Abstract
The size and sensitivity of the T-cell repertoire governs the effectiveness of immune responses against invading pathogens. Both are modulated by T-cell receptor (TCR) activity through molecular mechanisms, which remain unclear. Here, we provide genetic evidence that the SH2/SH3 domain containing proteins Nck lower the threshold of T-cell responsiveness. The hallmarks of Nck deletion were T-cell lymphopenia and hyporeactivity to TCR-mediated stimulation. In the absence of the Nck adaptors, peripheral T cells expressing a TCR with low avidity for self-antigens were strongly reduced, whereas an overall impairment of T-cell activation by weak antigenic stimulation was observed. Mechanistically, Nck deletion resulted in a significant decrease in calcium mobilization and ERK phosphorylation upon TCR engagement. Taken together, our findings unveil a crucial role for the Nck adaptors in shaping the T-cell repertoire to ensure maximal antigenic coverage and optimal T cell excitability.
Keywords: T-cell activation, T-cell receptor, signal transduction, repertoire development, immunodeficiency
The interaction between the T-cell receptor (TCR) and antigenic peptides presented in the context of self-major histocompatibility complex molecules (pMHC) is central to lymphocyte differentiation, maintenance of the peripheral T-cell pool, and acquisition of effector functions. Qualitative and quantitative gauging of TCR engagement shapes the responses of T cells throughout their entire life cycle.
In peripheral lymphoid organs, recurrent TCR engagement by self-pMHC complexes is required for maintaining the naïve T-cell pool, through enhanced T-cell survival and increased homeostatic expansion (1), and for shaping the threshold of T-cell responsiveness (2, 3). The strength of TCR interaction with self-pMHC regulates peripheral expansion of a given T-cell clone and balances its frequency within the peripheral T-cell repertoire (1).
Active proliferation and terminal differentiation of T lymphocytes into effector cells is triggered by recognition of foreign antigens in the presence of costimulation (4). The potency of TCR/pMHC interactions plays a crucial role in modulating T-cell expansion and exit from the lymphoid organs, as well as contraction of the activated T-cell pool (5). In a molecular mimicry model of autoimmunity, immunization with a virus expressing a lower affinity variant of the autoantigen failed to trigger autoimmunity (6). Therefore, the molecular mechanisms enhancing TCR signal potency may regulate the efficiency of immune responses, and may control their switch from harmless to harmful.
Here, we demonstrate that Nck proteins may orchestrate such a mechanism. The Nck proteins are a highly homologous and widely expressed family of adaptor molecules that contain three N-terminal Src homology (SH) 3 domains and a single C-terminal SH2 domain (7). Most mammalian cells contain two homologs: Nck1 and Nck2. In mice, targeted deletion of Nck1 or Nck2 demonstrates no obvious phenotype, suggesting functional redundancy of the two molecules. However, deletion of both Nck molecules results in very early embryonic lethality due to defects in mesoderm derived structures, showing the crucial role of Nck in mammalian development (8).
In several cell types, the Nck proteins link phosphotyrosine signals to actin cytoskeleton remodeling (9–11). Nck couples receptor tyrosine kinases (RTKs) or tyrosine phosphorylated docking proteins, to proteins controlling actin cytoskeletal reorganization, such as the Wiskott–Aldrich syndrome protein (WASP), WAVE-1, and the p21 activated kinase (PAK). Their ability to bind to CD3ε (12) and SLP-76 (13) is compatible with their involvement in the TCR signaling apparatus. In T lymphocytes, biochemical (13) and live colocalization (14) analyses indicate that the Nck adaptors are recruited through their SH2 domain to phosphorylated SLP-76, forming a trimolecular complex of Vav/SLP-76/Nck, involved in actin cytoskeletal rearrangement. Binding of the first SH3 domain of Nck to CD3ε from lysates of activated, but not resting Jurkat T cells, suggests that this interaction depends on a conformational change of CD3ε that exposes its proline-rich sequence (PRS) (12) upon TCRαβ engagement. However, deletion of the CD3ε.PRS (15–17) had no effect on the peripheral T-cell compartment, questioning the proposed mechanism of Nck regulation in mature T cells.
Despite these biochemical studies, the exact role of the Nck adaptors in peripheral T-cell activation remains elusive. In this study, a conditional genetic model was used to selectively delete both Nck adaptors in the T-cell compartment. This model was used to investigate the role of Nck in TCR signal strength in two TCR transgenic models, the HY-TCR (18) and P14-TCR (19) transgenic mice, which differ in their functional properties, as a result of their intrinsic TCR avidity for self antigens (20, 21).
We found that Nck deletion resulted in a severe T-cell lymphopenia, which was particularly profound in mice expressing a transgenic TCR with low avidity to self antigens, and in an overall loss of T-cell reactivity to weak antigenic stimulation. The hyporesponsiveness of Nck-deficient T cells was associated with reduced calcium mobilization and ERK phosphorylation upon TCR engagement. Taken together, our findings demonstrate that the Nck adaptors are required for optimal T-cell activation and point toward their pivotal role in fine-tuning the threshold of T-cell reactivity and shaping the T-cell repertoire.
