Abstract
The anterior cingulate cortex (ACC) is part of a network implicated in the development of self-regulation and whose connectivity changes dramatically in development. In previous studies we showed that 3 h of mental training, based on traditional Chinese medicine (integrative body–mind training, IBMT), increases ACC activity and improves self-regulation. However, it is not known whether changes in white matter connectivity can result from small amounts of mental training. We here report that 11 h of IBMT increases fractional anisotropy (FA), an index indicating the integrity and efficiency of white matter in the corona radiata, an important white-matter tract connecting the ACC to other structures. Thus IBMT could provide a means for improving self-regulation and perhaps reducing or preventing various mental disorders.
Keywords: anterior cingulate cortex, anterior corona radiata, integrative body–mind training, relaxation training, self-regulation
Previous studies on effects of long-term training on white matter assessed by diffusion tensor imaging (DTI) have had mixed results. Musicians show positive relations between fractional anisotropy (FA) and training in widespread white-matter regions such as the pyramidal tract (1, 2); however, the opposite result of lower FA in musicians than in nonmusicians in this region of the brain was also reported (3, 4). One study has reported that long-term abacus training from an early age enhances the integrity in white-matter tracts related to motor and visuospatial processes (5). Working memory is an important capacity involved in the short-term maintenance and manipulation of information. A recent study found months of working memory training increases FA associated with the white matter adjacent to the frontoparietal regions critical in working memory (6). Much of the research in training effects on white-matter plasticity have compared experts and novices without explicit training and when training has been used has generally required months to years to produce changes in FA.
The anterior cingulate cortex (ACC) is part of a network implicated in monitoring and resolving conflict among competing response tendencies (7, 8). During infant and child development this structure has been shown to change its connectivity (9, 10). These changes have been related to the increasing ability of children to regulate their own emotions and behavior (11).
Deficits in activation of the ACC have been associated with attention deficit disorder, addiction, dementia, depression, schizophrenia, and other disorders (12–16). In addiction, hypoactivation of the ACC has been found to be critical to symptoms of craving (17). In tobacco addiction, a circuit involving the ACC and striatum has been shown to have lower than normal connectivity (15). Thus evidence related to increasing the activation and strengthening connectivity of the ACC may be useful as a treatment or prevention of addiction and other disorders.
The anterior corona radiata has been identified as one important white-matter tract connecting the ACC to the striatum and other structures (18, 19). Recently individual differences in FA used as a measure of anterior corona radiata connectivity have been specifically related to individual differences in executive attention as measured by the time to resolve conflict during the Attention Network Test (20).
In our previous work, as little as 3 h of integrative body–mind training (IBMT), a meditation method adopted from traditional Chinese medicine (21), in comparison with a randomly assigned control group given relaxation training (RT), reduced the time to resolve conflict in the Attention Network Test and increased ACC activation (22, 23). However, neither 3 nor 6 h of training changed white-matter FA or gray-matter volume as measured by voxel-based morphometry (24). Recently we reported that 11 h of training with IBMT over a 1-mo period improved the efficiency of executive attention and alerting attention networks (21). IBMT also improved the basal immune system in a dose-dependent fashion as the amount of training increased from 3 to 11 h (25).
On the basis of the previous evidence, we hypothesized that 11 h of training with IBMT over 1 mo would increase FA in the anterior corona radiata. To test this hypothesis, we randomized 45 undergraduates to an IBMT or relaxation group for 11 h of training, 30 min per session over a 1-mo period. Before and after training we acquired brain images from each participant at rest for analysis of white matter by diffusion tensor imaging and gray matter by voxel-based morphometry (Materials and Methods).
Results
We examined all brain areas showing FA changes between pre- and posttraining. No areas showed significantly greater FA after relaxation training but a number of areas (Table 1) showed significantly greater FA following IBMT.
Table 1.
Significant FA increases in the IBMT group after 11 h of training
| Name | Voxel | X | Y | Z | P |
| Genu of corpus callosum | 111 | −17 | 23 | 24 | 0.033 |
| Body of corpus callosum | 493 | −13 | 19 | 24 | 0.036 |
| Anterior corona radiata L | 565 | −23 | 22 | 24 | 0.026 |
| Superior corona radiata R | 66 | 18 | 0 | 39 | 0.042 |
| Superior corona radiata L | 169 | −23 | 15 | 30 | 0.030 |
| Superior longitudinal fasciculus L | 46 | −35 | 6 | 21 | 0.037 |
Shown are the number of voxels, coordinates of the center of the mass, and P values of significant FA increases after 11 h of IBMT, all P < 0.05. FA, fractional anisotropy; IBMT, integrative body–mind training.
