Abstract
Both increase and decrease of cardiac inward rectifier current (IK1) are associated with severe cardiac arrhythmias. Flecainide, a widely used antiarrhythmic drug, exhibits ventricular proarrhythmic effects while effectively controlling ventricular arrhythmias associated with mutations in the gene encoding Kir2.1 channels that decrease IK1 (Andersen syndrome). Here we characterize the electrophysiological and molecular basis of the flecainide-induced increase of the current generated by Kir2.1 channels (IKir2.1) and IK1 recorded in ventricular myocytes. Flecainide increases outward IKir2.1 generated by homotetrameric Kir2.1 channels by decreasing their affinity for intracellular polyamines, which reduces the inward rectification of the current. Flecainide interacts with the HI loop of the cytoplasmic domain of the channel, Cys311 being critical for the effect. This explains why flecainide does not increase IKir2.2 and IKir2.3, because Kir2.2 and Kir2.3 channels do not exhibit a Cys residue at the equivalent position. We further show that incubation with flecainide increases expression of functional Kir2.1 channels in the membrane, an effect also determined by Cys311. Indeed, flecainide pharmacologically rescues R67W, but not R218W, channel mutations found in Andersen syndrome patients. Moreover, our findings provide noteworthy clues about the structural determinants of the C terminus cytoplasmic domain of Kir2.1 channels involved in the control of gating and rectification.
Keywords: cardiac IK1, Kir2.2 channel, Kir2.3 channel, Andersen mutations, inward rectifying channel
The cardiac inwardly rectifying K+ current (IK1) stabilizes resting membrane potential (RMP) close to the reversal potential of K+ (EK) and shapes the final repolarization phase of the action potential (AP) (1). Three inwardly rectifying channels (Kir2.1, Kir2.2, and Kir2.3) contribute to IK1 in the human heart assembled as homo- and/or heterotetramers (2). Experimental data suggest that in humans, Kir2.1 is the major isoform underlying ventricular IK1, whereas its relative contribution to atrial IK1 seems to be lower (3). The strong inward rectification of Kir2.x channels, i.e., the preferential conduction of inward compared with outward current, depends on the binding of intracellular Mg2+ and polyamines to the cytoplasmic pore and to the inner vestibule of the channel (4).
Gain- and loss-of-function mutations in the gene that encodes Kir2.1 (KCNJ2) have been reported, and both the IK1 increase and decrease produced by these mutations are associated with severe ventricular arrhythmias (1). Furthermore, experimental data showed that as the amplitude of the outward component of the IK1 increases, the frequency of the fast and stable reentry of spiral waves (rotors) increases. Indeed, the importance of IK1 in the establishment of rotors and ventricular fibrillation dynamics has been shown (5).
Flecainide is a class I antiarrhythmic drug that, besides its Na+ channel-blocking properties, exhibits class III antiarrhythmic effects [i.e., prolongs AP duration (APD) and refractoriness] at the atrial but not at the ventricular level (6, 7). Flecainide is widely used to suppress recent onset atrial fibrillation (AF) (8), though it is associated with an increased risk of ventricular proarrhythmia, especially in patients with coronary artery disease and/or heart failure. Conversely, in a limited number of patients, it has been reported that flecainide is effective in controlling ventricular tachycardia associated with the autosomal dominant trait Andersen syndrome (AS), which is produced by loss-of-function mutations in KCNJ2 (9, 10).
We hypothesized that a putative differential flecainide effect on atrial and ventricular IK1 (blockade of atrial and increase of ventricular IK1) can account for the selective prolongation of the atrial APD, the ventricular proarrhythmic effects, and the antiarrhythmic effects in the AS patients. Therefore, we have analyzed the flecainide effects on the currents generated by human WT and mutated Kir2.1, Kir2.2, and Kir2.3 channels (IKir2.x) and compared its effects on atrial and ventricular IK1. Acutely applied flecainide selectively increased ventricular IK1 and IKir2.1, an effect determined by the presence of Cys311 at the cytoplasmic domain of Kir2.1 channels. Binding of flecainide reduced the Kir2.1 polyamine blockade, thus reducing the inward rectification. Additionally, incubation with flecainide increased functional ion channel density, an effect also determined by Cys311. Overall, the findings show that Kir2.1 channels can be affected through the pharmacological modulation of their polyamine blockade by a therapeutically used drug.
Results
Flecainide Increases Kir2.1 Currents.
