Abstract
Extracellular superoxide dismutase (ECSOD or SOD3) is highly expressed in lungs and functions as a scavenger of O2• ─. ECM fragmentation, which can be triggered by oxidative stress, participates in the pathogenesis of chronic obstructive pulmonary disease (COPD) through attracting inflammatory cells into the lungs. The level of SOD3 is significantly decreased in lungs of patients with COPD. However, the role of endogenous SOD3 in the development/progression of emphysema is unknown. We hypothesized that SOD3 protects against emphysema by attenuating oxidative fragmentation of ECM in mice. To test this hypothesis, SOD3-deficient, SOD3-transgenic, and WT C57BL/6J mice were exposed to cigarette smoke (CS) for 3 d (300 mg total particulate matter/m3) to 6 mo (100 mg/m3 total particulate matter) or by intratracheal elastase injection. Airspace enlargement, lung inflammation, lung mechanical properties, and exercise tolerance were determined at different time points during CS exposure or after elastase administration. CS exposure and elastase administration caused airspace enlargement as well as impaired lung function and exercise capacity in SOD3-null mice, which were improved in mice overexpressing SOD3 and by pharmacological SOD mimetic. These phenomena were associated with SOD3-mediated protection against oxidative fragmentation of ECM, such as heparin sulfate and elastin, thereby attenuating lung inflammatory response. In conclusion, SOD3 attenuates emphysema and reduces oxidative fragmentation of ECM in mouse lung. Thus, pharmacological augmentation of SOD3 in the lung may have a therapeutic potential in the intervention of COPD/emphysema.
Keywords: antioxidants, cigarette smoke, chronic obstructive pulmonary disease, oxidants, glutathione
Extracellular superoxide dismutase (ECSOD or SOD3) is one of the three SOD antioxidant enzyme isoforms, and is highly expressed in lungs and vessels (1, 2). It is located in the ECM, the junctions of airway epithelial cells, the surface of airway smooth muscle, and the lining of vessels of the lung. SOD3 functions as a superoxide anion scavenger, thereby attenuating oxidative stress, which may play an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD). Acute loss of SOD3 in adult mice causes death, whereas low mortality rates are seen in SOD3-overexpressing animals exposed to hyperoxia, suggesting an essential role of SOD3 for survival (3, 4). Accumulating evidence shows that SOD3 polymorphism is associated with a decline in lung function in rodents and humans, and susceptibility to COPD, which may be a result of alteration of its encoding protein structure, function, and level (5–14). However, it is unclear whether endogenous SOD3 participates in the development/progression of the inflammatory response, leading to COPD/emphysema.
Cigarette smoke (CS) is the major etiological factor in the pathogenesis of COPD, which is characterized by chronic lung inflammation, parenchymal destruction (i.e., emphysema), and accelerated decline in lung function. In addition to being a direct exogenous source of reactive oxygen species (ROS), CS also induces endogenous production of ROS from activated inflammatory cells in the lung. ROS can fragment ECM components, such as elastin, heparan sulfate, and hyaluronan, which is an important factor in triggering lung inflammation and causing subsequent airspace enlargement (15–18). SOD3 has been shown to bind to ECM through its positively charged C-terminal, thereby preventing oxidative fragmentation of ECM components (15, 19). However, whether CS exposure is able to fragment ECM leading to lung inflammation and emphysema, and/or SOD3 can exhibit protection against these processes, are unclear. Hence, we hypothesize that CS-induced lung inflammatory and injurious responses would be protected by SOD3 via the prevention of oxidative fragmentation of ECM. To test this hypothesis, SOD3-null (SOD3 KO), SOD3 overexpressing/transgenic (SOD3 Tg), and WT mice were exposed to CS for 3 d [at 300 mg total particulate matter (TPM)/m3] to 6 mo (at 100 mg TPM/m3 for 2–6 mo of exposure) or by intratracheal elastase injection, and the lung inflammatory and injurious responses were assessed. Furthermore, SOD mimetic (MnTE-2-PyP) or N-acetyl-L-cysteine (NAC) was administered to WT and SOD3 KO mice to study the effect of these antioxidants on elastase-induced emphysema. Additionally, the oxidative shedding and fragmentation of ECM were determined to investigate the molecular mechanism of how SOD3 protects against CS-induced lung inflammation and injury.
Results
SOD3 Protected Lung Against Emphysema.