Results
Targeted Deletion of the Nck Adaptors in Developing and Mature T Cells.
To obtain deletion of both Nck adaptors in the T-cell compartment, Nck1−/− (8) and Nck2flx/flx (10) mice were crossed to Lck-Cre transgenic mice (22). The resulting Lck-Cre × Nck1−/−Nck2flx/flx mouse strain will be referred to as Nck.T−/− (see SI Methods for details of experimental and controls animals). The selective expression of the Cre recombinase in T cells (Fig. S1) resulted in a highly efficient Nck2 deletion in peripheral T cells (Fig. S2A), as assessed by RT-PCR. At the protein level, Nck2 was not detectable in mature T cells from Nck.T−/− mice (Fig. S2B).
Nck.T−/− Mice Are Lymphopenic and Hyporesponsive to TCR-Mediated Stimulation.
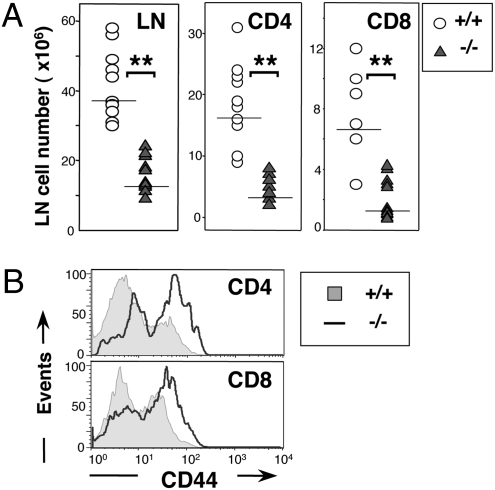
Nck.T−/− mice were lymphopenic and characterized by a 50% decrease in peripheral CD4+ and CD8+ T cells (Fig. 1A), compared with Nck.T+/+ controls. The surface levels of CD3ε, TCRβ, and CD62L remained unaltered in Nck-deficient T cells (Fig. S3C), whereas CD44 expression was significantly increased (Fig. 1B and Fig. S3 A and B), possibly as a result of lymphopenia-driven homeostatic proliferation (23). To assess whether the reduction in the number of Nck-deficient mature T cells was due to the selective depletion of T cells expressing specific TCR Vβ chains, we analyzed by flow cytometry CD4+ and CD8+ T cells from Nck.T−/− and Nck.T+/+ mice. Our findings indicate that Nck deletion did not induce any significant alterations in the frequency of a large set of Vβ chains in peripheral T cells (Fig. S4).
Fig. 1.
Nck deletion results in peripheral T-cell lymphopenia. (A) Total lymph node (LN) cells were markedly decreased in Nck.T−/− mice (n = 16, triangles), in comparison with Nck.T+/+ controls (n = 11, circles; P < 0.0001). The number of Nck-deficient CD4+ and CD8+ T cells was also significantly reduced compared with that of Nck.T+/+ mice. (B) The expression of CD44 was increased on gated CD4+ and CD8+ T cells from Nck.T−/− mice (line) compared with Nck.T+/+ controls (shaded histograms).
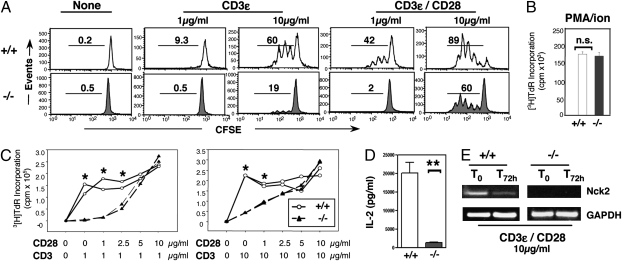
To test the functional properties of Nck-deficient T cells, we assessed their in vitro responsiveness to various stimuli, including plate-bound anti-CD3ε, the combination of anti-CD3ε/anti-CD28, and PMA/ionomycin. Nck-deficient T cells failed to respond to anti-CD3ε stimulation (Fig. 2A). In contrast, we found no alterations in the response to the stimulation by PMA and ionomycin (Fig. 2B), which, bypassing the TCR signaling machinery, induces RasGRP activation and intracellular calcium mobilization. Interestingly, CD28 costimulation rescued CD3-mediated proliferation of Nck-deficient T cells in a dose-dependent fashion (Fig. 2C). This compensatory effect was not due to proliferation of a rare T-cell subset harboring nondeleted Nck2 allele(s), but to expansion of bona fide Nck-deficient T cells, as determined by semiquantitative RT-PCR (Fig. 2E). Interestingly, the impairment of Nck-deficient T-cell responses to TCR-mediated stimulation was associated to a reduction in the expression of the high-affinity IL-2 receptor (CD25; Fig. S5) and to the levels of IL-2 present in the T-cell culture supernatant (Fig. 2D). Thus, Nck deletion induces T-cell lymphopenia and a selective impairment of TCR-driven T-cell proliferation, which can be rescued by CD28 costimulation or bypassing the TCR signaling apparatus.
