Abstract
Immunophilin FK506-binding protein 52 (FKBP52) is a cochaperone that binds to the progesterone receptor (PR) to optimize progesterone (P4)-PR signaling. We recently showed that Fkbp52-deficient (Fkbp52−/−) mice have reduced uterine PR responsiveness and implantation failure which is rescued by excess P4 supplementation in a genetic background-dependent manner. This finding led us to hypothesize that FKBP52 has functions in addition to optimizing PR activity. Using proteomics analysis, we found that uterine levels of peroxiredoxin-6 (PRDX6), a unique antioxidant, are significantly lower in Fkbp52−/− mice than in WT and PR-null (Pgr−/−) mice. We also found that Fkbp52−/− mice with reduced uterine PRDX6 levels are susceptible to paraquat-induced oxidative stress (OS), leading to implantation failure even with P4 supplementation. The same dose of paraquat did not interfere with implantation in WT mice. Moreover, treatment with antioxidants α-tocopherol and N-acetylcysteine (NAC) attenuated paraquat-induced implantation failure in P4-treated Fkbp52−/− mice. Functional analyses using mouse embryonic fibroblasts show that Fkbp52 deficiency associated with reduced PRDX6 levels promotes H2O2-induced cell death, which is reversed by the addition of NAC or by forced expression of PRDX6, suggesting that Fkbp52 deficiency diminishes the threshold against OS by reducing PRDX6 levels. These findings provide evidence that heightened uterine OS in Fkbp52−/− females with reduced PRDX6 levels induces implantation failure even in the presence of excess P4. This study shows that FKBP52–PRDX6 signaling protects pregnancy from overt OS.
Keywords: embryo implantation, mouse, uterus
Infertility is a global social and economic concern. It is estimated that by 2050 15% of couples worldwide will be childless because of infertility. Although many causes of infertility have been overcome by the application of in vitro fertilization and embryo transfer (IVF-ET), implantation failure is still a major obstacle for optimal pregnancy outcome. Pregnancy rates remain low in patients undergoing IVF-ET because of the transfer of embryos into a nonreceptive uterus, resulting in implantation failure (1). Heterogeneous uterine cell types respond differentially to estrogen and progesterone (P4) to prepare the uterus to the receptive state. For successful implantation to occur, the uterus must be transiently receptive to implantation-competent blastocysts. P4 signaling is an absolute requirement for implantation and pregnancy maintenance in most mammals studied (2). P4 acts via the nuclear progesterone receptor (PR) to activate transcription of genes involved in ovulation, uterine receptivity, implantation, decidualization, and pregnancy maintenance (3).
We recently found that the immunophilin FK506-binding protein 52 (FKBP52) serves as a cochaperone to govern normal PR function in the mouse uterus, where its expression overlaps with that of PR. Immunophilins are so named because they bind to certain immunosuppressive drugs to mediate their actions. They are grouped into two families, FKBP and cyclosporin A-binding (cyclophilin, CyP) proteins. Some FKBP and CyP family members have a tetratricopeptide repeat (TPR) domain that targets binding to the conserved C terminus of Hsp90. FKBP52 is one such TPR-containing cochaperone that influences steroid hormone receptor function (4). The mature PR complex bound to FKBP52 binds to P4 with high affinity and efficiency, although some basal PR responsiveness to P4 is retained in the absence of FKBP52. In addition, FKBP52 plays a role in immunoregulation and basic cellular processes involving protein stability, folding, and trafficking (5–7).
We found that Fkbp52−/− females on both C57BL6/129 mixed and CD1 backgrounds have implantation failure with normal ovulation (8, 9). However, P4 supplementation rescues implantation and decidualization in CD1, but not in C57BL6/129, Fkbp52−/− females. In CD1 Fkbp52−/− females, P4 at higher-than-normal levels confers PR signaling sufficient for implantation, but even higher levels of P4 are required to rescue full-term pregnancy (9). How P4 overcomes reduced PR responsiveness to allow implantation in CD1 Fkbp52−/− mice is still unknown. Because FKBP52 positions PR in optimal conformation for binding to P4, one possibility is that excess P4 in the absence of FKBP52 increases the probability of random binding of P4 to the PR complex even in its less optimal configuration. Alternatively, FKBP52 may influence other pathways along with PR signaling, because exogenous P4 cannot rescue implantation in C57BL6/129 Fkbp52−/− mice and significantly higher P4 levels are necessary to restore implantation and full-term pregnancy in CD1 Fkbp52−/− females (9). Moreover, Fkbp52 also is expressed in the placenta with no detectable PR expression (9), implying that FKBP52 has other functions in addition to its role in optimizing PR activity. Our present study, using proteomics analysis, found that FKBP52 regulates oxidative stress (OS) by regulating the levels of a unique antioxidant, peroxiredoxin-6 (PRDX6), which operates independently of other peroxiredoxins and antioxidant proteins (10–12). Here we show that uterine FKBP52–PRDX6 signaling is a major player in countering the adverse effects of OS during early pregnancy.