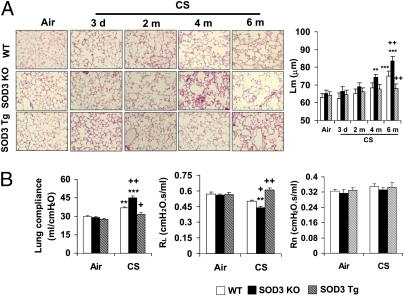
To determine the role of SOD3 in development/progression of experimental COPD/emphysema, we exposed SOD3 KO, SOD3 Tg, and WT mice to CS for 3 d to 6 mo. Lungs from air-exposed WT, SOD3 KO, and SOD3 Tg mice showed normal structure, and there was no significant difference in mean linear intercept (Lm) of airspace among these mice (Fig. 1A). WT C57BL/6J mice exhibited modest airspace enlargement only after 6 mo of CS exposure, which was ameliorated in SOD3 Tg mice. SOD3 KO mice started to show airspace enlargement after 4-mo CS exposure, which was further increased in response to 6-mo CS exposure (Fig. 1A). Moreover, exposure to CS for 6-mo increased lung compliance in WT mice, which was augmented in SOD3 KO mice, whereas overexpression of SOD3 significantly attenuated the increased lung compliance in response to chronic (6-mo) CS exposure compared with WT mice (Fig. 1B). There was a decrease in trend (not significant) in total lung resistance (RL) of WT mice after 6-mo CS exposure, which was significantly improved by SOD3 (Fig. 1B). However, the resistance of central airway (Rn) was not altered either by chronic CS exposure or by SOD3 deficiency/overexpression (Fig. 1B). Exercise capacity is a useful parameter to evaluate cardiopulmonary and skeletal muscle function, which is impaired in patients with COPD/emphysema (20, 21). We also found that 2-, 4- and 6-mo CS exposures significantly decreased the exercise capacity in WT mice, which was improved by SOD3 (Fig. S1). Moreover, SOD3 overexpression protected against 6 mo of CS-induced decrease in arterial oxygen saturation in mice (WT-air, 98.1%; WT-CS, 93.8%; SOD3 KO-air, 97.8%; SOD3 KO-CS, 90.2%; SOD3 Tg-air, 98.5%; SOD3 Tg-CS, 97.1%; P < 0.05).
Fig. 1.
SOD3 attenuated CS-induced airspace enlargement and lung function decline. SOD3 KO, SOD3 Tg, and WT mice were exposed to CS for 3 d to 6 mo (m), and killed at 24 h following their last exposure. (A) SOD3 KO mice were susceptible to develop airspace enlargement whereas overexpression of SOD3 attenuated increased Lm of airspace in response to chronic CS exposure. H&E-stained pictures represent three separate experiments. Original magnification, 100×. (B) Overexpression of SOD3 restored the abnormal increase of lung compliance in mice exposed to CS for 6 mo. SOD3 exhibited a protective effect on RL, although 6-mo CS exposure showed only a decreasing trend in RL in WT mice. However, Rn was not altered by either CS exposure or SOD3. Data are shown as mean ± SEM (n = 3–4 per group). **P < 0.01 and ***P < 0.001 versus the corresponding air-exposed groups; +P < 0.05 and ++P < 0.01 versus the corresponding CS-exposed WT mice.
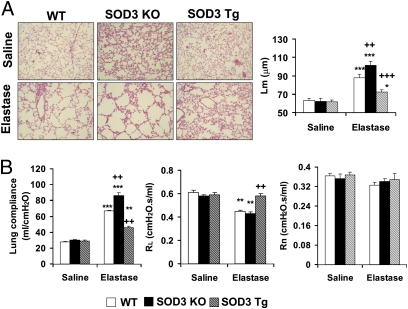
Elastase was intratracheally injected into WT, SOD3 KO, and SOD3 Tg mice to further document the protective role of SOD3 in COPD/emphysema. Overexpression of SOD3 protected against intratracheal injection of elastase-induced airspace enlargement and decline in lung function versus WT and SOD3 KO mice (Fig. 2 A and B). Furthermore, administration of SOD mimetic (MnTE-2-PyP) (22) significantly reduced airspace enlargement as well as redressed abnormal lung mechanical properties in both WT and SOD3 KO mice (Fig. 3 A and B). Interestingly, NAC did not exhibit any beneficial effects in response to elastase-induced emphysema in SOD3 KO mice, although the airspace enlargement was reduced and abnormal lung compliance as well as resistance were redressed by NAC in WT mice exposed to elastase (Fig. 3 A and B). Furthermore, the efficacy of MnTE-2-PyP was higher than that of NAC in attenuating elastase-induced airspace enlargement (MnTE-2-PyP, 12.9% vs. NAC, 7.3%; P < 0.05) and increased lung compliance (MnTE-2-PyP, 28.6% vs. NAC, 14.2%; P < 0.01) in WT mice. Elastase-induced reduction in exercise endurance was also improved by MnTE-2-PyP, but not by NAC, in SOD3 KO mice (Fig. S2). These results suggest that SOD3 and SOD mimetic are beneficial in attenuating pulmonary emphysematous changes caused by both CS and elastase exposures in mice.
Fig. 2.