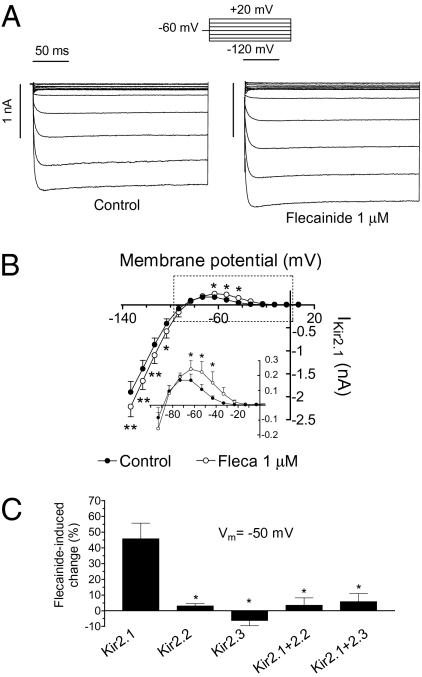
Figure 1A shows IKir2.1 traces recorded in transiently transfected Chinese hamster ovary (CHO) cells by applying 250-ms pulses from −60 mV to potentials ranging −120 and +20 mV in the absence and presence of 1 μM flecainide. Flecainide increased IKir2.1 at voltages negative (16.5 ± 2.4% at −120 mV) and, more importantly, at voltages positive to EK (45.8 ± 9.8% at −50 mV, n = 8, P < 0.05; Fig. 1 A and B). These effects were completely reversible upon washout (Fig. S1). The concentration that produces the half-maximum effect (EC50) and the maximum effect (Emax) were calculated by fitting the Hill equation to the increase produced by different flecainide concentrations and averaged 0.8 ± 0.01 μM (nH= 1.6 ± 0.4) and 22 ± 1.9% at −120 mV, respectively (Fig. S1). At −50 mV, the EC50 and the Emax averaged 0.4 ± 0.01 μM (nH = 2.2 ± 0.2) and 53.9 ± 3.6%, respectively (Fig. S1). Therefore, flecainide preferentially increased the outward IKir2.1 generated at physiological potentials.
Fig. 1.
Flecainide increases IKir2.1. (A) IKir2.1 traces recorded by applying the protocol shown in the absence and presence of flecainide. (B) I–V curves for currents measured at the end of the pulses. (Inset) Data at potentials positive to EK in an expanded scale. *P < 0.05 and **P < 0.01 vs. control. (C) Flecainide-induced change on the current recorded at −50 mV in cells expressing homotetramers of Kir2.1, Kir2.2, or Kir2.3 channels or heterotetramers of Kir2.1 + Kir2.2 and Kir2.1 + Kir2.3. *P < 0.05 vs. Kir2.1. Each point/bar represents the mean ± SEM of five or more experiments.
To determine the effect of flecainide in a physiologically relevant setting, we used an epicardial AP voltage-clamp protocol (Fig. S2). Under these conditions, flecainide increased the charge crossing the membrane estimated from the integral of the current traces, reaching 137 ± 28% at 1 μM (n = 8; Fig. S2).
Flecainide Does Not Modify Kir2.2 and Kir2.3 Currents.
Because Kir2.2 and Kir2.3 proteins also contribute to cardiac IK1, the effects of flecainide on these channels were studied. The findings showed that flecainide failed to modify IKir2.2 and IKir2.3 (P > 0.05 vs. control, n = 10; Fig. 1C and Fig. S3). We also studied the flecainide effects on cells cotransfected (1:1 ratio) with both Kir2.1 and Kir2.2 (Kir2.1/Kir2.2) and with Kir2.1 and Kir2.3 (Kir2.1/Kir2.3). Kir2.1/Kir2.2 and Kir2.1/Kir2.3 currents displayed activation kinetics significantly different from those of the respective homotetrameric channels (Fig. S4), demonstrating the heterotetrameric nature of the channels. Under these conditions, flecainide failed to increase inward and outward currents (P > 0.05 vs. control, n = 10; Fig. 1C and Fig. S4), demonstrating that its effects were only apparent in channels composed of four Kir2.1 subunits.
Flecainide Increases IK1 in Ventricular but Not in Atrial Myocytes.
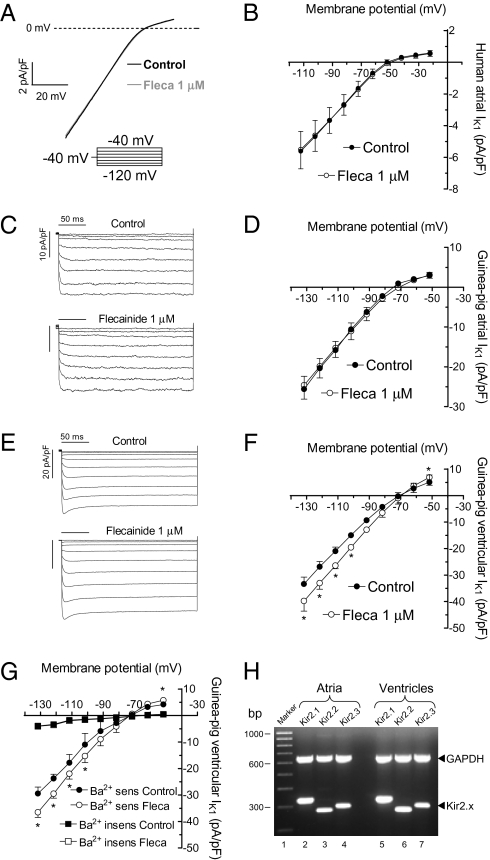
Considering that flecainide selectively increases IKir2.1, we surmised that flecainide will differentially affect atrial and ventricular IK1. Figure 2 A and B show original recordings and mean I–V relationships for IK1 obtained in isolated human atrial myocytes by applying a voltage ramp from −100 to −10 mV in the absence and presence of flecainide (1 μM). Flecainide did not modify either the inward (−5.5 ± 1.2 vs. −5.6 ± 1.2 pA/pF at −100 mV; P > 0.05, n = 5) or outward (0.5 ± 0.1 vs. 0.6 ± 0.1 pA/pF at −10 mV; P > 0.05) current.