Results
PRDX6 Levels Are Reduced in Fkbp52−/− Uteri.
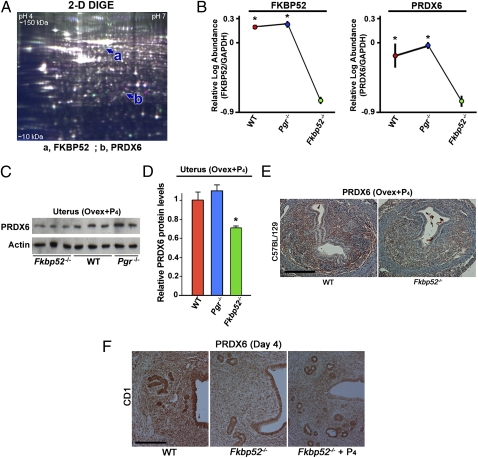
To explore downstream targets of FKBP52 independent of PR, we used proteomics approaches. Ovariectomized WT, Fkbp52−/−, and PR-null (Pgr−/−) mice on the C57BL6/129 background were treated with P4 (2 mg/d) for 2 d, and uteri were collected 24 h after the second injection. Uterine protein lysates were subjected to 2D difference in gel electrophoresis (2D-DIGE) analysis with detection limits from 10- to 150-kDa proteins (pH 4–7) (Fig. 1A). Among the differentially expressed proteins, we found that PRDX6 levels are markedly down-regulated in Fkbp52−/− uteri compared with Pgr−/− and WT uteri, with the exception of FKBP52, serving as an internal control (Table S1 and Fig. 1B). This finding led us to focus on PRDX6 as a potential downstream mediator of FKBP52 function, independent of its PR cochaperone activity, and to assess uterine expression of Prdx6 in early pregnancy. Notably, we found that levels of six proteins increased in Fkbp52−/− uteri compared with WT uteri, but no differences were detected between WT and Pgr−/− uteri (Table S2). We confirmed down-regulated PRDX6 protein levels in Fkbp52−/− uteri compared with Pgr−/− and WT uteri by Western blotting (Fig. 1 C and D) and immunohistochemistry (Fig. 1E). In addition, both stromal and epithelial localization of PRDX6 was reduced in ovariectomized Fkbp52−/− uteri treated with P4 (Fig. 1E).
Fig. 1.
PRDX6 expression is reduced in uteri of Fkbp52−/− mice. (A) A representative DIGE gel comprising protein extracts from WT and Fkbp52−/− uteri shows proteins of isoelectric points between pH 4–7 and apparent molecular mass between 10–150 kDa. a, FKBP52; b, PRDX6. (B) Decreased protein levels of PRDX6 and FKBP52 in P4-treated uteri of Fkbp52−/− ovariectomized mice (n = 4) compared with WT (n = 3) and Pgr−/− (n = 3) ovariectomized mice on a C57BL6/129 background. Values are mean ± SEM. *P < 0.05 compared with Fkbp52−/− mice. (C and D) Decreased PRDX6 protein levels in P4-treated uteri of Fkbp52−/− ovariectomized mice compared with WT and Pgr−/− ovariectomized females as determined by Western blotting. Band intensities are normalized against actin and expressed as relative ratios compared with WT samples on the C57BL6/129 background. Values are mean ± SEM of two or three independent samples. *P < 0.05 compared with WT mice. (E) Differential expression patterns of PRDX6 protein in P4-treated ovariectomized mouse uteri of WT and Fkbp52−/− mice on the C57BL6/129 background. (Scale bar, 200 μm.) (F) Differential expression patterns of PRDX6 protein in day 4 uteri of WT and Fkbp52−/− mice on a CD1 background. (Scale bar, 200 μm.)
To examine the effects of ovarian hormones on Prdx6 expression in mouse uteri, we performed Northern and in situ hybridization using uteri from ovariectomized WT mice. Neither P4 nor estradiol-17β (E2) significantly altered Prdx6 expression levels (Fig. S1A), but P4 changed the expression domain from the epithelium to the stroma (Fig. S1B).
PRDX6 Is Aberrantly Expressed in Fkbp52−/− Uteri.