Fig. 2.
Nck-deficient T cells are hyporesponsive to TCR-mediated stimulation. (A) Proliferation of CFSE-labeled T cells from Nck.T−/− (n = 9, Lower) and Nck.T+/+ (n = 7, Upper) mice was assessed upon in vitro stimulation with low (1 μg/mL) or high (10 μg/mL) doses of anti-CD3ε ± CD28. Nck-deficient T-cell responses were reduced compared with those of Nck+/+ T cells. (B) However, upon stimulation with PMA and a calcium ionophore, T cells from Nck.T−/− (n = 4, filled bar) and Nck+/+ (n = 5, open bar) mice exhibited similar levels of proliferation, as assessed by [3H]TdR incorporation. (C) Nck.T−/− (n = 6) T cells failed to respond to stimulation with low (1 μg/mL, P < 0.0001, Left) and high (10 μg/mL, P = 0.003, Right) doses of anti-CD3ε compared with Nck.T+/+ controls (n = 6). At low doses of anti-CD3ε, low doses of anti-CD28 failed to rescue Nck-deficient T-cell proliferation (1–2.5 μg/mL, P < 0.005). At high doses of anti-CD3ε, Nck-deficient T-cell proliferation was restored by lower doses of anti-CD28 (1 μg/mL, P < 0.001; anti-CD28 > 2.5 μg/mL, P = n.s.). (D) T-cell cultures (αCD3ε/αCD28, 1 μg/mL, 60 h) from Nck.T−/− (n = 6, filled bar) mice exhibited a 6- to 18-fold reduction in the content of IL-2 compared with Nck.T+/+ (n = 6, open bar) T-cell cultures (P < 0.0001), as assessed by ELISA. Data are representative of three independent experiments. (E) Nck2 expression was assessed by semiquantitative RT-PCR on resting (T0) and stimulated (T72h) T cells from Nck.T+/+ (n = 2, Left) and Nck.T−/− (n = 3, Right) mice. T cells from Nck.T−/− mice lacked Nck2 expression before or after stimulation with high doses of anti-CD3/CD28.
Nck Adaptors Are Differentially Required for Low- and High-Avidity T Cells.
The peripheral repertoire comprises T cells that differ in their homeostatic and functional profiles as a result of their intrinsic avidity for self-pMHC complexes (1). To assess the effect of Nck deletion on T cells of different TCR signal strength, we exploited two TCR transgenic systems. The HY-TCR transgenic model (18) is characterized by high receptor editing, low homeostatic proliferation (21), and strong susceptibility to mutations of the TCR signaling apparatus (20) and was therefore chosen to exemplify T cells with low TCR avidity for self pMHC; in contrast, P14-TCR transgenic mice (19) exhibit low receptor editing, high homeostatic proliferation, and resistance to mutations of the TCR/CD3 complex (20), and were selected as a model for T cells with high TCR avidity for self pMHC.
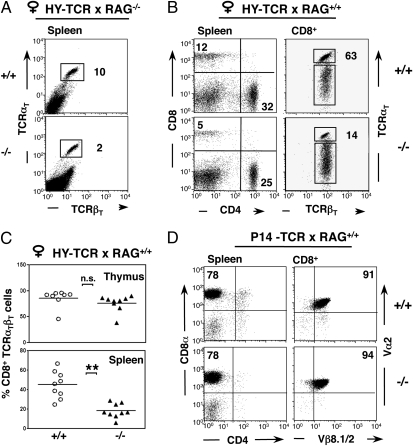
HY-TCR.RAG−/−Nck.T−/− females displayed a drastic decrease in peripheral CD8+ HY-TCR T cells (Fig. 3A), when compared with HY-TCR.RAG−/−Nck.T+/+ females. Such a decrease was also observed in RAG-sufficient HY.Nck.T−/− females, in which the percentage of CD8 T cells expressing high levels of the transgenic TCRα and TCRβ chains were strongly reduced compared with HY.Nck.T+/+ controls (Fig. 3B). To investigate the potential role of peripheral events in the decrease of HY-TCR T cells, we compared the percentage of CD8+ T cells expressing both transgenic TCRα and TCRβ chain (TCRαTβT) in the thymus and in the spleen. Interestingly, the percentage of TCRαTβT+ single positive CD8 thymocytes was not significantly different in the presence/absence of Nck (Fig. 3C Upper), while the percentage of TCRαTβT+ splenic T cells was drastically reduced in Nck.T−/− mice when compared with wild-type controls (Fig. 3C Lower).
Fig. 3.
