] [PubMed] [Google Scholar]
- 10.Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 12.den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider NB, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24(2):127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 14.Sancho-Pelluz J, et al. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol. 2008;38:253–269. doi: 10.1007/s12035-008-8045-9. [DOI] [PubMed] [Google Scholar]
- 15.Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- 16.Albert AD, Young JE, Paw Z. Phospholipid fatty acyl spatial distribution in bovine rod outer segment disk membranes. Biochim Biophys Acta. 1998;1368(1):52–60. doi: 10.1016/s0005-2736(97)00200-9. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson P, et al. Nonsense-mediated mRNA decay in human cells: Mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci. 2010;67:677–700. doi: 10.1007/s00018-009-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9(2):162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 20.Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 21.Bazan NG. Cell survival matters: Docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.LaVail MM. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 23.Watts A, Davoust J, Marsh D, Devaux PF. Distinct states of lipid mobility in bovine rod outer segment membranes. Resolution of spin label results. Biochim Biophys Acta. 1981;643:673–676. doi: 10.1016/0005-2736(81)90365-5. [DOI] [PubMed] [Google Scholar]
- 24.Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73(1–2):159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 25.Hayasaka T, et al. Matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-based imaging mass spectrometry reveals a layered distribution of phospholipid molecular species in the mouse retina. Rapid Commun Mass Spectrom. 2008;22:3415–3426. doi: 10.1002/rcm.3751. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, et al. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33:2596–2602. [PubMed] [Google Scholar]
- 27.Leat WM, Curtis R, Millichamp NJ, Cox RW. Retinal function in rats and guinea-pigs reared on diets low in essential fatty acids and supplemented with linoleic or linolenic acids. Ann Nutr Metab. 1986;30(3):166–174. doi: 10.1159/000177190. [DOI] [PubMed] [Google Scholar]
- 28.Ebert S, et al. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J Neurochem. 2009;110:1863–1875. doi: 10.1111/j.1471-4159.2009.06286.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman DR, et al. A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. Am J Ophthalmol. 2004;137:704–718. doi: 10.1016/j.ajo.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Li F, et al. DHA does not protect ELOVL4 transgenic mice from retinal degeneration. Mol Vis. 2009;15:1185–1193. [PMC free article] [PubMed] [Google Scholar]
- 31.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: Significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: The Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–4881. doi: 10.1167/iovs.07-0918. biography 4864–4865. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Hyatt BA, Mucenski ML, Mason RJ, Shannon JM. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci USA. 2006;103:11724–11729. doi: 10.1073/pnas.0604946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi H, et al. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J Biol Chem. 2006;281:20140–20147. doi: 10.1074/jbc.M600225200. [DOI] [PubMed] [Google Scholar]
- 34.Bridges JP, et al. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J Clin Invest. 2010;120:1736–1748. doi: 10.1172/JCI38061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler PL, Mallampalli RK. Cross-talk between remodeling and de novo pathways maintains phospholipid balance through ubiquitination. J Biol Chem. 2009;285:6246–6258. doi: 10.1074/jbc.M109.017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem. 2008;283:11097–11106. doi: 10.1074/jbc.M708909200. [DOI] [PubMed] [Google Scholar]
- 37.Mansilla F, et al. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) overexpression in human colorectal cancer. J Mol Med. 2009;87(1):85–97. doi: 10.1007/s00109-008-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Han X, Shi Y. A regulatory role of LPCAT1 in the synthesis of inflammatory lipids, PAF and LPC, in the retina of diabetic mice. Am J Physiol Endocrinol Metab. 2009;297:E1276–E1282. doi: 10.1152/ajpendo.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tummala P, Mali RS, Guzman E, Zhang X, Mitton KP. Temporal ChIP-on-Chip of RNA-Polymerase-II to detect novel gene activation events during photoreceptor maturation. Mol Vis. 2010;16:252–271. [PMC free article] [PubMed] [Google Scholar]
- 40.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry RJ, Cañada FJ, Rando RR. Solubilization and partial purification of retinyl ester synthetase and retinoid isomerase from bovine ocular pigment epithelium. J Biol Chem. 1989;264:9231–9238. [PubMed] [Google Scholar]
- 42.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 43.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004;117:761–771. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Yuan Q, Li S, Travis GH. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J Biol Chem. 2007;282:20915–20924. doi: 10.1074/jbc.M701432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weltzien HU. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979;559:259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 46.Wilson-Ashworth HA, et al. Formation of transient non-protein calcium pores by lysophospholipids in S49 Lymphoma cells. J Membr Biol. 2004;200(1):25–33. doi: 10.1007/s00232-004-0691-x. [DOI] [PubMed] [Google Scholar]
- 47.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration—Emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: An immunologically driven disease. Curr Opin Investig Drugs. 2009;10:434–442. [PubMed] [Google Scholar]
- 49.Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: From genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: A review and summary. Semin Immunopathol. 2008;30(2):127–134. doi: 10.1007/s00281-008-0114-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27(1):89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 52.Agbaga MP, et al. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc Natl Acad Sci USA. 2008;105:12843–12848. doi: 10.1073/pnas.0802607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 54.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seabra MC, Ho YK, Anant JS. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem. 1995;270:24420–24427. doi: 10.1074/jbc.270.41.24420. [DOI] [PubMed] [Google Scholar]
- 56.Maw MA, et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9(1):27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- 57.Pang JJ, et al. Retinal degeneration 12 (rd12): A new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- 58.Bechtold LS, Smith RS. In: Systematic evaluation of the mouse eye. Smith RS, editor. Boca Raton: CRC Press; 2002. pp. 272–277. [Google Scholar]
- 59.Friedman JS, et al. Premature truncation of a novel protein, RD3, exhibiting subnuclear localization is associated with retinal degeneration. Am J Hum Genet. 2006;79:1059–1070. doi: 10.1086/510021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.