On day 4 of pregnancy the uterus is under the influence of P4 produced by the newly formed corpus luteum. PRDX6 is localized in both the stroma and epithelium in WT CD1 mice on day 4 of pregnancy (Fig. 1F). In contrast, in Fkbp52−/− uteri PRDX6 is restricted primarily to the epithelium but is localized in the subluminal stroma after P4 supplementation (Fig. 1F). These results suggest that FKBP52 deficiency attenuates PRDX6 levels in the stroma that are compensated to some extent by P4.
PRDX6 Is Differentially Expressed in Uteri During Early Pregnancy.
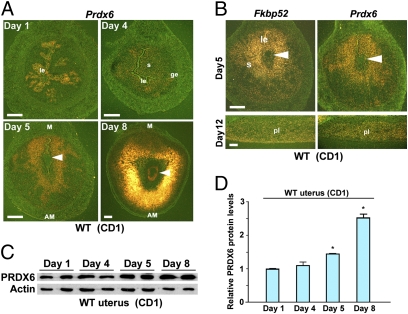
The uterus is under the influence of preovulatory estrogen with increased epithelial cell proliferation on day 1 of pregnancy. On day 4, the uterus is exposed to heightened P4 levels along with a small amount of estrogen, resulting in epithelial cell differentiation with stromal cell proliferation. The first molecular and physical interaction between the blastocyst and receptive uterus is observed on the evening of day 4, and the attachment process continues through day 5. The attachment reaction coincides with increased stromal vascular permeability solely at the site of the blastocyst and can be demarcated by distinct blue bands along the uterus after i.v. injection of a blue dye solution (13). The proliferating stromal cells surrounding the implanting blastocyst start to differentiate to decidual cells in the afternoon of day 5. Differentiating stromal cells in the immediate vicinity of the implanting blastocyst begin to form the primary decidual zone (PDZ) at this time. The PDZ is well formed on day 6 with the beginning of a proliferating secondary decidual zone (SDZ). Although cell proliferation ceases in the PDZ, it continues in the SDZ. The PDZ progressively degenerates as pregnancy progresses. On day 8, the implantation process is well advanced with maximal decidualization. We assessed Prdx6 expression on days 1, 4, 5, and 8 of pregnancy by in situ hybridization. We found that Prdx6 is detected mostly in the luminal epithelium on day 1 (Fig. 2A). On day 4, the expression is restricted to the stroma (Fig. 2A). Prdx6 expression on these days closely resembles that of Fkbp52 (14). On day 5, Fkbp52 and Prdx6 are expressed in a similar fashion in the decidualizing stroma surrounding the implanting blastocyst (Fig. 2 A and B). This overlapping expression of Fkbp52 and Prdx6 surrounding the implanting blastocyst suggests that the process of implantation may regulate these two molecules. On day 8, Prdx6 is expressed primarily in the SDZ cells and in the embryo. PRDX6 protein levels also are up-regulated in uteri on days 5 and 8 of pregnancy (Fig. 2 C and D). In the placenta, Fkbp52 and Prdx6 showed a similar expression pattern in the labyrinth layer (Fig. 2B), with undetectable levels of PR (9). The similar localization of FKBP52 and PRDX6 in the deciduum and placenta suggests their PR-independent roles in these tissues. Furthermore, pull-down assays show that FKBP52 is physically associated with PRDX6 in the deciduum (Fig. S2), suggesting that FKBP52 influences PRDX6 status through this interaction.
Fig. 2.
Uterine PRDX6 is expressed differentially during early pregnancy. (A) In situ hybridization showing spatiotemporal expression of Prdx6 in WT CD1 uteri on days 1, 4, 5, and 8 of pregnancy. Arrowheads indicate implanting embryos. AM, antimesometrial pole; ge, glandular epithelium; le, luminal epithelium; M, mesometrial pole; s, stroma. (Scale bar, 200 μm.) (B) Overlapping expression of Fkbp52 and Prdx6 in CD1 WT uteri on days 5 and 12 of pregnancy. Arrowheads indicate implanting embryos. le, luminal epithelium; pl, placenta; s, stroma. (Scale bars, 200 μm.) (C and D) Levels of uterine PRDX6 protein during early pregnancy as determined by Western blotting. Band intensities of PRDX6 were normalized against actin. Two independent samples from different mice were examined in each group. Data are expressed as mean ± SEM. *P < 0.05 compared with day 1 uteri.
In the human endometrium, both PRDX6 and FKBP52 are expressed in epithelial cells during the proliferative phase and in both epithelial and stromal cells in the secretory phase (Fig. S3A). The higher levels of Prdx6 in the secretory (receptive) phase suggest that uterine PRDX6 also plays antioxidant roles in human implantation (Fig. S3B).
Fkbp52−/− Uteri Are More Prone to Overt OS During Implantation.