Fig. 2.
Flecainide increases ventricular but not atrial IK1. Voltage ramp (800 ms) from −100 to −10 mV (A) and I–V curves (B) for human atrial IK1 in the absence and presence of flecainide. Representative IK1 traces recorded in guinea-pig atrial (C) or ventricular (E) myocytes by applying the protocol shown. I–V curves for guinea-pig atrial (D) or ventricular (F) IK1. (G) I–V of the Ba2+-sensitive and Ba2+-insensitive currents recorded in ventricular myocytes before and after application of flecainide. (H) mRNA expression level of Kir2.x channels in guinea-pig atrial and ventricular samples. First lane shows molecular weight marker (1,000–100 bp). Lanes 2–4 and 5–7 show Kir2.1 (325 bp), Kir2.2 (291 bp), and Kir2.3 (303 bp) mRNA expression in atrial and ventricular tissue, respectively. GAPDH gene was used as internal standard. Each point represents the mean ± SEM of four experiments in each group. *P < 0.05 vs. control.
Because human ventricular myocytes were not available, we compared the effects of flecainide on the IK1 recorded in guinea-pig atrial and ventricular myocytes (Fig. 2 C–F). As in humans, in guinea-pig ventricles, Kir2.1 expression is higher than in the atria (1, 11, 12). In ventricular myocytes, 1 μM flecainide significantly increased both the inward (19.5 ± 3.2% at −120 mV) and outward (38.0 ± 9.5% at −40 mV) current (P < 0.05, n = 4) without modifying atrial IK1 (P > 0.05, n = 4; Fig. 2 C–F). All these experiments were performed in the presence of atropine (1 μM) and glibenclamide (10 μM) to block the acetylcholine-activated component (IKACh) and the ATP-sensitive (IKATP) inward rectifier currents, respectively. Identical findings were obtained when the ventricular IK1 was measured as the Ba2+-sensitive current (Fig. 2G).
A previous report proposed that Kir2.1 is the only Kir2.x channel expressed in guinea-pig atria (11). Conversely, our findings suggested that guinea-pig ventricular IK1 is mainly carried by Kir2.1 homotetramers, whereas Kir2.x heterotetramers generate atrial IK1. For testing whether Kir2.1 is the only Kir2.x channel present in the atria, we analyzed the mRNA expression of Kir2.x in guinea-pig atria and ventricles. In agreement with another report (12), we could detect Kir2.1, Kir2.2, and Kir2.3 mRNA in both the atria and the ventricles (Fig. 2H).
Flecainide Increases Open Probability of Kir2.1 Channels.
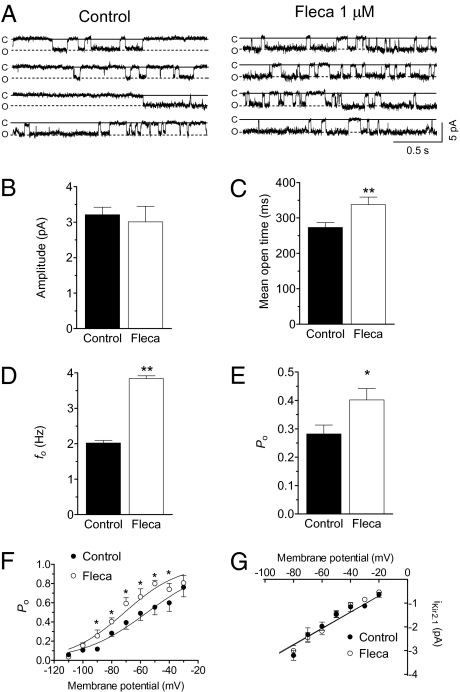
Figure 3A shows single-channel recordings in a CHO cell expressing Kir2.1 channels by applying 10-s pulses to −80 mV from a holding potential of 0 mV in the absence and presence of 1 μM flecainide. In control conditions, Kir2.1 channel activity was characterized by few and long events, leading to an opening frequency (fo) of 2.0 ± 0.1 Hz and a mean open probability (Po) of 0.27 ± 0.01 (n = 6; Fig. 3 D and E). Flecainide did not modify unitary current amplitude (Fig. 3B) but changed channel gating by significantly increasing the mean open time and fo (Fig. 3 C and D), which eventually resulted in a significant increase in the Po (Fig. 3E). Figure 3F compares the voltage dependence of the Po (Po–V curves) in control conditions and in the presence of flecainide. As described, Po decreased as the membrane potential became more negative (1, 13). Flecainide shifted the midpoint of the curve to more negative potentials (from −55.3 ± 1.8 to −71.1 ± 2.4 mV; P < 0.05) without modifying the slope. In Fig. 3G, the voltage dependence of the single-channel current amplitude is depicted. Flecainide did not modify the slope conductance values (γ = 34.7 ± 1.7 pS) yielded by the fit of a linear function to the data. All of these effects resemble those produced by PIP2 (14), suggesting that flecainide potentiates the activating PIP2 effects on the channel. To test this hypothesis, we studied the effects of flecainide on L222I Kir2.1 channels, a mutation that decreases the channel affinity for PIP2 but renders functional channels (14). Fig. S5 shows that the decrease of the channel affinity for PIP2 suppressed the flecainide IKir2.1- increasing effects at potentials negative to the EK, whereas increasing effects at positive potentials were still apparent.