SOD3 protected against elastase-induced airspace enlargement and lung function decline. (A) Elastase administration significantly increased the value of Lm, which was decreased by SOD3 overexpression. H&E-stained panels represent three separate experiments. Original magnification, 100×. (B) Deficiency of SOD3 increased lung compliance, whereas lung compliance was decreased in SOD3 transgenic mice compared with WT mice administered with elastase. Elastase-induced decrease of RL was attenuated in SOD3 Tg mice compared with WT mice. However, Rn was not altered by either CS exposure or SOD3. Data are shown as mean ± SEM (n = 3–4 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 versus corresponding saline solution–exposed groups; ++P < 0.01 and +++P < 0.001 versus corresponding elastase-exposed WT mice.
Fig. 3.
SOD mimetic attenuated elastase-induced airspace enlargement and lung function decline in WT and SOD3 KO mice. (A) Elastase administration increased the value of Lm in WT and SOD3 KO mice, which was lowered by SOD mimetic. Treatment with NAC reduced elastase-induced airspace enlargement only in WT mice. H&E-stained panels represent three separate experiments. Original magnification, ×100. (B) s.c. injection of SOD mimetic reduced lung compliance and increased RL in both WT and SOD3 KO mice after elastase injection. Elastase-induced reduction in RL was significantly attenuated by NAC only in WT mice. However, Rn was not altered by either SOD mimetic or NAC in both WT and SOD3 KO mice. Data are shown as mean ± SEM (n = 3 per group). **P < 0.01 and ***P < 0.001 versus corresponding saline solution–exposed groups; +P < 0.05, ++P < 0.01, and +++P < 0.001 versus corresponding elastase-exposed control mice; $P < 0.05, $$P < 0.01, and $$$P < 0.001 versus corresponding elastase-exposed WT mice. Con, control; Mimetic, SOD mimetic.
SOD3 Attenuated Lung Inflammatory Response Induced by CS and Elastase.
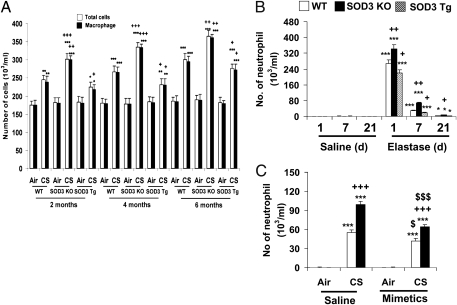
Abnormal lung inflammation is a hallmark of COPD/emphysema. We therefore assessed lung inflammation in SOD3 KO, SOD3 Tg, and WT mice exposed to CS or administered with elastase. As shown in Fig. S3A, overexpression of SOD3 reduced the 3-d CS-induced neutrophil influx in bronchoalveolar lavage (BAL) fluid. Macrophage influx into BAL fluid was not altered in WT and SOD3 Tg mice in response to 3-d CS exposure. Interestingly, disruption of SOD3 decreased macrophage influx in BAL fluid compared with CS-exposed WT mice (Fig. S3B). This was a result of greater macrophage infiltration into lung interstitium of SOD3 KO mice than that in WT mice exposed to CS for 3 d (Fig. S3C). The number of total cells and macrophages in BAL fluid was significantly lowered by SOD3 overexpression in response to CS exposure for 2 to 6 mo using the Teague smoking machine (Model TE-10) (Fig. 4A). Furthermore, overexpression of SOD3 attenuated CS exposure (3 d)-induced release of proinflammatory mediators (e.g., GM-CSF, INF-γ, IL-13, IL-17, IL-1β, IL-6, keratinocyte-derived chemokine, monocyte chemotactic protein–1, macrophage inflammatory protein-2, and TNF-α) in the lungs (Fig. S4). In addition, elastase-induced neutrophil influx into BAL fluid was significantly reduced by SOD3 overexpression (Fig. 4B). Overall, attenuation of emphysema by SOD3 was associated with reduction of lung inflammation in mice.
Fig. 4.
SOD3 protected lung against inflammation in mice exposed to CS and elastase. Inflammatory cell influx into BAL fluid were decreased in SOD3 Tg mice, whereas deficiency of SOD3 enhanced the lung inflammatory response compared with WT mice exposed to CS for 2, 4, and 6 mo (A), or intratracheally injected with elastase (B). (C) s.c. injection of SOD mimetic significantly attenuated 3-d CS-induced neutrophil influx in lungs of both WT and SOD3 KO mice. Data are shown as mean ± SEM (n = 3–5 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 versus corresponding air- or saline solution–exposed groups; +P < 0.05, ++P < 0.01, and +++P < 0.001 versus corresponding CS- or elastase-exposed WT mice; $P < 0.05 and $$$P < 0.001 versus corresponding air- or CS-exposed control groups.