Given our hypothesis, we examined differences due to training in the anterior corona radiata. Only the left anterior corona radiata showed a significant change in FA so we ran a 2 × 2 repeated-measures ANOVA with group (IBMT and relaxation) and training session (before and after) factors, showed a significant group × session interaction in the left anterior corona radiata [F(1, 42) = 13.441; P = 0.001], indicating that white-matter changes from training in this structure were significantly greater after 11 h of IBMT than those following relaxation training.
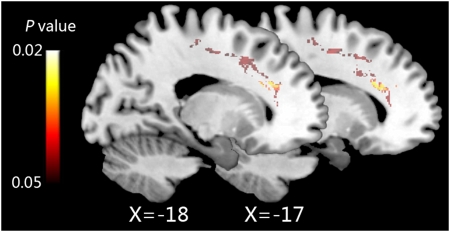
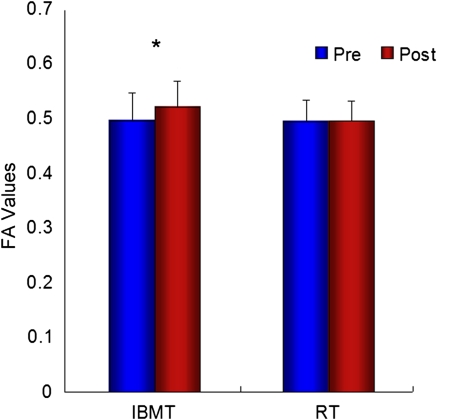
The largest significant increase in FA following IBMT was found in the left anterior corona radiata (Voxel = 565, x = −23, y = 22, z = 24, P = 0.026; Fig. 1). We found no significant increase in the right anterior corona radiata (P > 0.05). Fig. 2 shows FA values in the left anterior corona radiata before and after the IBMT or relaxation training.
Fig. 1.
Eleven hours of IBMT increases fiber integrity in the left anterior corona radiata (after versus before training, two sagittal sections, x = −17 and −18).
Fig. 2.
FA in the left anterior corona radiata before and after IBMT or RT. Changes are shown in FA values in the left anterior corona radiata before and after 11 h of IBMT (P < 0.01) or relaxation training (P > 0.05), indicating the training effects on the integrity and efficacy of the white matter in the region.
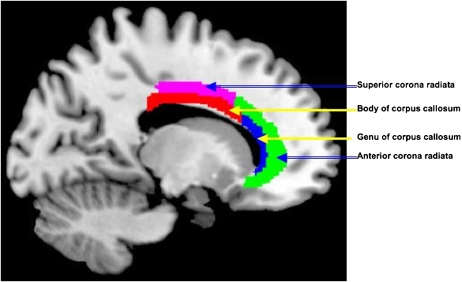
We also found significant FA increases in the body and genu of the corpus callosum, superior corona radiata, and superior longitudinal fasciculus (Fig. 3 and see details in Table 1). There were no significant changes in FA in the relaxation group after 11 h of training (all P > 0.05).
Fig. 3.
Demonstration of brain regions with significant FA increases after 11 h of IBMT. The demonstration map shows the significant FA increases in the left anterior corona radiata (green area), the left superior corona radiata (purple area), the genu of corpus callosum (blue area), and the body of corpus callosum (red area) after 11 h of IBMT, all P < 0.05.
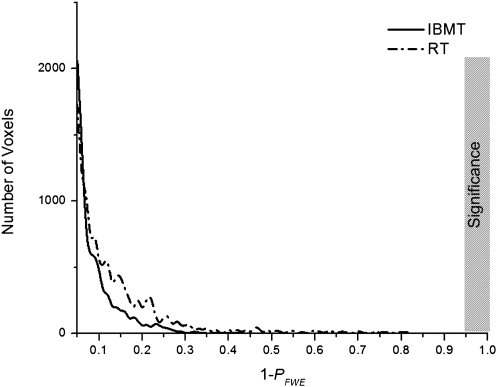
We used voxel-based morphometry to assess gray-matter differences between the two training conditions after 11 h of practice (26). As shown in Fig. 4, the horizontal axis illustrated the 1 − PFWE (corrected) value of the paired t test result, whereas the vertical axis showed the number of voxels with the corresponding P value. The difference between posttest versus pretest was significant, if the 1 − PFWE (corrected) value was larger than 0.95 (PFWE < 0.05). Clearly, neither group showed significant changes in gray-matter volume (all P > 0.05).
Fig. 4.