Fig. 3.
Flecainide 1 μM increases mean open time, fo, and Po, of Kir2.1 channels. (A) Single-channel recordings under control conditions and after perfusion with flecainide. Closed- and open-channel levels are indicated by C and O, respectively. Unitary current amplitude (B), mean open time (C), fo (D), and Po (E) in the absence and presence of flecainide. (F) Po–V in control conditions and in the presence of flecainide. Solid lines represent the fit of a Boltzmann function to the data. (G) Single-channel current-voltage relationships in the absence and presence of flecainide. Solid lines represent the fit of a linear function to the data. Each point/bar represents the mean ± SEM of six experiments. *P < 0.05 and **P < 0.01 vs. control.
Flecainide Increases Kir2.1 Currents by Decreasing the Polyamine Blockade.
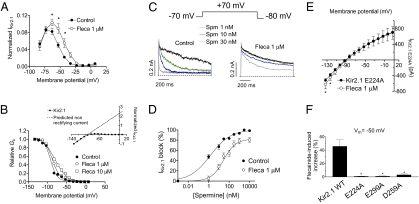
Figure 4A illustrates the I–V curve normalized to the amplitudes at −120 mV in control conditions and in the presence of 1 μM flecainide at potentials positive to EK. As can be observed, flecainide induced a marked increase of the outward IKir2.1 and shifted the potential at which the outward current peaks, suggesting that it decreased the channel rectification.
Fig. 4.
Flecainide decreases polyamine blockade. (A) IKir2.1 normalized to the amplitudes at −120 mV at potentials positive to EK in control conditions and in the presence of flecainide. (B) Mean relative Gc in control conditions and in the presence of flecainide. Solid lines represent the fit of a Boltzmann function to the data. (Inset) Mean I–V curve and the current predicted, assuming a linear unblocked current in control conditions. (C) Current traces recorded at +70 mV in excised inside-out patches from HEK-293 cells expressing Kir2.1 channels in control conditions and after cytoplasmic surface application of Spm in the absence and presence of flecainide. Dashed lines represent the zero current level. (D) Percentage of current inhibition at +70 mV in excised inside-out patches as a function of Spm concentrations in the absence and presence of flecainide. (E) I–V curves for E224A Kir2.1 channels in the absence and presence of flecainide. (F) Flecainide-induced change on the current recorded at −50 mV in CHO cells expressing WT, E224A, E299A, and D259A Kir2.1 channels. *P < 0.05 vs. control. Each point/bar represents the mean ± SEM of five or more experiments.
The degree of rectification of Kir2.1 channels was estimated as the relative chord conductance (Gc; Fig. 4B) in control conditions and in the presence of flecainide. Flecainide shifted the midpoint of the curves to more positive potentials (from −91.9 ± 1.8 to −87.1 ± 1.8 and −80.5 ± 1.4 mV at 1 and 10 μM flecainide, respectively; P < 0.05) and decreased the steepness of rectification, reflected by a lower z value in the presence of flecainide (2.4 ± 0.3 and 1.6 ± 0.6 for 1 and 10 μM flecainide, respectively) than in control conditions (3.0 ± 0.5; Fig. 4B).
Because the strong rectification of Kir2.1 channels is explained by the voltage-dependent block produced by intracellular polyamines (4), our findings suggested that flecainide decreases the affinity of the channel for polyamines. Therefore, flecainide effects were further tested in excised inside-out macropatches in the presence of increasing concentrations of spermine (Spm, 1 nM–10 μM). Figure 4C shows that in control conditions, Spm produced a concentration-dependent block of the outward current at +70 mV. The concentration that produces the half-maximum inhibition (IC50) was 1.5 ± 0.1 nM (Fig. 4D; n = 8). In the presence of flecainide (1 μM), blockade produced by all of the Spm concentrations tested was decreased, an effect that shifted rightward (29.0 ± 2.1 nM, n = 8) the concentration-effect curve of Spm (Fig. 4D). Importantly, in the presence of flecainide, Emax produced by Spm reached saturation at 82.1 ± 5.5%.
Finally, we analyzed the effects of flecainide on E224A, D259A, and E299A Kir2.1 channels, three cytoplasmic residues that are important in determining the extent of Kir2.1 inward rectification (15). Figure 4E shows the E224A I–V relationship in the absence and presence of flecainide. The mutation disrupted the rectification mechanism of the channel and completely abolished the flecainide increase at potentials positive to the EK. Identical findings were obtained when analyzing the effects of flecainide on D259A and E299A Kir2.1 channels (Fig. 4F).