Consistent with its protective effects against emphysema, pharmacological administration of MnTE-2-PyP significantly attenuated 3 d of CS-induced neutrophil influx into BAL fluid in both WT and SOD3 KO mice (Fig. 4C). The protection of MnTE-2-PyP against acute CS-induced neutrophils influx was stronger in SOD3 KO mice than that in WT mice (23.6% inhibition in WT mice vs. 35.4% inhibition in SOD3 KO mice; P < 0.05). Similarly, elastase-induced neutrophil influx into BAL fluid was also significantly reduced by MnTE-2-PyP, but not by NAC, in SOD3 KO mice, although both NAC and MnTE-2-PyP decreased neutrophil influx in WT mice exposed to elastase (Fig. S5). Hence, pharmacological SOD mimetics are effective in reducing lung inflammation even in SOD3 KO mice in response to CS and elastase exposures.
SOD3 Protected Against CS-Induced Heparan Sulfate Shedding and Elastin Fragmentation in the Lung.
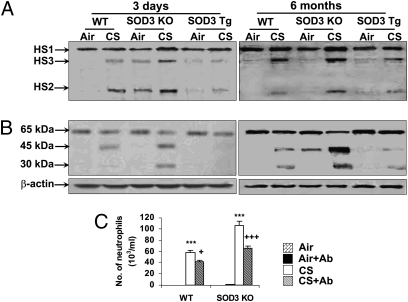
SOD3 has high affinity to ECM components, and their shedding and fragmentation trigger the inflammatory response caused by a variety of stimuli (15–18, 23–25). Hence, immunoblotting was performed to determine whether the protective effect of SOD3 on lung inflammation and subsequent emphysema is caused by an increased integrity of ECM in mouse lung exposed to CS. Both 3-d and 6-mo CS exposures significantly increased the levels of heparan sulfate and its fragmentations in BAL fluid of WT mice, which were reduced by SOD3 overexpression (Fig. 5A). Furthermore, CS exposure increased the levels of elastin fragments in lung of WT mice, indicated by 45- and 30-kDa bands (Fig. 5B). Deficiency of SOD3 enhanced CS-induced elastin fragmentation, whereas the levels of elastin fragments were decreased in SOD3 Tg mice compared with WT mice exposed to CS for 3 d and 6 mo (Fig. 5B). To further study whether enhanced lung inflammation was related to increased ECM fragmentation in SOD3 KO mice in response to CS exposure, intratracheal instillation of antielastin antibody was performed daily for 3 d at 2 h before CS exposure to WT and SOD3 KO mice. These experiments revealed that increased neutrophil influx was significantly attenuated by anti-elastin antibody treatment in WT and SOD3 KO mice exposed to CS for 3 d (Fig. 5C). Furthermore, the efficacy of antielastin antibody against CS-induced neutrophils influx into the BAL fluid was higher in SOD3 KO mice than that in WT mice (WT, 27.5%; SOD3 KO, 37.5%; P < 0.05). These results suggest that protection against CS-induced ECM shedding and fragmentation by SOD3 contributes to the decreased lung inflammatory response and subsequent lung damage, implicating a role of SOD3 in elastin-mediated inflammation and autoimmunity.
Fig. 5.
SOD3 protected against CS-induced shedding and fragmentation of heparan sulfate and elastin, resulting in reduction of neutrophil influx into the lung. SOD3 KO, SOD3 Tg, and WT mice were exposed to CS for 3 d and 6 mo, and the levels of heparan sulfate in BAL fluid (A) and elastin in lung tissues (B) were determined using immunoblotting. (A and B) CS exposure increased the levels/fragments of heparan sulfate in BAL fluid and elastin fragmentation in lung tissues in WT mice, which was prevented by SOD3 overexpression. Gel pictures shown are representative of at least three separate experiments. HS1 (heparan sulfate 1), HS2 (heparan sulfate 2), and HS3 (heparan sulfate 3), which are the species of heparan sulfate, are predicted by molecular mass. (C) Three days of CS-induced neutrophil influx into BAL fluid was attenuated by intratracheal antielastin antibody administration in WT and SOD3 KO mice. Data are shown as mean ± SEM (n = 3–5 per group). ***P < 0.001 versus corresponding air-exposed groups; +P < 0.05 and +++P < 0.001 versus corresponding CS-exposed WT or SOD3 KO mice.
SOD3 Attenuated CS Exposure-Induced Oxidative Stress in the Lung.