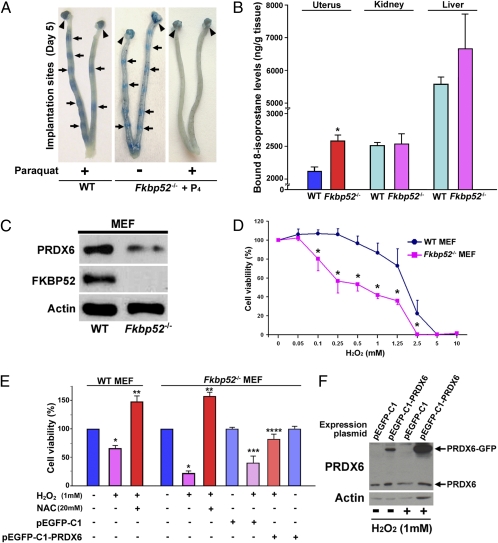
Because PRDX6 levels are reduced in Fkbp52−/− uteri on day 4 of pregnancy, especially in the stroma (Fig. 1F), we speculated that Fkbp52−/− uteri are more susceptible to OS during implantation. We have shown previously that P4 levels are comparable in WT and Fkbp52−/− mice. However, excess P4 supplementation that markedly elevates circulating P4 levels is required for implantation in Fkbp52−/− females (9). Although P4 supplementation rescues implantation failure in CD1 Fkbp52−/− mice (9), concomitant administration of the OS-inducing agent paraquat (15 or 20 mg/kg) prevented this rescue (Table 1 and Fig. 3A). In contrast, similar doses of paraquat failed to affect implantation in WT mice (Table 1 and Fig. 3A). To minimize the detrimental effects of paraquat on embryo growth and activation, we also performed embryo-transfer experiments. Day 4 WT blastocysts were transferred into day 4 WT or P4-treated Fkbp52−/− recipients that received paraquat 7 h before the embryo transfer. Again, Fkbp52−/− mice had reduced numbers of implantation sites, whereas WT recipients showed normal implantation when examined on day 5 (Table 2). It is interesting that even a smaller dose of paraquat (2 mg/kg) inhibited implantation in P4-treated Fkbp52−/− mice (Table 3). These results suggest that Fkbp52−/− mice are quite prone to OS in the context of implantation. More interestingly, a combined treatment of two antioxidants, N-acetylcysteine (NAC) and α-tocopherol (αTCP), significantly attenuated paraquat-induced implantation failure in P4-treated Fkbp52−/− mice (Table 3), indicating the contribution of OS to implantation failure. Our speculation that Fkbp52−/− mice are sensitive to OS is supported by the finding of higher levels of bound 8-isoprostane, a lipid peroxidation marker, in C57BL6/129 Fkbp52−/− uteri than in WT uteri on day 4 of pseudopregnancy (Fig. 3B).
Table 1.
Paraquat adversely affects implantation in Fkbp52−/− mice
| Genotype | Paraquat (mg/kg) | No. of mice | No. of mice with IS (%) | No. of IS | No. of blastocysts recovered |
| WT | 15 | 7 | 7 (100%) | 12.1 ± 1.7 | N/A |
| 20 | 7 | 7 (100%) | 12.0 ± 0.7 | N/A | |
| Fkbp52−/− + P4 | 0 | 8 | 8 (100%) | 10.6 ± 0.6 | N/A |
| 15 | 7 | 2 (29%)* | 12.0 ± 2.3 | 25† | |
| 20 | 5 | 1 (20%)* | 5.0 | 17‡ |
WT and Fkbp52 −/− females on a CD1 background were mated with CD1 WT fertile males, and the number of implantation sites (IS) was examined on day 5 of pregnancy. Fkbp52−/− females were treated with P4 (2 mg/d) on days 2–4 of pregnancy. Paraquat was injected on day 4. Uteri without IS were flushed with saline to recover any unimplanted blastocysts. Values are mean ± SEM.
*P < 0.05 compared with WT mice treated with the same dose of paraquat; Student's t test. N/A = not applicable.
†Twenty-five blastocysts were recovered from five mice.
‡Seventeen blastocysts were recovered from four mice.
Fig. 3.