Nck is required for low-avidity T cells. (A) The percentage of splenic CD8+ T cells expressing the transgenic TCRβ (TCRβT, Vβ8.1/2) and TCRα (TCRαT, T3.70) chains was reduced in RAG2−/−.HY-TCR.Nck.T−/− females (n = 5, Lower) in comparison with RAG2−/−.HY-TCR.Nck.T+/+ females (n = 5; Upper). (B) In RAG2+/+.HY-TCR.Nck.T−/− females (Lower), an overall decrease in splenic CD8+ T cells (Left) was associated to a drastic reduction in T cells expressing both TCRαT and TCRβT (Right) compared with Nck.T+/+ controls (Upper). (C) The percentage of TCRαT βT+ cells was assessed in on gated CD8 single positive thymocytes (Upper) and CD8+ splenic T cells (Lower) from RAG2+/+.HY-TCR.Nck.T−/− females (n = 9, triangles) and RAG2+/+.HY-TCR.Nck.T+/+ controls (n = 9, circles). Nck deletion did not affect the percentage of TCRαT βT+ in mature CD8+ thymocytes (P = 0.24) but resulted in a strong reduction of splenic TCRαT βT+CD8+ T cells (P < 0.0001). Lines represent the arithmetic mean. (D) In the spleen, the percentage of CD8+ T cells was comparable in Nck.T−/− (n = 9) and Nck.T+/+ (n = 9) P14-TCR mice (Left). Gated CD8+ T cells expressed similar levels of the transgenic TCRα (Vα2) and TCRβ (Vβ8.1/2) chains in the presence/absence of Nck (Right). All data are representative of at least three independent experiments.
Unlike in the low-avidity HY-TCR model, the loss of Nck function did not modify the percentage and absolute number of CD8+ T cells in the high-avidity P14-TCR transgenic model (Fig. 3D Left). Despite the possibility of producing T cells with different specificities through TCRα chain recombination, P14-TCRαTβT T cells were strongly predominant in the peripheral repertoire and did not exhibit any alterations in the levels of TCRαT expression or CD8 (Fig. 3D Right).
Taken together, our data point toward a stringent requirement of the Nck adaptors for the inclusion of low- but not high-avidity T cells in the peripheral T-cell repertoire.
Reduced Reactivity of Nck-Deficient T Cells to Weak Antigenic Stimulation.
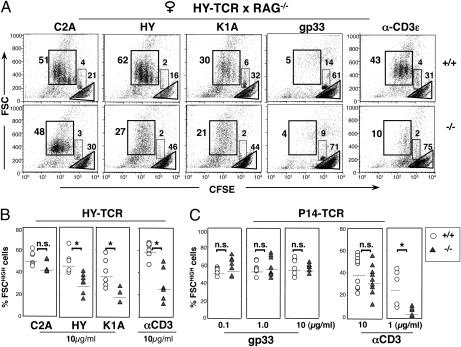
To investigate the role of Nck in T-cell responsiveness to stimuli of different strengths, CD8+ T cells from female HYRAG Nck.T−/− and Nck.T+/+ mice were challenged with dendritic cells presenting peptides of various affinity—notably, the agonist HY, the hyperagonist C2A, the weak agonist K1A (24), and the H-2Db-binding “null” peptide gp33. Nck-deficient HY-TCR T cells failed to respond to the HY and K1A peptides, whereas they vigorously proliferated upon stimulation with the C2A hyperagonist (Fig. 4 A and B). The reduced proliferation of Nck-deficient HY-TCR T cells to the HY peptide was associated with an increase in the percentage of FSClow cells, which, according to Topro3 staining, were apoptotic or no longer viable (Fig. S6).
Fig. 4.
Differential requirement for Nck of low- and high-avidity T-cell responses. (A) CFSE-labeled HY-TCR T cells from RAG−/−. HY-TCR.NckT−/− (n = 6) and Nck.T+/+ (n = 7) females were stimulated with anti-CD3ε (10 μg/mL, Right) or with D1 dendritic cells loaded with peptides of different affinity—namely, the hyperagonist C2A, the agonist HY, the weak agonist K1A, and the “null” peptide gp33. In the CFSE/FSC dot plots, the gates define actively proliferating cells (FSChighCFSEint), viable nondividing cells (FSCintCFSEhigh), and apoptotic/dead cells (FSClow). Nck-deficient T cells exhibited a lower proliferative activity than Nck.T+/+ T cells in response to the HY and K1A peptides (A and B) (HY: P = 0.002; K1A: P = 0.048). In contrast, Nck.T+/+ and Nck.T−/− T cells mounted comparable responses to the C2A hyperagonist. The response of Nck-deficient HY-TCR T cells (n = 5) to optimal CD3 stimulation was impaired, when compared with that of control T cells (A) (n = 7, P = 0.004). Data are representative of three separate experiments, collectively depicted in B. (C) CFSE-labeled P14-TCR T cells from Nck.T−/− and Nck.T+/+ mice were stimulated with dendritic cells loaded with different doses of the agonist peptide gp33. The percentage of actively proliferating (FSChigh) Nck.T−/− (n = 9) and Nck.T+/+ (n = 9) P14-TCR+ T cells was comparable. Proliferation of Nck-deficient (n = 5) P14-TCR T cells was impaired in response to suboptimal doses of anti-CD3ε (P = 0.06). Cumulative results from five (gp33) or three (anti-CD3ε) independent experiments are shown.