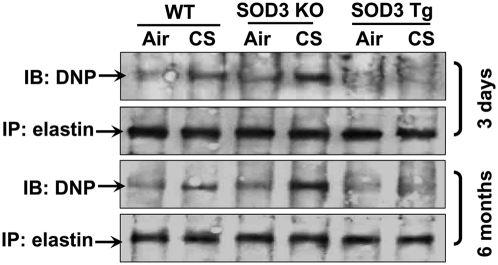
ECM can be subjected to oxidation, which affects its stability, leading to ECM fragmentation and shedding (15, 18, 26). Therefore, we determined elastin oxidation/carbonylation in lungs of WT, SOD3 KO, and SOD3 Tg mice after CS exposures. Both 3-d and 6-mo CS exposures led to elastin oxidation/carbonylation, which was protected by SOD3 overexpression in mouse lung (Fig. 6). Lung levels of lipid peroxidation products [4-hydroxy-2-nonnenal (4-HNE) and malondialdehyde (MDA)] were also attenuated by SOD3 in response to 3-d and 6-mo CS exposures (Fig. S6). Furthermore, SOD3 protected against CS-induced ROS release in BAL cells in response to 3-d CS exposure (Fig. S7). Both acute and chronic CS exposures increased the levels of GSSG in lungs of WT mice, which were augmented in SOD3 KO mice whereas overexpression of SOD3 protected against CS-induced increase in oxidized glutathione (GSSG; Fig. S8). The level of reduced glutathione (GSH) was not altered by SOD3, although increased levels of reduced GSH were observed in mouse lung in response to CS exposure for 3 d (Fig. S8). However, chronic CS exposure significantly decreased the levels of reduced GSH in WT mouse lung, which was further lowered in SOD3 KO mice. The level of reduced GSH was increased in lungs of mice overexpressing SOD3 compared with WT mice exposed to CS for 6 mo (Fig. S8). Both 3-d and 6-mo CS exposures led to a decrease in SOD3 activity in lungs of WT and SOD3 Tg mice (Fig. S9A). Interestingly, the level of SOD3 was increased in response to 3-d CS exposure, whereas 6 mo of CS exposure significantly decreased SOD3 protein levels in lungs of WT mice (Fig. S9B). There was no detectable expression/activity of SOD3 in SOD3 KO mouse lung, whereas the level/activity of SOD3 was significantly increased in lungs of SOD3 Tg mice compared with WT mice (Fig. S9 A–C). In addition, the expression of SOD3 was mainly observed in extracellular areas, such as connective tissues around bronchi and vessels in WT and SOD3 transgenic mice by using an antibody that can recognize both human and mouse SOD3 (Fig. S9C). This was identical to the pattern of murine SOD3 distribution in mouse lung (27). These results suggest that CS-induced reduction of SOD3 activity contributes to enhanced oxidative stress and elastin oxidation/carbonylation in mouse lung.
Fig. 6.
SOD3 attenuated CS-induced elastin carbonylation in the lung. Exposure of WT mice to CS increased elastin carbonylation in lungs of WT mice, which was significantly attenuated in SOD3 Tg mice. Gel pictures shown are representative of at least three separate experiments. DNP, 2,4-dinitrophenylhydrazine.
Discussion
The level of SOD3 is significantly decreased in lungs of patients with COPD (28). We also found the reduction of SOD3 level/activity in lungs of mouse model of emphysema. However, the role of endogenous SOD3 in the development/progression of COPD/emphysema remains unclear. We hypothesized that SOD3 protects the lung against emphysema through decreasing oxidative stress-mediated ECM fragmentation and inflammation. Our findings show that SOD3 KO mice were susceptible to develop airspace enlargement and abnormal lung mechanics, whereas overexpression of SOD3 improved these pathophysiological abnormalities in response to CS exposure or intratracheal elastase administration compared with WT mice. Interestingly, SOD3 also improved CS- and elastase-induced reduction of exercise capacity and arterial oxygen saturation, which are the characteristic features of COPD (20, 21). Hence, SOD3 exhibits a beneficial role in attenuating pulmonary emphysema in mouse models of this disease.
Sustained lung inflammatory response is a key feature in driving the progression of COPD/emphysema. Hence, we determined whether protection against emphysema by SOD3 is a result of decreased lung inflammation in mice exposed to CS or elastase. Lung inflammation was significantly decreased by SOD3 overexpression in mice exposed to CS and elastase. This is in agreement with previous studies showing a protective effect of SOD3 on lung inflammatory responses to hyperoxia, bleomycin, and asbestos (4, 17, 29, 30). Furthermore, the SOD mimetic exhibited a protective effect in lung inflammation in SOD3 KO as well as in WT mice. The efficacy of SOD mimetic against lung neutrophil influx in SOD3 KO mice was higher than that in WT mice exposed to CS and elastase. This may be attributed to increased oxidative stress in lungs of SOD3 KO mice compared with WT mice in response to these stimuli. These results indicate that attenuation of pulmonary emphysema in mice by SOD3 overexpression and SOD mimetic is associated with the reduction of lung inflammation. In agreement with previous observations (31, 32), we did not observe neutrophil influx into BAL fluid in WT mice after 2, 4, and 6 mo of smoke exposures using a Teague smoking machine, whereas 3-d CS exposure significantly increased neutrophil influx using a Baumgartner-Jaeger smoking machine in WT mice. In contrast, the number of macrophages was not altered after 3-d CS exposure, whereas subchronic and chronic CS exposure induced a significant increase in macrophages influx in BAL fluid of C57BL/6J mice. These discrepancies are likely caused by differences in CS doses, and pattern/composition of smoke delivered by different CS generating systems (31, 32).