Fkbp52−/− uteri are more prone to OS. (A) OS inducer paraquat blocks embryo implantation in P4-primed CD1 Fkbp52−/− females on day 5 of pregnancy, whereas paraquat-treated CD1 WT uteri have normal numbers of implantation sites as demarcated by blue bands. Fkbp52−/− females were treated with P4 (2 mg/d) on days 2–4 of pregnancy. Paraquat was injected on day 4. Arrows and arrowheads indicate implantation sites and ovaries, respectively. (B) Increased levels of bound 8-Isoprostane, a lipid peroxidation marker, in C57BL6/129 Fkbp52−/− uteri on day 4 of pseudopregnancy. Values are mean ± SEM of three independent samples. *P < 0.05 compared with WT mice. (C) Decreased levels of PRDX6 in Fkbp52−/− MEFs. Actin was used as a loading control. (D) Heightened sensitivity of Fkbp52−/− MEFs to H2O2-induced OS. Cells were treated with different concentrations of H2O2 for 24 h, and cell viability was evaluated by MTT assay. Values are mean ± SEM of six replicates from three independent experiments. *P < 0.05 compared with WT mice. (E) H2O2-induced cell death in Fkbp52−/− MEFs is protected by addition of NAC and forced expression of PRDX6. Cell viability was evaluated by MTT assay. Values are mean ± SEM of six replicates from three independent experiments. *P < 0.05, compared with vehicle-treated cells derived from mice of the same genotype. **P < 0.05, compared with H2O2-treated cells derived from mice of the same genotype. ***P < 0.05, compared with Fkbp52−/− cells transfected with pEGFP-C1 control plasmids; ****P < 0.05 compared with H2O2-treated Fkbp52−/− cells with transfection of pEGFP-C1 control plasmids. (F) PRDX6-GFP fused protein is induced effectively by transfection of pEGFP-C1-PRDX6 plasmids in Fkbp52−/− MEFs. pEGFP-C1 is a control plasmid.
Table 2.
Paraquat inhibits implantation of transferred WT blastocysts in Fkbp52−/− recipients
| Genotype of blastocysts | Genotype of recipients | Paraquat (mg/kg) | No. of blastocysts transferred | No. of recipients | No. of mice with IS (%) | No. of IS (%) | No. of blastocysts recovered |
| WT | WT | 15 | 77 | 6 | 6 (100%) | 58/77 (75%) | N/A |
| WT | Fkbp52−/−+P4 | 15 | 60 | 5 | 1 (20%) | 1/60 (2%) | 30* |
Day 4 CD1 WT blastocysts were transferred into uteri of CD1 WT or CD1 Fkbp52−/− recipients on day 4 of pseudopregnancy. Fkbp52−/− recipients were treated with P4 (2 mg/d) on days 2–4 of pregnancy. Paraquat was injected into the recipients 5 h before embryo transfer on day 4 of pregnancy. Uteri without IS were flushed with saline to recover any unimplanted blastocysts. N/A, not applicable.
*Thirty blastocysts were recovered from four mice.
Table 3.
Reversal of paraquat-induced implantation failure by treatment of antioxidants
| Genotype | Paraquat (2 mg/kg) | NAC + αTCP | No. of mice | No. of mice with IS (%) | No. of IS (mean ± SEM) | No. of blastocysts recovered |
| Fkbp52−/− + P4 | + | − | 10 | 1 (10%) | 13.0 | 35† |
| + | + | 8 | 5 (63%)* | 6.2 ± 1.7 | 22‡ |
Mice were given an i.p. injection of paraquat on day 4 of pregnancy. P4 (2 mg/d) was administrated s.c. into each CD1 Fkbp52−/− mouse on days 2–4. NAC (50 mg/kg) and α-tocopherol (αTCP) (1 g/kg) were injected i.p. on days 2–4. Mice were killed on day 5, and their IS were examined by the blue dye method.
*P < 0.05 compared with Fkbp52−/− mice treated with the same dose of paraquat, P4; Student's t test.
†Thirty-five blastocysts were recovered from nine mice.
‡Twenty-two blastocysts were recovered from three mice. Dormant-looking blastocysts were recovered from mice in which blue bands were not present or were very weak.
Fkbp52−/− Mouse Embryonic Fibroblasts with Reduced PRDX6 Levels Are Susceptible to OS.
Because PRDX6 protects cells from membrane damage associated with phospholipid peroxidation, we speculated that reduced PRDX6 would reduce cell viability if challenged with overt OS. Indeed, in vitro studies with WT and Fkbp52−/− mouse embryonic fibroblasts (MEFs) show that FKBP52 deficiency is associated with reduced PRDX6 levels (Fig. 3C) and enhances H2O2-induced cell death (Fig. 3D). This adverse effect in Fkbp52−/− MEFs was reversed substantially by supplementation of NAC (Fig. 3E) and, more importantly, by forced expression of PRDX6 (Fig. 3 E and F), suggesting that Fkbp52 deficiency lowers the threshold against reactive oxygen species (ROS) by reducing PRDX6 levels. Because PR expression is undetectable in these MEFs (Fig. S4), these results provide evidence for FKBP52’s role independent of PR function.