In contrast, Nck-deficient P14-TCR T cells did not display any alterations in their proliferative responses to dendritic cells loaded with the agonist peptide gp33, irrespective of the dose of peptide used (Fig. 4C). Thus, loss of Nck function selectively compromises T-cell responses to weak antigenic stimulation.
We reasoned that the ability of dendritic cells to deliver costimulatory signals may mask potential defects of Nck-deficient P14-TCR T cells. Thus, we challenged Nck.T−/− and Nck.T+/+ P14-TCR T cells with different doses of plate-bound anti-CD3ε. Loss of Nck function impaired P14-TCR T-cell responses (Fig. 4C) to low (1 μg/mL) but not high doses of anti-CD3ε (10 μg/mL). In contrast, the low-affinity HY-TCR T cells failed even to respond to high doses of anti-CD3ε stimulation (Fig. 4 A and B). Thus, two points emerge from these observations: first, the Nck adaptors endow the entire T-cell repertoire with enhanced sensitivity to TCR-mediated stimulation; second, their deletion unveils a difference in the threshold of activation by TCR-mediated stimuli of low- and high-affinity T cells.
TCR Internalization and “Capping” Are Not Altered in the Absence of Nck.
Optimal T-cell activation requires actin cytoskeleton remodeling and immune synapse (IS) formation. Because the Nck adaptors regulate actin cytoskeleton in a number of different cell types (7), we tested the efficiency of TCR internalization and “capping,” both dependent on actin cytoskeleton remodeling, in T cells from Nck.T−/− and Nck.T+/+ mice. The kinetics and efficiency of TCR internalization (Fig. S7A) and “capping” (Fig. S7B) were comparable in the presence/absence of Nck, as assessed by phalloidin staining, followed by flow cytometric or microscopic analyses. Thus, the Nck adaptors seem to be dispensable for actin cytoskeleton rearrangement in T cells stimulated by TCR-mediated stimulation.
Nck Deletion Impairs Calcium Mobilization and ERK Phosphorylation in Activated T Cells.
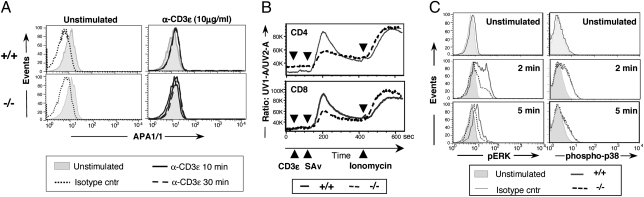
Several lines of evidence link the Nck adaptors to the conformational change and phosphorylation of CD3ε upon TCR engagement. The APA1/1 Ab recognizes the “open” conformer of CD3ε, accessible to Nck binding. APA1/1 staining of freshly isolated peripheral T cells revealed a low but significant “accessibility” of this region to Nck binding under resting conditions (Fig. 5A Left), unmodified by TCR engagement (Fig. 5A Right), similarly to previously reported findings (17). Interestingly, Nck deletion did not modify the pattern of APA1/1 staining (Fig. 5A Lower), indicating that Nck is not required for exposure of CD3ε.PRS epitope in mature resting T cells.
Fig. 5.
Altered signaling in Nck-deficient thymocytes and mature T cells. (A) Peripheral T cells from Nck.T+/+ (n = 6, Upper) and Nck.T−/− (n = 6, Lower) mice were stained with the CD3ε.PRS-specific APA1/1 mAb, under resting conditions (0 min; shaded histogram) and 10 min (solid line) or 30 min (broken line) after CD3ε engagement (10 μg/mL, Right). Isotype controls under resting (dotted line) and stimulating (not shown) conditions had superimposable profiles. (B) Nck-deficient CD4+ and CD8+ T cells (n = 3, dotted line) exhibited lower intracellular calcium flux than Nck.T+/+ T cells (n = 3, solid line) upon CD3ε cross-linking. Data are representative of three separate experiments. (C) Reduced levels of ERK phosphorylation (Left) were detected upon CD3ε stimulation of CD8+ and CD4+ (not shown) peripheral T cells from Nck.T−/− mice (n = 6) compared with Nck.T+/+ (n = 6) T cells. In contrast, no differences were identified in the kinetics and levels of p38 phosphorylation (Right) upon CD3ε stimulation in CD4+ (data not shown) and CD8+ peripheral T cells from Nck.T−/− mice (n = 3) compared with Nck.T+/+ (n = 3) T cells. Dotted lines represent isotype controls.