ECM fragments are shown to trigger lung epithelial proinflammatory response, thereby inducing chemotactic activity for inflammatory cells or to generate neoantigens leading to an autoimmune response, thereby driving the progression of COPD/emphysema (16, 33–35). In the present study, CS exposure significantly induced ECM shedding and fragmentation in mouse lung, which was protected by SOD3. Importantly, pretreatment of antielastin antibody, which neutralizes elastin and its fragments, was more effective in decreasing neutrophil influx in lungs of SOD3 KO mice than in WT mice in response to CS exposure. This was a result of increased elastin fragmentation in SOD3 KO mice after CS exposure compared with WT mice. Moreover, the levels of ECM fragments were increased in lungs of patients with COPD and in mouse lungs with emphysema (16, 33, 34). Hence, inhibition of ECM fragmentation by SOD3 contributes to its protection against CS- and elastase-induced lung inflammation and subsequent emphysema.
In addition to proteases, oxidative stress is also shown to cause ECM oxidation, degradation, and fragmentation (15, 18). We therefore proposed that SOD3 attenuates CS-induced ECM oxidation, degradation, and fragmentation in mouse lung through reduction of oxidant stress. As expected, overexpression of SOD3 protected against CS-induced elastin oxidation/carbonylation in mouse lung. Furthermore, decreased levels of lipid peroxidation products by SOD3 would further reduce the formation of MDA- or 4-HNE-elastin adducts, which possibly ameliorate ECM fragmentation/shedding or autoimmunity in mouse lung exposed to CS. Further study is required to investigate whether these oxidized/carbonylated ECM or ECM adducts (with MDA, 4-HNE, or acrolein) are key factors to induce its fragmentation or autoimmunity in the progression of COPD. Enhanced oxidative stress is also involved in the development of elastase-induced emphysema (36, 37). Hence, decreased oxidative stress by SOD3 contributes to reduced ECM oxidation/carbonylation and subsequent fragmentation/shedding leading to inflammatory response in mouse lung in response to CS exposure. It is also likely that SOD3 may indirectly intervene the inflammatory signaling (e.g., redox-dependent NF-κB activation) via maintaining the physiological intracellular GSH/GSSG redox status (e.g., lowering GSH depletion and oxidation) in response to CS exposure. The protection against oxidative stress by SOD3 was not a result of compensatory regulation of SOD1, SOD2, catalase, or glutathione peroxidase, as these enzymes were not influenced by SOD3 deficiency or overexpression (4, 38, 39). Interestingly, the germline SOD3 KO mice used in the present study survived without lung inflammation and injury, whereas acute loss of SOD3 in adult mice results in lung damage and 85% mortality in the presence of ambient air (3, 38). The mechanism for these discrepancies is unclear and remains to be investigated.
Antioxidants and antiinflammatory agents are promising therapies for COPD/emphysema because oxidative stress and abnormal inflammation initiate and aggravate COPD progression. Unfortunately, currently available antioxidants (e.g., NAC) and antiinflammatory therapies (e.g., corticosteroids and the anti–TNF-α antibody infliximab) are ineffective or show limited efficacy in improving lung function and quality of life, with patients becoming resistant or having side effects from the use of these therapies (40–42). Recently, a redox-sensitive transcription factor, Nrf2, has been suggested to be an interesting target to intervene in the progression of experimental COPD/emphysema (43). However, it remains to be seen how effective Nrf2 activators will be in the treatment of COPD/emphysema, as it is posttranslationally modified, destabilized, and degraded in lungs of patients with COPD, and in rodent lungs exposed to CS (44–47). In light of this, we compared the efficacy of SOD mimetic with NAC on elastase-induced emphysema in WT C57BL/6J and SOD3 KO mice. We found that SOD mimetic was more efficacious than NAC in attenuating elastase-induced airspace enlargement and lung function decline in WT mice. This is corroborated by the studies that show a slight protective effect of NAC against rat emphysema induced by elastase and CS exposure (48, 49). Interestingly, SOD mimetic, but not NAC, exhibited a protective effect on elastase-induced emphysematous changes in SOD3 KO mice. These results indicate the importance of SOD3 in comparison with NAC in attenuating elastase-induced emphysema. Overall, specific SOD3 mimetics could be promising drug candidates to prevent ROS-mediated ECM oxidation and fragmentation, as well as to protect against COPD.