Cys311 Is Critical for the Flecainide-Induced Increase of IKir2.1.
To explore the putative binding site of flecainide within Kir2.1 channels, a molecular model was developed. A blind docking for flecainide with a full-length channel composed of the crystal structure of one cytoplasmic domain of Kir2.1 (15) and a modeled transmembrane domain based on KirBac1.1 crystal structure was performed. The findings showed that the most practical binding site on Kir2.1 channels was located on the βI strand within the C terminus of the cytoplasmic domain (Fig. S6 and Table S1). In contrast, when blind docking was performed on Kir2.2 and Kir2.3, none of the conformations obtained were located on the βI (Fig. S7). Thereafter, flecainide was manually docked into the Kir2.1 βI strand. In the lowest-energy pose (−11.3 kcal/mol), flecainide was predicted to bind strongly to Cys311 by forming three hydrogen bridge bonds (one between the carbonyl oxygen atom of flecainide and the thiol group of the Cys residue, and two between the carbonyl oxygen atom of the amino acid and the nitrogen atoms of the amide and of the piperidine groups of flecainide (Fig. S6). Interestingly, the βI strand is highly conserved among Kir2.x channels, Cys311 being the only different residue between Kir2.1 and Kir2.2 and Kir2.3 (Fig. S6).
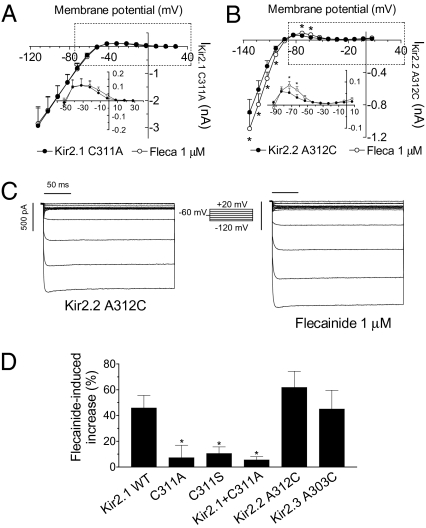
When Cys311 was substituted by Ala (C311A), the residue present in Kir2.2 and Kir2.3 channels at equivalent position (312 and 303, respectively), the flecainide-induced increase was abolished at potentials negative and positive to EK (Fig. 5 A and D). Similar findings were obtained when it was substituted by Ser (C311S) (Fig. 5D). Flecainide also failed to increase IKir2.1 generated in cells cotransfected with both WT and C311A Kir2.1 channels (Fig. 5D). As the Cys-to-Ala substitution abrogates flecainide sensitivity of Kir2.1, a reverse mutation in Kir2.2 and Kir2.3 ought to have the opposite effect. Here we show that this is indeed the case. Flecainide significantly increased the inward and the outward A312C IKir2.2 (Fig. 5 B and C; n = 5) and A303C IKir2.3 (Fig. 5D; n = 5). These findings verify and underscore the importance and specificity of the Cys residue for flecainide sensitivity of Kir2.1 channels.
Fig. 5.
Cys311 is critical for the flecainide-induced increase of IKir2.1. The I–V curves for currents recorded in cells expressing C311A Kir2.1 (A) and A312C Kir2.2 (B) channels in the absence and presence of flecainide. (C) Current traces recorded in a cell expressing A312C Kir2.2 channels in control conditions and in the presence of flecainide. (D) Flecainide-induced change on the current recorded at 40 mV positive to EK in cells expressing WT or mutant Kir2.x channels. *P < 0.05 vs. control. Each point/bar represents the mean ± SEM of five or more experiments.
Flecainide Increases Functional Kir2.1 Channel Density.
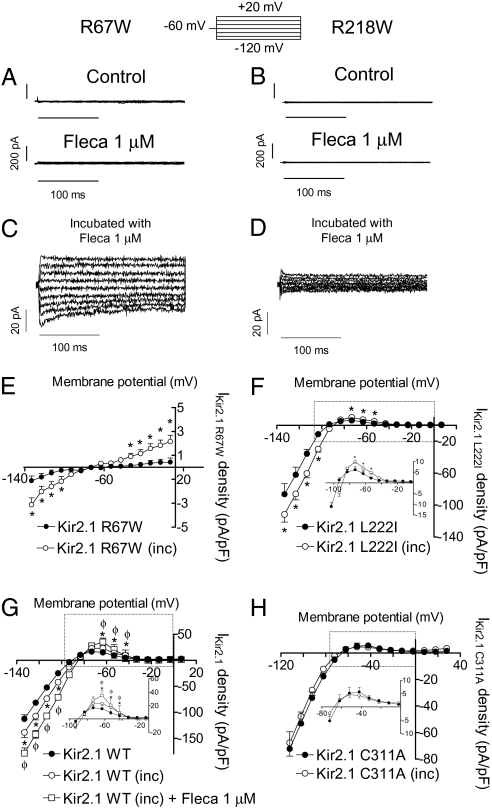
To test whether flecainide could also increase the current generated by Kir2.1 channels with AS mutations, we tested the effects of flecainide in R67W and R218W channels—two mutations found in AS patients (16). Flecainide did not increase R67W or R218W currents, which were almost undetectable (Fig. 6 A and B). R67W IKir2.1 markedly increased when increasing the [K+]o to 140 mM (Fig. S8; n = 5, P < 0.001), suggesting that channels were present in the plasma membrane. Conversely, R218W channels did not generate any current at 140 mM [K+]o (Fig. S8), even when it has been described that the channels are able to reach the cell membrane (17). Next we tested the effects produced by the incubation of R67W and R218W Kir2.1 transiently transfected cells with flecainide (1 μM) for 24 h (Fig. 6 C and D). R67W, but not R218W, flecainide-incubated cells generated a small but detectable BaCl2-sensitive current (Fig. S8) that was significantly greater than the current generated by nonincubated cells (Fig. 6E; n = 21, P < 0.01). Loss of function of R67W and R218W channels has been attributed to a decreased affinity of the channels for PIP2 (17); therefore, we also tested the effects of the 24-h incubation with flecainide on L222I channels. Figure 6F shows that flecainide also significantly increased L222I IKir2.1 density.