Discussion
The highlight of the present study is that Fkbp52−/− uteri are susceptible to OS because of reduced PRDX6 levels. Although further investigation is warranted to explore the mechanism by which FKBP52 regulates PRDX6 levels, our observation of physical interaction between FKBP52 and PRDX6 suggests that FKBP52 stabilizes PRDX6 protein and attenuates its degradation as a function of its chaperone activity. FKBP52’s roles in protein stability, folding, and trafficking have been reported previously (5–7).
The reduced tolerance to OS in Fkbp52−/− mice results in paraquat-induced failure of implantation and higher levels of ROS generation in the uterus. Because excess P4 cannot prevent implantation failure in the face of overt OS induced by paraquat in CD1 Fkbp52−/− females, we believe that FKBP52 regulates OS, in addition to its function in optimizing PR activity and other potential functions. In fact, FKBPs have several physiological roles, including binding and sequestration of calcineurin, protein folding and assembly, protein trafficking, and direct regulation of protein activity (15). Our present study adds another function to the list of various activities that have been reported for FKBP52.
PRDX6 is a unique nonredundant antioxidant that acts independently of other PRDXs and antioxidant enzymes (10–12). A recent study shows that Prdx6-deficient (Prdx6−/−) mice have normal development, but paraquat-induced OS causes abnormal phenotypes such as lower survival rate, again not resulting from changes in gene expression and/or activity of other antioxidant enzymes (12). Thus, PRDX6 might be a critical antioxidant against exogenous OS. Our present finding of increased susceptibility of Fkbp52−/− uteri to OS during implantation because of reduced PRDX6 levels underscores the importance of uterine FKBP52–PRDX6 signaling during the periimplantation period. Although Prdx6−/− females were reported to be fertile (12), it is possible that exposure to OS will impair fertility in these mice. Indeed, it is known that certain genes serve critical functions during physiologically altered conditions. For example, deficiency of prolactin (PRL)-like protein A (PLP-A), a nonclassical member of the PRL family expressed in the mouse placenta, has little effect on female fertility under normal conditions, but PLP-A mutants show compromised pregnancy when exposed to a stressor (16). Thus, fertility in Prdx6−/− females could be compromised if PRDX6 deficiency is superimposed with OS. Future studies are warranted to characterize the female fertility phenotype in Prdx6−/− mice in a more systematic manner under normal and OS conditions. Collectively, our observations suggest that implantation failure in Fkbp52−/− mice not only is caused by the reduced PR activity but also is compounded by increased OS levels.
There is evidence that smoking and alcohol consumption reduce fertility in women by elevating OS (17, 18). Evidence also indicates that dietary antioxidant and diet-related exposures to ROS affect the timing and maintenance of pregnancy (19). These findings, together with our observation that commercial rodent diets influence uterine gene expression and the timing of implantation in mice (20), suggest the possibility of ROS-induced alterations in uterine functions related to implantation. Because Prdx6 is expressed primarily in the stroma in WT uteri on day 4 of pregnancy, the reduction of stromal Prdx6 in Fkbp52−/− uteri even with P4 supplementation may attenuate stromal function more drastically when exposed to OS. Hoxa10 and Ihh are expressed in the stroma and epithelium, respectively, in day 4 pregnant mouse uteri (21, 22). Our results show that paraquat down-regulates Hoxa10 expression without altering Ihh expression in Fkbp52−/− uteri (Fig. S5). In this respect, stromal Prdx6 could be more important in protecting the uterus from overt OS and allowing implantation to proceed. Interestingly, we found a similar expression pattern of PRDX6 and FKBP52 in human endometrium and higher expression of Prdx6 during the secretory phase, suggesting that these proteins may have physiological roles in human endometrium and implantation. It will be interesting to see whether endometria from patients with recurrent implantation failure have higher ROS because of low PRDX6 levels.
During the periimplantation period, embryos are exposed to low oxygen tension (23). Because preimplantation embryos are sensitive to OS (24), it is believed that a low oxygen condition is conducive to normal embryonic growth and development. This notion is consistent with the finding that antioxidants prevent ROS-induced abnormal embryo growth in culture (25, 26). Thus, the embryonic environment facing excess OS interferes with successful implantation. It also is a distinct possibility that the uterine environment is hypoxic during the periimplantation period to protect embryos from OS. Our findings that WT, but not Fkbp52−/−, pregnant females treated with paraquat have normal implantation suggest that the uterus has a built-in protective system to combat OS to a degree and that PRDX6 is a critical player in this system. With increased angiogenesis after implantation, oxygen tension in the uterus and placenta increases, gradually exposing the developing embryo to higher ROS levels. Because a major role of the deciduum and placenta is to accommodate and protect the growing embryos, our data showing strong expression of Prdx6 in these tissues in WT mice suggest that decidual and placental PRDX6 has an antioxidant function and protects embryos against increased oxygen tension at that time.