In conclusion, SOD3 was shown to protect lung against emphysema induced by CS and elastase, which was attributed to reduction of oxidative ECM fragmentation and oxidative posttranslational modifications of elastin fragments (leading to autoantibody production) in the lung. Administration of SOD mimetic significantly attenuated elastase-induced emphysema in both WT and SOD3 KO mice. Furthermore, the level/activity of SOD3 was decreased in the emphysematous mouse lung. Therefore, the development of specifically pharmacological mimetics to replenish/augment SOD3 in the lung may have a therapeutic potential in the intervention of COPD/emphysema.
Materials and Methods
Mice and CS Exposure.
The generation of SOD3 KO and SOD3 Tg mice were described previously, with their background WT mice being the C57BL/6J strain (38, 39, 50). All animal procedures described in this study were approved by the University Committee on Animal Research Committee of the University of Rochester. For studies involving 3-d CS exposure, research-grade cigarettes (3R4F; University of Kentucky, Lexington, KY) were used to generate smoke, and 8-wk-old male mice were exposed to mainstream CS using a Baumgartner-Jaeger CSM2072i automatic CS generating machine (CH Technologies) as described previously (31). The smoke concentration was set at a value of approximately 300 mg TPM/m3 (31, 37). Mice received two 1-h exposures (1 h apart) daily for 3 consecutive d, and were killed at 24 h after last exposure. For 2-, 4-, and 6-mo CS exposures, 3R4F cigarettes were used to generate a mixture of sidestream smoke (89%) and mainstream smoke (11%) by a Teague smoking machine (model TE-10; Teague Enterprises) at a concentration of approximately 100 mg TPM/m3 to avoid the possible toxicity to mice at a high concentration of long-term CS exposure (32, 43). Mice received 5-h exposures per day, 5 d/wk, for 2, 4, or 6 mo, and were killed at 24 h after last CS exposure. Control mice were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure.
Intra/Endotracheal Administration of Porcine Pancreatic Elastase and Anti-Elastin Antibody in Mice.
Fifty microliters of saline solution alone or saline solution containing 1 U of porcine pancreatic elastase (Sigma) was sprayed into the trachea (36, 51). Mice were killed at day 1, 7, or 21 after elastase injection. Similarly, 50 μL of saline solution alone or saline solution containing 10 μg of antielastin antibody (Santa Cruz Biotechnology) was intratracheally injected daily at 2 h before CS exposure for 3 d (16). Mice were killed at 24 h after the last CS exposure.
Administration of SOD Mimetic and NAC in Mice.
SOD mimetic (MnTE-2-PyP) was administered daily through s.c. injection at 2 h before CS exposure (two 1-h exposures, 1 h apart) for 3 d. The dose of MnTE-2-PyP was chosen based on our previous study with slight modification (52). Briefly, a 2.5 mg/kg body mass loading dose of MnTE-2-PyP was used on day 1, then 5 and 10 mg/kg of SOD mimetic was administered on days 2 and 3, respectively. Animals that did not receive the drug were injected with equivalent volumes of sterile saline solution as control. To study the effect of SOD mimetic or NAC on elastase-induced emphysema, MnTE-2-PyP (5 mg/kg) was s.c. injected daily or NAC (Sigma) was included in drinking water at the dose equivalent to 2.0 g/kg/d at 2 d before the saline solution or elastase instillation until the time points at which the animals were killed (49, 53).
Statistical Analysis.
Statistical analysis of significance was calculated using one-way ANOVA followed by Tukey post hoc test for multigroup comparisons using StatView software. For details of other experimental methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Saravanan Rajendrasozhan and Ms. Suzanne E. Cook for their technical assistance. This study was supported by National Institutes of Health Grants R01-HL085613, R01-HL097751, and 1R01HL092842 (to I.R.); Grant U01 HL089897 (to J.D.C.); and National Institute of Environmental Health Sciences Grant P30-ES01247. V.L.K. was supported by the governmental subsidy for health science research (EVO) of the Helsinki University Hospital, Finnish Antituberculosis Association Foundation, and Yrjö Jahnsson Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This work was presented in part at the American Thoracic Society International Conference, May 15–20, 2009, San Diego, and the European Respiratory Society Lung Science Conference, May 26–28, 2010, Estoril, Portugal. It was published in abstract form (Am J Respir Crit Care Med 179:A4162).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007625107/-/DCSupplemental.