Fig. 6.
Effects of flecainide on Kir2.1 channel density. Current traces recorded in a cell expressing R67W (A) and R218W (B) Kir2.1 in the absence and presence of flecainide. Current traces recorded in cells expressing R67W (C) and R218W (D) Kir2.1 channels incubated with flecainide for 24 h. The I–V curves for currents recorded in cells expressing R67W (E), L222I (F), WT (G), and C311A (H) Kir2.1 channels in control conditions and after incubation with flecainide. In G, squares represent acute effects of flecainide produced in cells incubated with flecainide. *P < 0.01 vs. control. ΦP < 0.05 vs. incubated cells. Each point represents the mean ± SEM of eight experiments.
These findings suggested that flecainide increased the functional ion channel density. To test this hypothesis, we incubated Kir2.1 WT transfected cells with flecainide, which significantly increased IKir2.1 density (Fig. 6G). Importantly, acute addition of flecainide increased IKir2.1 generated by the incubated cells, producing the same increase as in nonincubated cells (42.7 ± 9.5% at −50 mV, n = 4, P < 0.05). This suggests that acute effects of flecainide are additive to its effects on channel density. Conversely, incubation with flecainide did not increase C311A Kir2.1 channel density (Fig. 6H), indicating that the presence of Cys311 is also critical for this effect. Indeed, incubation with flecainide failed to increase density of Kir2.2 and Kir2.3 channels, whereas it increased those of A312C Kir2.2 and A303C Kir2.3 (Fig. S9). Kir2.1/Kir2.2 and Kir2.1/Kir2.3 densities were also not modified with the flecainide incubation (Fig. S9).
Discussion
Our findings show that flecainide acutely increased human IKir2.1 mainly by reducing the polyamine blockade of the channel. Additionally, incubation with flecainide increased functional Kir2.1 channel density. Flecainide did not modify either IKir2.2 and IKir2.3 or Kir2.2 and Kir2.3 channel density. This selectivity could be attributed to the specific presence of Cys311 on the C terminus of the cytoplasmic region of Kir2.1 channels.
Flecainide Increases IKir2.1 but Not IKir2.2 and IKir2.3.
Acutely applied flecainide increased IKir2.1, and this effect was only apparent in Kir2.1 homotetrameric channels. Therefore, flecainide-increasing effects would be apparent only in those species and tissues in which IK1 is mainly generated by homotetrameric Kir2.1 channels. Thus, our pharmacological findings suggested that guinea-pig ventricular IK1 is mainly generated by homotetrameric Kir2.1 channels. Recent data showed that Kir2.1 is the major isoform underlying human ventricular IK1, whereas its relative contribution to atrial IK1 seems to be lower (3). This could explain why flecainide did not increase human atrial IK1.
Flecainide preferentially increased the outward IKir2.1 generated at potentials positive to the EK, which influences cardiac RMP, excitability, and APD. Our findings suggested that flecainide decreased polyamines’ affinity for the channel in a dose-dependent manner, thus reducing the strength of rectification. Furthermore, the effects of flecainide on the concentration dependence of the Spm-induced block suggested that it decreases the Spm block by a “noncompetitive” mechanism. Indeed, in the presence of flecainide, Emax produced by Spm reached saturation at ≈82%. This finding suggests that flecainide does not compete with Spm for the same binding site at the channel level, but that interaction of flecainide to its own receptor site allosterically reduces the binding of polyamines. Previous studies demonstrated that the Glu224, Asp259, and Glu299 cytoplasmic residues help to maximize the rate of polyamine channel block (4, 15). Thus, we tested whether mutation of these residues decreased the outward IKir2.1 flecainide increase. The findings show that in E224A, D259A, and E299A nonrectifying channels, flecainide did not increase the IKir2.1. It could be possible that flecainide interaction to the Cys311 at the βI strand modifies the position of the rings of negatively charged amino acids that create a complimentary electrostatic match for the binding of positively charged polyamines (4, 18). Therefore, this shows that a therapeutically used drug can affect Kir2.1 channels by modulating their interaction with polyamines.