The intensity and number of voxels after versus before training. The horizontal axis illustrates the 1 − PFWE (corrected) value of a paired t test result, whereas the vertical axis shows the number of voxels with the corresponding P value. The difference between posttest versus pretest was significant, if the 1 − PFWE (corrected) value was larger than 0.95. The gray bar indicates the significance area. No significance was detected in both IBMT (solid line) and RT (dashed line) groups.
Discussion
Eleven hours of IBMT can induce changes in fractional anisotropy in the anterior corona radiata associated with the ACC, a key node of self-regulation network (8, 12, 13, 27, 28). IBMT has been shown to improve the basal immune system as the amount of training increases from 3 to 11 h (25). Because no white matter changes were found after 3 or 6 h of IBMT training (24), the current finding suggests that white-matter changes require more than 6 but less than 11 h of training.
Because changes in myelination lead to FA changes in diffusion tensor imaging, a possible mechanism for the observed FA change is increased myelination after training (6). However, these changes may also reflect differences in the organization of white-matter tracts rather than changes in myelination. It might also be possible that these white-matter changes were due to changes in ventricle volume induced by training. Although this seems unlikely, we performed structural analysis for cerebral spinal fluid using voxel-based morphometry before and after 11 h of training, neither the IBMT nor the relaxation group showed significance in ventricular volume (P > 0.05).
In addition to the increased FA in left anterior corona radiata following 11 h of IBMT, we also found significant effects in adjacent regions of left and right superior corona radiata. We do not know whether this difference in laterality between the anterior and superior corona radiata reflects different functions or differential sensitivity, but the result warrants further investigation. The increased white matter FA in the body and genu of the corpus callosum could lead to increased interhemispheric transfer between the ventral and dorsal anterior cingulate.
We found clear changes in FA after 11 h of training with IBMT, but no change in gray matter. One possible explanation of the result is that the methods used to detect alterations in white and gray matter (FA from diffusion tensor imaging and voxel-based morphometry from T1 images) may have different sensitivities. It is also possible that the training can result in changes in both white and gray matter, but with different time courses. We plan to study this possibility in future experiments.
Because deficits in activation of the ACC have been associated with many disorders (12–16), the ability to strengthen cingulate connectivity through training could provide a means for improving self-regulation and might serve as a possible therapy or prevention tool (13). Further, these findings suggest a use of IBMT as a vehicle for understanding how training influences brain plasticity observed in functional activation, functional connectivity, white matter anisotropy, EEG coherence, gray matter volume, and other measures. There are studies showing all of these changes after various amounts of training in different domains (4, 6, 29, 30), but no way of systemically understanding either their sequence or what behavioral changes accompany them. We believe IBMT might induce with time all of these changes and thus be a good vehicle for basic understanding of their functional significance. Further research may allow us to learn the sequence of events in brain plasticity and how they relate to the behavioral and physiological changes we have reported with IBMT practice.
Materials and Methods
Participants.
Forty-five healthy undergraduates [28 male, mean age, 20.58 ± 1.57 (SD) yr, excluded from neurological or psychiatric disorders] at University of Oregon were recruited and randomly assigned to an IBMT group (22 subjects, 13 male) or a relaxation group (23 subjects, 15 male). The participants had no previous training experience and received 30-min of IBMT or relaxation training group practice every night from Monday through Friday for 1 mo, with a total of 11 h of training. The experiment was approved by the Institutional Review Board at University of Oregon and informed consent was obtained from each participant.
Training Methods.
IBMT involves body relaxation, mental imagery, and mindfulness training, accompanied by selected music background. Cooperation between the body and the mind is emphasized in facilitating and achieving a meditative state. The trainees concentrated on achieving a balanced state of body and mind guided by an IBMT coach and the compact disc. The method stresses no effort to control thoughts, but instead a state of restful alertness that allows a high degree of awareness of body, mind, and external instructions from a compact disc (21, 22, 31). Relaxation training involves the relaxing of different muscle groups over the face, head, shoulders, arms, legs, chest, back, and abdomen, etc., guided by a tutor and compact disc. With eyes closed and in a sequential pattern, one is forced to concentrate on the sensation of relaxation such as the feelings of warmth and heaviness. This progressive training helps the participant achieve physical and mental relaxation and calmness (21, 22).
Data Acquisition and Analysis.
Diffusion tensor imaging.