References
- 1.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 2.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 3.Gongora MC, et al. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: A potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganguly K, et al. Superoxide dismutase 3, extracellular (SOD3) variants and lung function. Physiol Genomics. 2009;37:260–267. doi: 10.1152/physiolgenomics.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl M, et al. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med. 2008;178:906–912. doi: 10.1164/rccm.200804-549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul K, Tybjaerg-Hansen A, Marklund S, Lange P, Nordestgaard BG. Genetically increased antioxidative protection and decreased chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:858–864. doi: 10.1164/rccm.200509-1387OC. [DOI] [PubMed] [Google Scholar]
- 8.Young RP, et al. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax. 2006;61:394–399. doi: 10.1136/thx.2005.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcaroli JJ, et al. Extracellular superoxide dismutase haplotypes are associated with acute lung injury and mortality. Am J Respir Crit Care Med. 2009;179:105–112. doi: 10.1164/rccm.200710-1566OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganguly K, et al. Candidate genes controlling pulmonary function in mice: transcript profiling and predicted protein structure. Physiol Genomics. 2007;31:410–421. doi: 10.1152/physiolgenomics.00260.2006. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard C, et al. Genomewide linkage analysis identifies novel genetic Loci for lung function in mice. Am J Respir Crit Care Med. 2005;171:880–888. doi: 10.1164/rccm.200409-1204OC. [DOI] [PubMed] [Google Scholar]
- 12.Bentley AR, Emrani P, Cassano PA. Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: A systematic review. Thorax. 2008;63:956–961. doi: 10.1136/thx.2007.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandström J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 14.Chu Y, et al. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation. 2005;112:1047–1053. doi: 10.1161/CIRCULATIONAHA.104.531251. [DOI] [PubMed] [Google Scholar]
- 15.Kliment CR, et al. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houghton AM, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283:6058–6066. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi A, Ryu A, Suzuki T, Kawada A, Tajima S. In vitro degradation of tropoelastin by reactive oxygen species. Arch Dermatol Res. 1998;290:497–500. doi: 10.1007/s004030050342. [DOI] [PubMed] [Google Scholar]
- 19.Folz RJ, Crapo JD. Extracellular superoxide dismutase (SOD3): Tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- 20.Montes de Oca M, Rassulo J, Celli BR. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med. 1996;154:1284–1289. doi: 10.1164/ajrccm.154.5.8912737. [DOI] [PubMed] [Google Scholar]
- 21.Oga T, et al. Exercise capacity deterioration in patients with COPD: Longitudinal evaluation over 5 years. Chest. 2005;128:62–69. doi: 10.1378/chest.128.1.62. [DOI] [PubMed] [Google Scholar]
- 22.Oury TD, et al. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SV, et al. Extracellular superoxide dismutase (EC-SOD) binds to type I collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 24.Kliment CR, et al. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 26.Mattana J, Margiloff L, Chaplia L. Oxidation of extracellular matrix modulates susceptibility to degradation by the mesangial matrix metalloproteinase-2. Free Radic Biol Med. 1999;27:315–321. doi: 10.1016/s0891-5849(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.Ookawara T, et al. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275:C840–C847. doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- 28.Stark H, et al. Decreased levels of extracellular superoxide dismutase in the lung and sputum supernatants of the patients with COPD. Am J Respir Crit Care Med. 2008;177:A657. [Google Scholar]
- 29.Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2006;40:601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 31.Yao H, et al. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–L1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- 32.Rangasamy T, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 34.Weathington NM, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 35.van Houwelingen AH, et al. Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB J. 2008;22:3403–3408. doi: 10.1096/fj.07-096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita T, et al. Thioredoxin prevents the development and progression of elastase-induced emphysema. Biochem Biophys Res Commun. 2007;354:712–719. doi: 10.1016/j.bbrc.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Foronjy RF, et al. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oury TD, Ho YS, Piantadosi CA, Crapo JD. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Natl Acad Sci USA. 1992;89:9715–9719. doi: 10.1073/pnas.89.20.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rennard SI, et al. COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 41.Decramer M, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 42.Pauwels RA, et al. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 43.Sussan TE, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goven D, et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra D, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kode A, et al. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki M, et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 48.Cai S, Chen P, Zhang C, Chen JB, Wu J. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology. 2009;14:354–359. doi: 10.1111/j.1440-1843.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 49.Rubio ML, Martin-Mosquero MC, Ortega M, Peces-Barba G, González-Mangado N. Oral N-acetylcysteine attenuates elastase-induced pulmonary emphysema in rats. Chest. 2004;125:1500–1506. doi: 10.1378/chest.125.4.1500. [DOI] [PubMed] [Google Scholar]
- 50.Demchenko IT, Oury TD, Crapo JD, Piantadosi CA. Regulation of the brain's vascular responses to oxygen. Circ Res. 2002;91:1031–1037. doi: 10.1161/01.res.0000043500.03647.81. [DOI] [PubMed] [Google Scholar]
- 51.Bivas-Benita M, Zwier R, Junginger HE, Borchard G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm. 2005;61:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Makinde AY, et al. Effect of a metalloporphyrin antioxidant (MnTE-2-PyP) on the response of a mouse prostate cancer model to radiation. Anticancer Res. 2009;29:107–118. [PubMed] [Google Scholar]
- 53.Podowski M, et al. Complex integration of matrix, oxidative stress, and apoptosis in genetic emphysema. Am J Pathol. 2009;175:84–96. doi: 10.2353/ajpath.2009.080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.