Our findings strongly suggest that Cys311 located in the βI strand of the C terminus cytoplasmic domain of the Kir2.1 channel determines the flecainide binding. Furthermore, the presence of this Cys is critical for the increasing effects absent in Kir2.2 and Kir2.3 channels that do not exhibit a Cys residue at the equivalent position. The βI and βH strands form the HI loop or G loop (residues 300–315) (15). Mutations in the HI loop disrupted gating and affected inward rectification of Kir2.1 channels (15), and thus it seems reasonable to assume that binding of flecainide to this region can produce the observed effects. Moreover, the HI loop is structurally distinct from but functionally coupled to the PIP2 binding site (13–15). Indeed, the adjacent residue to Cys311 within the βI strand (Arg312 in Kir2.1 channels) modulates the PIP2-channel interactions (14, 18). Furthermore, it has been previously shown that substitution of Cys311 by polar residues strongly modifies the Kir2.1 channel kinetic properties, producing long-lasting closed-time intervals that decreased the channel Po—effects that were attributed to a destabilization of PIP2-Kir2.1 interaction (13). Therefore, we surmised that flecainide interaction with Cys311 could increase the channel-activating PIP2 effects. Our findings demonstrated that flecainide increased the Po of the channel by shifting the Po–V relationship to more negative potentials. Moreover, it did not modify the unitary current amplitude or the single-channel conductance, but augmented the mean open time and the fo of the channel. All these effects resemble those produced by PIP2 (14). Finally, the L222I mutation, which decreased Kir2.1 affinity for PIP2, suppressed the flecainide-increasing effects at potentials negative to the EK, leaving increasing effects at positive potentials unaltered. Overall, these findings suggest that flecainide also potentiates the activating PIP2 actions on the channel, which contributes to its increasing effects—particularly at potentials negative to EK.
Flecainide Increases Kir2.1 Channel Density.
Incubation for 24 h with flecainide increased functional Kir2.1 channel density. This effect was also dependent on the presence of Cys311 and, importantly, was additive to the acute flecainide-increasing effects. Therefore, flecainide would substantially increase homotetrameric Kir2.1 IK1 by two mechanisms. To test whether flecainide increases the current and the functional density of AS-mutated channels, we tested its effects on R67W and R218W channels. As expected, acutely applied flecainide did not increase either R67W or R218W IKir2.1, because both channels do not exhibit affinity for PIP2. However, incubation with flecainide for 24 h significantly increased R67W and L222I but not R218W IKir2.1 densities. Therefore, these preliminary findings suggest that in AS patients who carry mutations that produce functional channels, flecainide would increase IKir2.1 generated by WT and mutated channels by increasing their membrane density (i.e., flecainide would pharmacologically rescue some AS mutations).
Growing evidence suggests that ion-channel density can be modified pharmacologically. It has been described that two therapeutically used drugs that acutely block Kir2.1 also modify the expression of the channels in the membrane, i.e., incubation with chloroquine increases, and with pentamidine decreases, the IKir2.1 density of transfected cells (19). Further studies are needed to elucidate whether flecainide increases the anterograde delivery to or decreases the endocytosis of channel protein from the membrane.
Therapeutic Implications.
Flecainide is a potent Na+ channel blocker that also blocks several voltage-dependent K+ channels (Kv). Indeed, flecainide inhibits the Ca2+-independent transient outward K+ current (Ito1) and the rapid component of the delayed rectifier current (IKr) at concentrations close to those needed for blocking Na+ channels (20, 21). Flecainide frequency-dependently increases the human atrial APD and refractoriness (6) without modifying human ventricular APD, as measured from the QT interval of the electrocardiogram (7). The selective increase of the ventricular IK1 could account for this differential effect on atrial and ventricular APD. Indeed, because IK1 plays a critical role in the final phase of repolarization, it could be speculated that the ventricular IK1 increase overcomes the putative APD prolongation produced by the simultaneous blockade of other Kv channels. However, in the atrial tissue, in which flecainide did not increase the IK1, the APD prolongation produced by the blockade of Kv channels will be apparent. Furthermore, the ventricular IK1 increase could contribute to the ventricular proarrhythmic effects of flecainide. The importance of IK1 in the establishment and maintenance of the stability of rotors and ventricular fibrillation dynamics has been demonstrated (5). The prediction is that an IK1 increase accelerates and stabilizes the reentry (5).
Finally, it is noteworthy that the flecainide effects here described were produced at concentrations that are therapeutically relevant, because peak plasma concentrations after administration of therapeutic doses are between 0.4 and 2.2 μM (22).
Conclusions
Overall, our findings show that Kir2.1 channels can be positively modulated through the decrease of their polyamine blockade by a therapeutically used drug. Moreover, flecainide increases the density of Kir2.1 channels, an effect that could produce the pharmacological rescue of functional AS-mutated channels. Both effects were based on the presence of a Cys residue at position 311 within the βI strand, which further stresses the role of the cytoplasmic domain and Cys residues of Kir2.x channels in controlling gating and rectification.