Brain imaging experiments were performed on an Allegra 3 Tesla scanner (Siemens) at the Lewis Center for Neuroimaging, University of Oregon. Whole brain diffusion weighted volumes (60 directions; b = 700 s/mm2; 60 slices; voxel size 2 × 2 × 2 mm3; TR/TE = 10900/113 ms) plus 10 volumes without diffusion weighting (b = 0 s/mm2) were acquired while the participant was at rest with eyes closed. DTI data were processed using the FSL 4.1 Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/) following the procedures: (i) motion and eddy current corrections, (ii) removal of the skull and nonbrain tissue using the brain extraction tool, and (iii) voxel-by-voxel calculation of the diffusion tensors. Voxelwise statistical analysis of the FA data was carried out using the latest tract-based spatial statistics (TBSS), part of FSL (32, 33), and included: (i) nonlinear alignment of each participant's FA volume to the 1 × 1 × 1 mm3 standard MNI152 space via the FMRIB58_FA template using the FMRIB's nonlinear registration tool (34), (ii) calculation of the mean of all aligned FA images, (iii) creation of a representation of white-matter tracts common to all subjects (white-matter skeleton) by perpendicular nonmaximum-suppression of the mean FA image and setting the FA threshold to 0.2, and (iv) perpendicular projection of the highest FA value (local center of tract) onto the skeleton, separately for each subject. The between group t test was conducted using the randomize tool, which tests the t value at each voxel against a null distribution generated from 5,000 random permutations of group membership. The output contained statistical maps corrected for multiple comparisons (PFWE < 0.05) using threshold-free cluster enhancement (TFCE) (35). Significant clusters from the FA analysis were separately masked and labeled with reference to the JHU ICBM-DTI-81 white-matter labels (36, 37). Forty-four subjects had usable data for 11 h of DTI analysis.
Voxel-based morphometry.
A high-resolution (1 × 1 × 1 mm3) T1-weighted whole-brain image was collected from every subject at rest with eyes closed (with TR/TE/TI = 2,500/4.38/1,100 ms; flip angle, 8°; slice thickness, 1 mm). Voxel-based morphometry analysis (26) was carried out using FSL tools (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html). First, structural images were brain extracted using the BET algorithm. Next, tissue segmentation was carried out using the FAST4 algorithm. The resulting gray matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT, followed by nonlinear registration FNIRT, which uses a b-spline representation of the registration warp field. The resulting images were averaged to create a study-specific template, to which the native gray-matter images were then nonlinearly reregistered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a full width at half maximum of 3 mm. Finally, voxel-wise repeated-measures ANOVAs were applied using permutation-based nonparametric testing, correcting for multiple comparisons across space.
Acknowledgments
Comments on this manuscript from Mary Rothbart (University of Oregon) are gratefully acknowledged. This study was supported by the James S. Bower and John Templeton Foundations, National Natural Science Foundation of China Grants 60971096 and R21DA030066, and the National Institute on Drug Abuse-Intramural Research Program.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 2.Han Y, et al. Gray matter density and white matter integrity in pianists’ brain: A combined structural and diffusion tensor MRI study. Neurosci Lett. 2009;459:3–6. doi: 10.1016/j.neulet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Ullén F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, et al. (2010) Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp. doi: 10.1002/hbm.20996. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi H, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 8.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 9.Fair DA, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothbart MK, et al. Developing mechanisms of self-regulation in early life. Emot Rev. 2010 doi: 10.1177/1754073910387943. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posner MI, Rothbart MK, Sheese BE, Tang YY. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 13.Posner MI, Rothbart MK. Educating the Human Brain. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- 14.Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer's disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- 15.Hong LE, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal D, et al. Diffusion tensor anisotropy in the cingulate gyrus in schizophrenia. Neuroimage. 2010;50:357–365. doi: 10.1016/j.neuroimage.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein RZ, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci USA. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori S, Wakana S, van Zijl PC, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier Science, Amsterdam, The Netherlands; 2005. [Google Scholar]
- 19.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 20.Niogi S, Mukherjee P, Ghajar J, McCandliss BD. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat. 2010;4:2. doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang YY. Exploring the Brain, Optimizing the Life. Beijing: Science Press; 2009. [Google Scholar]
- 22.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci USA. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang YY, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang YY, et al. Does short-term mental training induce grey matter change? Prog Mod Biomed. 2010 in press. [Google Scholar]
- 25.Fan Y, Tang YY, Ma Y, Posner MI. Mucosal immunity modulated by integrative meditation in a dose-dependent fashion. J Altern Complement Med. 2010;16:151–155. doi: 10.1089/acm.2009.0234. [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs A, van Turennout M, Coles MG. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proc Natl Acad Sci USA. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty A, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 29.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 31.Tang YY, Posner MI. Attention training and attention state training. Trends Cogn Sci. 2009;13:222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Rueckert D, et al. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burzynska AZ, et al. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]