TCR-mediated stimulation results in the activation of protein tyrosine kinases (PTKs), which associate with the CD3 and TCRζ subunits. Crucial signaling events include tyrosine phosphorylation of enzymes and adaptor proteins by members of the Src kinases (Lck), Syk kinases (ZAP-70), Tec (Itk), and Csk families of nonreceptors PTKs (25). The overall pattern of TCR-mediated tyrosine phosphorylation was similar in Nck.T+/+ and Nck.T−/− mice (Fig. S8), although some differences in the levels of phosphorylation could be identified and are currently under investigation. Production of IP3 and DAG, respectively, results in Ca2+ mobilization and activation of the Ras/MAPK pathway, which are essential for T-cell proliferation, cytokine production, and terminal differentiation (25). Indo-1 staining and flow-cytometric analysis revealed that Ca2+ mobilization was impaired upon TCR-mediated stimulation in Nck-deficient T cells (Fig. 5B). Activation of ERK1/2 was assessed by flow cytometry on Nck.T−/− and Nck.T+/+ peripheral T cells, using phosphospecific Abs, recognizing the active forms of ERK1 and ERK2. Phosphorylation of ERK1/2 was reduced upon activation of Nck-deficient T cells (Fig. 5C Left). In contrast, the levels of p38 phosphorylation induced by TCR cross-linking were comparable in the presence/absence of Nck (Fig. 5C Right). Loss of Nck function did not alter the kinetic of Akt phosphorylation upon TCR-mediated stimulation (Fig. S9).
Thus, our findings point toward a crucial role of the Nck adaptors in regulating key molecular events leading to T-cell cytokine production and proliferation—notably, TCR-mediated calcium mobilization and ERK1/2 phosphorylation.
Discussion
Our data establish a previously unrecognized function of the Nck adaptors as positive regulators of the size and sensitivity of the T-cell repertoire. The powerful combination of a conditional genetic approach with two different TCR transgenic models provides compelling evidence that the Nck adaptors are crucial regulators of the basal activity and TCR-mediated response of mature T cells. Indeed, loss of Nck function affected (i) the number of mature T cells, (ii) the presence of low-avidity T cells in the peripheral repertoire, (iii) the sensitivity of the entire T cell repertoire to antigen-specific stimulation, and (iv) the levels of ERK phosphorylation and intracellular calcium mobilization upon TCR engagement.
How do the Nck adaptors affect the composition of the peripheral T-cell repertoire? A preliminary analysis of the phenotype and TCR Vβ usage of Nck-deficient T cells did not reveal any significant defect. Thus, we focused our attention on the potential effects of the Nck adaptors on TCR signal strength. To this end, we exploited two TCR transgenic models, the HY- and P14-TCR systems, which differ in their avidity for endogenous ligands and in their respective “fitness” for positive selection in the thymus, survival and homeostatic expansion in the periphery. These models enabled us to demonstrate that the Nck adaptors are selectively required for inclusion of low-avidity T cells in the peripheral T-cell repertoire. Indeed, whereas the number and proportion of peripheral P14-TCR T cells remained unchanged upon Nck deletion, low-avidity HY-TCR T cells were drastically reduced in Nck.T−/− female mice and out-competed by T cells expressing endogenously rearranged TCRα chains in HY-TCR RAG+/+ Nck.T−/− females. Our finding that CD8+ T cells expressing endogenously rearranged TCRα chains were greatly enriched in the periphery of Nck.T−/− females compared with their proportion in the CD8 single positive thymocytes, ready to be exported in the periphery, is compatible with the hypothesis that the Nck adaptors enhance the “fitness” of low-avidity T cells in the periphery and that, in the absence of Nck, peripheral events lead to loss of HY-TCR T cells. Thus, it is tempting to speculate that loss of low-avidity T cells may be a major factor in the pathogenesis of lymphopenia in Nck.T−/− mice.
Several lines of evidence suggest that the recurrent interaction of peripheral T cells with self-ligands is necessary for their survival, homeostatic expansion (1), and readiness to respond to incoming stimuli (2, 3). Adoptive transfer experiments will clarify to which extent the Nck adaptors are involved in low-avidity T-cell survival and homeostatic expansion.