Methods
This study was approved by the Investigation Committee of the Hospital Universitario Gregorio Marañón (CNIC-13) and conforms to the principles outlined in the Declaration of Helsinki. Human atrial myocytes were enzimatically isolated from right atrial appendages obtained from patients that underwent cardiac surgery (Table S2) (12). Guinea-pig cardiomyocytes were also enzimatically isolated (23). Reverse transcription-PCR analysis of Kir2.x channels was developed with the primers depicted in Table S3. WT and mutated human Kir2.1, Kir2.2, and Kir2.3 channels were transiently transfected in CHO cells (24). Macroscopic and single-channel currents were recorded using the whole-cell and cell-attached patch-clamp configurations, respectively. Inside-out recordings of IKir2.1 were developed in transiently transfected HEK-293 cells. All I–V curves were corrected according to the calculated liquid junction potential. Blind and manual dockings of flecainide on a modeled Kir2.1 channel were performed with AutoDock 4.0 and QUANTA/CHARMm software, respectively (23). Detailed SI Methods are available online.
Supplementary Material
Acknowledgments
This work was supported by Ministerio de Educación y Ciencia Grant SAF2008-04903; Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III Grants Red HERACLES RD06/0009 and PI080665; Universidad Complutense de Madrid (4195); Fundación LILLY; and Centro Nacional de Investigaciones Cardiovasculares (CNIC-13).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004021107/-/DCSupplemental.
References
- 1.Anumonwo JM, Lopatin AN. Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol. 2010;48:45–54. doi: 10.1016/j.yjmcc.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–2428. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 3.Gaborit N, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 5.Jalife J. Inward rectifier potassium channels control rotor frequency in ventricular fibrillation. Heart Rhythm. 2009;6(11, Suppl):S44–S48. doi: 10.1016/j.hrthm.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZG, Pelletier LC, Talajic M, Nattel S. Effects of flecainide and quinidine on human atrial action potentials. Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues. Circulation. 1990;82:274–283. doi: 10.1161/01.cir.82.1.274. [DOI] [PubMed] [Google Scholar]
- 7.Katritsis D, et al. Effect of flecainide on atrial and ventricular refractoriness and conduction in patients with normal left ventricle. Implications for possible antiarrhythmic and proarrhythmic mechanisms. Eur Heart J. 1995;16:1930–1935. doi: 10.1093/oxfordjournals.eurheartj.a060850. [DOI] [PubMed] [Google Scholar]
- 8.Crijns HJ, et al. Acute conversion of atrial fibrillation to sinus rhythm: Clinical efficacy of flecainide acetate. Comparison of two regimens. Eur Heart J. 1988;9:634–638. doi: 10.1093/oxfordjournals.eurheartj.a062553. [DOI] [PubMed] [Google Scholar]
- 9.Pellizzón OA, Kalaizich L, Ptácek LJ, Tristani-Firouzi M, Gonzalez MD. Flecainide suppresses bidirectional ventricular tachycardia and reverses tachycardia-induced cardiomyopathy in Andersen–Tawil syndrome. J Cardiovasc Electrophysiol. 2008;19:95–97. doi: 10.1111/j.1540-8167.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- 10.Bökenkamp R, Wilde AA, Schalij MJ, Blom NA. Flecainide for recurrent malignant ventricular arrhythmias in two siblings with Andersen–Tawil syndrome. Heart Rhythm. 2007;4:508–511. doi: 10.1016/j.hrthm.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Dhamoon AS, et al. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 12.Liu GX, et al. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garneau L, Klein H, Parent L, Sauvé R. Contribution of cytosolic cysteine residues to the gating properties of the Kir2.1 inward rectifier. Biophys J. 2003;84:3717–3729. doi: 10.1016/S0006-3495(03)75100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A. Phosphoinositide-mediated gating of inwardly rectifying K(+) channels. Pflugers Arch. 2007;455:83–95. doi: 10.1007/s00424-007-0276-5. [DOI] [PubMed] [Google Scholar]
- 15.Pegan S, et al. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- 16.Plaster NM, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 17.Lopes CM, et al. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- 18.Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K(+) channels. Nat Rev Neurosci. 2003;4:957–967. doi: 10.1038/nrn1244. [DOI] [PubMed] [Google Scholar]
- 19.de Boer TP, Houtman MJ, Compier M, van der Heyden MA. The mammalian KIR2.x inward rectifier ion channel family: Expression pattern and pathophysiology. Acta Physiol (Oxf) 2010;199:243–256. doi: 10.1111/j.1748-1716.2010.02108.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Fermini B, Nattel S. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- 21.Follmer CH, Colatsky TJ. Block of delayed rectifier potassium current IK by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- 22.Slama R, LeClercq JF. The clinical use of oral flecainide. Drugs. 1985;29(Suppl 4):28–29. doi: 10.2165/00003495-198500294-00007. [DOI] [PubMed] [Google Scholar]
- 23.Núñez L, et al. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 2006;72:80–89. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Gómez R, et al. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res. 2009;105:383–392. doi: 10.1161/CIRCRESAHA.109.197558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.