Endometriosis, the growth of endometrium-like tissues outside the uterus, is a common gynecological disease often associated with pelvic pain and infertility (27). Accumulating evidence shows that OS is associated with the pathogenesis of endometriosis and endometriosis-related infertility including implantation failure (28). Because our recent study has shown that FKBP52 levels are down-regulated in endometria of women with endometriosis compared with women without endometriosis (29), reduced FKBP52 levels may lead to endometrial PRDX6 deficiency, increasing uterine susceptibility to ROS, aggravating endometriosis, and adversely affecting endometrial receptivity in patients with endometriosis.
In conclusion, we show here that uterine FKBP52–PRDX6 signaling is a key player against ROS during pregnancy, an intriguing finding regarding a role of FKBP52–PRDX6 in uterine biology in the context of implantation.
Materials and Methods
Mice.
C57BL/6/129 and CD1 Fkbp52−/− mice (9, 30) and C57BL6/129 Pgr−/− mice (31) were used in this study. FKBP52 and PR mutant mice were originally obtained from David Smith (Mayo Clinic, Scottsdale, AZ) and Bert O’Malley (Baylor College of Medicine, Houston, TX), respectively. All protocols for the present study were reviewed and approved by the Cincinnati Children's Research Foundation Institutional Animal Care and Use Committee.
Analysis of Implantation and Blastocyst Transfer.
Mice were examined for implantation as described previously (9). WT and Fkbp52−/− females (2- to 5-mo-old) were mated with WT fertile males to induce pregnancy (day 1 = vaginal plug). To supplement Fkbp52−/− females with exogenous P4, they were given an s.c. injection of P4 (2 mg/d, s.c.) on days 2–4 of pregnancy. For induction of OS, paraquat was given i.p. to WT or Fkbp52−/− mice on day 4 (0900 hours) of pregnancy. For antioxidant treatment, NAC (50 mg/kg body weight) and αTCP (1g/kg body weight) were given i.p. daily on days 2–4. Pregnant females were killed on day 5 (0900 hours), and implantation sites were evaluated by i.v. injection of a Chicago blue dye solution. Uteri without implantation sites were flushed with saline to recover any unimplanted blastocysts. For blastocyst transfer experiments, pseudopregnant recipients were generated by mating females with vasectomized males. Paraquat was injected into recipients 5 h before embryo transfer on day 4 (0700 hours) of pregnancy. Day-4 WT blastocysts were transferred into day 4 uteri of WT or Fkbp52−/− pseudopregnant recipients (1200 hours), and implantation sites were examined 24 h later (day 5, 1200 hours).
Treatment with P4 and/or E2.
To assess the effects of ovarian hormones on uterine Prdx6 expression, CD1 WT females were ovariectomized and rested for 2 wk. They then were given a single s.c. injection of P4 (2 mg) and/or E2 (100 ng) (32). The control group of mice received only the vehicle (oil). Mice were killed after 24 h, and uteri were collected for Northern and in situ hybridization.
Human Tissues.
Endometrial tissues were obtained from 24 women who had shown regular menstrual cycles without any hormonal treatment for at least 6 months. Endometrial samples were dated according to the women's menstrual history and standard histological criteria (33). The experimental procedures were approved by the institutional review board of the University of Tokyo, and signed informed consent for the use of tissues was obtained from each woman.
2D-DIGE Analysis and Protein Identification.
WT, Fkbp52−/−, and Pgr−/− mice on C57BL6/129 background were ovariectomized, rested for 2 wk, and then treated daily with P4 (2 mg) for 2 d. Mice were killed 24 h after the second P4 injection, and their ueri were collected and processed for 2D-DIGE. Proteins were extracted from uterine tissues from three independent WT and Pgr−/− mice and four Fkbp52−/− mice, and 2D-DIGE and protein identification were performed as previously described by us (SI Materials and Methods) (14).
Immunoblotting.
Protein extraction and Western blotting were performed as described (14). Antibodies to PRDX6, FKBP52 (kindly provided by Marc B. Cox, University of Texas, El Paso, TX), and actin were used. Actin served as a loading control.
Immunohistochemistry.
Immunostaining of PRDX6 was performed in formalin-fixed paraffin-embedded sections using a specific antibody to PRDX6 (kindly provided by Aron B. Fisher, University of Pennsylvania, Philadelphia).
In Situ Hybridization.
Paraformaldehyde-fixed frozen sections were hybridized with 35S-labeled cRNA probes as described (13).
Northern Hybridization.
Northern blotting was performed as described previously (13). Rpl7 served as a housekeeping gene.