Previous studies conducted on mice deprived of the CD3ε.PRS clearly indicated that abrogation of the putative in vivo interaction between Nck and the CD3ε.PRS did not affect the number and function of peripheral T cells (15, 16). Thus, at the molecular level, the impact of the Nck adaptors on peripheral T-cell functions may be mediated by their interaction with other binding partners. A candidate of major interest is the adaptor SLP-76, because biochemical (13) and live colocalization (14) analyses have shown the in vivo formation of a trimolecular complex SLP-76/Vav1/Nck, leading to WASP recruitment and actin cytoskeleton rearrangement. The phenotype of Nck.T−/− shares a number of aspects with those of Vav1−/− (26, 27) and WASP−/− (28, 29) mice—namely, the thymic and peripheral T cell lymphopenia and the T cell hyporeactivity. These similarities suggest that the interaction of Nck with SLP-76/Vav may be crucial in naïve T cells. The lack of gross alterations in actin cytoskeleton remodeling is reminiscent of WASP−/− T cells (30). In this scenario, a thorough analysis of the dynamic events underlying immune synapse formation, symmetry breaking, and reformation (31) warrants further investigation.
Our study has uncovered an essential role for the Nck adaptors in antigen-specific T-cell responses. Nck-deficient T cells failed to proliferate upon stimulation with low- but not high-affinity peptides and could be rescued by CD28 costimulation. Selective TCR tickling of Nck-deficient T cells revealed an overall reduction in T-cell excitability, with a more drastic decline in low-avidity T cells.
The differential requirement for Nck in low- versus high-avidity T cells and the unmodified response of Nck-deficient T cells to stimulation with PMA/ion suggest involvement of Nck in proximal TCR signaling. Experiments conducted on thymocytes (17) and in vitro “differentiated” effector T cells (16) have yielded contrasting results on the role of the CD3ε.PRS in CD3ε.ITAM phosphorylation upon TCR engagement. However, because a fraction of CD3ε molecules seems to be accessible for Nck binding under resting conditions and binding of the Nck.SH31 to a noncanonical PxxDY166 region of CD3ε, contiguous to the CD3ε.PRS (32), prevents CD3ε phosphorylation (33), we speculate that Nck may influence the threshold of peripheral T cell responsiveness by controlling ITAM phosphorylation in the resting CD3 signalosome.
The impairment of ERK phosphorylation and Ca2+ flux in Nck.T−/− T cells is reminiscent of the phenotype of Vav1−/− T cells (26) and indicates the importance of Nck in the activation of downstream pathways leading to cytokine production and T-cell proliferation. The role of Nck in the activation of PLC-γ1, which controls both pathways through the production of IP3 and DAG, warrants further investigation.
How is the Nck activity turned “on” in T cells? One possibility is that it may be activated by weak TCR/pMHC interactions; alternatively, it may be triggered by TCR occupancy per se, but major functional effects may only emerge in proximity to a biological threshold. Finally, Nck may endow T cells with a basal level of activity, a basal “tone,” which enhances their sensitivity to low potency antigenic stimuli. In conclusion, our data point toward a crucial role of the Nck adaptors in enhancing TCR signal potency, thereby allowing optimal responsiveness to weak antigenic simulation and maximal antigenic coverage, through inclusion of low-avidity T cells in the T-cell repertoire.
Methods
Antibodies.
For FACS staining, all monoclonal antibodies (mAbs) were purchased from BD Pharmingen, except those recognizing the HY-TCRα chain (T3.70; CliniSciences) and the CD3ε.PRS (APA1/1; Upstate). In cell signaling studies, short-term T-cell stimulation was induced upon cross-linking of biotinylated anti-CD3ε (500A.2) and anti-CD8α (53-6.7).
Flow Cytometry.
Surface staining was carried out according to standard procedures. In cell signaling studies, Alexa Fluor 647-conjugated antibodies specific for pERK (Cell Signaling 137F5) or phospho-p38 (Cell Signaling 28B10) were used for intracellular staining, according to the manufacturer's instructions. Intracellular Ca2+ flux was assessed by Indo-1 (5μM; Molecular Probes) staining, as a change in the ratio of bound/ unbound Indo-1 fluorescence. Baseline Ca2+ flux was assessed at 37 °C for 1 min. Cells were then treated with biotinylated anti-CD3ε (10 μg/mL; 1 min), followed by cross-linking with streptavidin (20 μg/mL; Sigma) and measurement of Ca2+ flux for 10 min. Ionomycin (100 ng/mL) was added and Ca2+ flux measured (2 min), as positive control. Data were acquired on a FACSVantage (BD).
Four-color FACS samples were acquired on a FACSCalibur (BD); eight-color FACS samples were acquired on an LSRII (BD), using Diva acquisition software. Data analysis was performed with FlowJo (Tree Star).
In Vitro T-Cell Proliferation.
Lymph node T cells were purified by magnetic cell sorting (Dynal) and activated by plate-bound αCD3 (145-2C11; 1–10 μg/mL) with or without αCD28 (37.51; 1–10 μg/mL), or PMA (20 ng/mL; Sigma)/calcium ionophore (A23187, 100 ng/mL; Sigma). T-cell assays were carried out in 96-well plates for 48–72 h at 37 °C, 5% CO2. Supernatants were harvested and IL-2 was quantified by ELISA (R&D Systems ELISA kit).