Immunoprecipitation.
Decidual cells were isolated as described previously (34). Protein lysates were immunoprecipitated with an anti-FKBP52 antibody, and complexes were run on SDS/PAGE and immunoblotted using antibodies specific to PRDX6 or FKBP52. The control immunoprecipitation was performed by incubating the lysates with rabbit IgG.
Quantitative PCR.
Quantitative PCR was performed as previously described (29). A housekeeping gene Gapdh was used as an internal standard.
RT-PCR.
RT-PCR was performed as previously described (14). Actb served as a housekeeping gene.
8-Isoprostane Assay.
Tissue levels of 8-isoprostane were assayed as described previously (35).
Isolation and Culture of MEFs.
WT and Fkbp52−/− MEFs (kindly provided by Marc B. Cox, University of Texas, El Paso, TX) were isolated as described previously (8). The cells were maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin.
MTT Assays.
MEFs were cultured for 24 h in DMEM containing various concentrations of H2O2, and cell viability was determined with MTT assay as described previously (Promega) (36). To examine the effects of NAC on H2O2-induced cell damage, MEFs were pretreated with NAC for 24 h and then were treated with H2O2. To examine the effects of PRDX6 on H2O2-induced cell damage, Fkbp52−/− MEFs were transiently transfected with pEGFP-C1 (control vector carrying GFP alone, Clontech) or pEGFP-C1-PRDX6 (carrying GFP-PRDX6 fusion construct) plasmids (kindly provided by Aron Fisher, University of Pennsylvania, Philadelphia, PA) by liposome transfection. The cells expressing GFP or PRDX6-GFP fusion proteins were incubated with H2O2 for 24 h.
Statistical Analysis.
Statistical analyses were performed using two-tailed Student's t test and ANOVA as appropriate. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Erin L. Adams for editing the manuscript. Tissue 8-isoprostane levels were measured by the late Dr. Jason Morrow (Vanderbilt University, Nashville, TN) who died in 2008. We are very grateful for his contributions to our research. Anti-PRDX6 antibody and pEGFP-C1 and pEGFP-C1-PRDX6 plasmids were kindly provided by Dr. Aron Fisher (University of Pennsylvania, Philadelphia, PA). Anti-FKBP52 antibody and WT and Fkbp52−/− MEFs were kindly provided by Dr. Marc B. Cox (University of Texas, El Paso, TX). This study was supported in part by National Institutes of Health Grants HD12304 and DA006668. Y.H. is supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. N.A. is supported by the 2214-Abroad Research Fellowship for PhD students from the Scientific and Technological Research Council of Turkey (TUBITAK).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009324107/-/DCSupplemental.
References
- 1.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–3043. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 2.Dey SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 4.Smith DF. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones. 2004;9:109–121. doi: 10.1379/CSC-31.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong L, et al. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J Biol Chem. 2004;279:12714–12723. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies TH, Sánchez ER. Fkbp52. Int J Biochem Cell Biol. 2005;37:42–47. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12:129–140. doi: 10.1016/s1074-7613(00)80166-1. [DOI] [PubMed] [Google Scholar]
- 8.Tranguch S, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tranguch S, et al. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Phelan SA. AOP2 (antioxidant protein 2): Structure and function of a unique thiol-specific antioxidant. Antioxid Redox Signal. 1999;1:571–584. doi: 10.1089/ars.1999.1.4-571. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, et al. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: A possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 14.Daikoku T, et al. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- 15.Kay JE. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem J. 1996;314:361–385. [PMC free article] [PubMed] [Google Scholar]
- 16.Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA. 2004;101:16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70:632–637. doi: 10.1016/s0015-0282(98)00257-x. [DOI] [PubMed] [Google Scholar]
- 18.Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: Findings in a large prospective study. Br Med J (Clin Res Ed) 1985;290:1697–1700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, et al. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 23.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 24.Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007;81:155–162. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 25.Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Protection from oxidative stress by thioredoxin and superoxide dismutase of mouse embryos fertilized in vitro. Hum Reprod. 1991;6:1305–1310. doi: 10.1093/oxfordjournals.humrep.a137532. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, et al. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78:1272–1277. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- 27.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 28.Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25:75–81. doi: 10.1080/09513590802485012. [DOI] [PubMed] [Google Scholar]
- 29.Hirota Y, et al. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung-Flynn J, et al. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 31.Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 32.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 33.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 34.Hirota Y, et al. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. (2nd) 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 36.Daikoku T, et al. Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferator-activated receptor-delta signaling in epithelial ovarian cancer. Cancer Res. 2007;67:5285–5292. doi: 10.1158/0008-5472.CAN-07-0828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.