Peptide-specific responses were assessed in cocultures with D1 dendritic cells (20:1 ratio) (a generous gift of P. Ricciardi-Castagnoli). Upon activation with LPS (1 μg/mL; 12–16 h), D1 cells (2 × 106 cells per mL) were incubated with the following peptides (10 μg/mL, 3 h): HY (KCSRNRQYL), C2A (KASRNRQYL), and K1A [ACSRNRQYL (24)] or lymphocytic choriomeningitis gp33 (KAVYNFATC). T-cell proliferation was assessed by [3H]TdR (1 μCi per well) incorporation or CFSE staining (0.5 μM; Invitrogen).
Western Blotting.
Short-term stimulation of freshly isolated thymocytes was induced by incubation with αCD3ε (500A.2)/αCD8α (53-6.7; 10/10 μg/mL), followed by cross-linking with streptavidin (20 μg/mL). Cells were lysed in buffer containing 0.5% Nonidet P-40, 150 mM NaCl, 20 mM Tris·HCl (pH 7.6), 1 mM EDTA, 1 mM EGTA, 1 mM β-glycerophosphate freshly supplemented with protease inhibitors (Roche), 1 mM NaF, 1 mM Na3VO4, and 1 mM okadaic acid. Abs specific for phospho-Akt (Cell Signaling 587F11), Akt (Cell Signaling), and phospho-tyrosine (4G10 Platinum; Millipore) were used according to standard procedures.
Statistics.
All two-group comparisons were performed with the exact Wilcoxon rank-sum test using SAS (Version 9.1; SAS Institute). In the graphs, P < 0.05 is indicated as significant (*) and P < 0.001 as highly significant (**).
Supplementary Material
Acknowledgments
We thank M. C. Parrini, V. Fraisier, Y. Lepelletier, S. Guegan, G. Caplat, C. Le Moellic, L. Guerri, and C. Hivroz for help in establishing various experimental systems. We are indebted to the Département de Cryopréservation, Distribution, Typage et Archivage animal and the Core Facilities of the Institut Curie for expert technical assistance. This work was supported by grants from INSERM (Avenir), Agence Nationale de la Recherche (ANR-05-MIME), and Fondation pour la Recherche Médicale, and by Excellence Grant “Marie Curie Action” EXT 024281 (to A.T.), and by Canadian Institutes of Health Research Grants MOP-6849 and MOP-13466 (to T.P.). E.R. was supported by fellowships from the FRM and Association pour la Recherche sur le Cancer. A.D.H. is a Special Fellow of the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009743107/-/DCSupplemental.
References
- 1.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13:687–698. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- 3.Stefanova I, Dorfman JR, Tsukamoto M, Germain RN. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronski MA, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 7.Buday L, Wunderlich L, Tamás P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 8.Bladt F, et al. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruenheid S, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- 10.Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett JP, et al. Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci USA. 2007;104:20973–20978. doi: 10.1073/pnas.0710316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil D, Schamel WW, Montoya M, Sánchez-Madrid F, Alarcón B. Recruitment of Nck by CD3 ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 13.Bubeck Wardenburg J, et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 14.Barda-Saad M, et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 15.Szymczak AL, et al. The CD3ε proline-rich sequence, and its interaction with Nck, is not required for T cell development and function. J Immunol. 2005;175:270–275. doi: 10.4049/jimmunol.175.1.270. [DOI] [PubMed] [Google Scholar]
- 16.Tailor P, et al. The proline-rich sequence of CD3ε as an amplifier of low-avidity TCR signaling. J Immunol. 2008;181:243–255. doi: 10.4049/jimmunol.181.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingueneau M, et al. The proline-rich sequence of CD3ε controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat Immunol. 2008;9:522–532. doi: 10.1038/ni.1608. [DOI] [PubMed] [Google Scholar]
- 18.Kisielow P, Teh HS, Blüthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 19.Brändle D, et al. Involvement of both T cell receptor V α and V β variable region domains and α chain junctional region in viral antigen recognition. Eur J Immunol. 1991;21:2195–2202. doi: 10.1002/eji.1830210930. [DOI] [PubMed] [Google Scholar]
- 20.Pitcher LA, van Oers NS. T-cell receptor signal transmission: Who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 22.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maile R, et al. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner M, et al. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 27.Fujikawa K, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotta-de-Almeida V, et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci USA. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol. 2004;173:1658–1662. doi: 10.4049/jimmunol.173.3.1658. [DOI] [PubMed] [Google Scholar]
- 31.Sims TN, et al. Opposing effects of PKCθ and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Kesti T, et al. Reciprocal regulation of SH3 and SH2 domain binding via tyrosine phosphorylation of a common site in CD3ε. J Immunol. 2007;179:878–885. doi: 10.4049/jimmunol.179.2.878. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi K, et al. Structural and functional evidence that Nck interaction with CD3ε regulates T-cell receptor activity. J Mol Biol. 2008;380:704–716. doi: 10.1016/j.jmb.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.