Abstract
Salmonella enterica serovar Typhi, the cause of typhoid fever, is host-adapted to humans and unable to cause disease in mice. Here, we show that S. Typhi can replicate in vivo in nonobese diabetic (NOD)-scid IL2rγnull mice engrafted with human hematopoietic stem cells (hu-SRC-SCID mice) to cause a lethal infection with pathological and inflammatory cytokine responses resembling human typhoid. In contrast, S. Typhi does not exhibit net replication or cause illness in nonengrafted or immunocompetent control animals. Screening of transposon pools in hu-SRC-SCID mice revealed both known and previously unknown Salmonella virulence determinants, including Salmonella Pathogenicity Islands 1, 2, 3, 4, and 6. Our observations indicate that the presence of human immune cells allows the in vivo replication of S. Typhi in mice. The hu-SRC-SCID mouse provides an unprecedented opportunity to gain insights into S. Typhi pathogenesis and devise strategies for the prevention of typhoid fever.
Keywords: pathogenesis, typhoid, virulence, animal model, bacterial infections
Typhoid fever is a disease of antiquity that continues to exact a considerable human toll today. The World Health Organization estimates that more than 16,000,000 new cases of typhoid each year result in ∼600,000 deaths (1). Typhoid is caused by Salmonella enterica serovar Typhi (S. Typhi), a Gram-negative bacterium transmitted through contaminated food or water. Humans are the only known reservoir for S. Typhi. Multidrug-resistant strains have created new challenges for typhoid treatment (2). Neither vaccine currently available for typhoid prevention is completely effective, with protection rates varying from 60% to 80% (3). The Vi parenteral vaccine has a high incidence of local adverse reactions, fails to induce mucosal responses, does not elicit a booster effect, and induces relatively short-lived protection. The Ty21a oral vaccine can elicit durable responses (4) but does not withstand storage under adverse conditions (5) and requires multiple doses.
Much has been learned about Salmonella pathogenesis in recent decades (6). Salmonella is distinguished from other enteric bacteria principally on the basis of acquired DNA in the form of genomic islands, smaller islets, plasmids, and bacteriophages. In particular, a type III secretory system (T3SS) encoded by Salmonella Pathogenicity Island-1 (SPI-1) allows Salmonella to invade host epithelial cells, induce intestinal inflammation, and cause macrophage death (7, 8); the SPI-2–encoded T3SS expressed in the intracellular environment interferes with vesicular trafficking and promotes bacterial survival (9–11), and SPI-7 encodes a capsule that enables S. Typhi to resist phagocytosis and complement killing (12) and suppresses innate inflammatory responses (13, 14). Salmonella virulence factors are controlled by a plethora of regulators, including two component systems (PhoQ-PhoP and SsrA-SsrB), counter silencers (SlyA), alternative sigma factors (σE and σS), and nucleoid-associated proteins (H-NS) (15–18).
Most current understanding of Salmonella pathogenesis comes from studies of S. Typhimurium in mice. S. Typhimurium–murine interactions are often stated to mimic S. Typhi–human interactions (19), but murine typhoid and human typhoid differ in a number of important respects. S. Typhi possesses virulence factors (e.g., Vi capsular polysaccharide and the CdtB cytolethal distending toxin) not shared with S. Typhimurium (20, 21) and likewise, has lost numerous S. Typhimurium genetic loci by genomic decay (22). Although aro and phoP mutations attenuate virulence in both S. Typhimurium and S. Typhi (23–27), other mutations that attenuate virulence in S. Typhimurium (e.g., galE and cya-crp) fail to attenuate the ability of S. Typhi to cause bacteremia and typhoid-like symptoms in humans (3, 28, 29). This complicates attempts to construct improved live attenuated typhoid vaccines on the basis of observations in mice infected with S. Typhimurium. Even more importantly, determinants of innate immunity to S. Typhimurium and S. Typhi are fundamentally distinct. Studies in mice have shown that IFNγ/IL-12 signaling and the NADPH phagocyte oxidase play a critical role in innate immunity to S. Typhimurium (30, 31). However, humans deficient in these host defenses exhibit enhanced susceptibility to S. Typhimurium but not S. Typhi (32–34). Similarly, mice lacking CD4+ T cells are highly susceptible to S. Typhimurium (30) as are humans with low CD4 T cell counts caused by HIV infection (35). However, HIV infection does not confer a risk of increased incidence or severity of typhoid because of S. Typhi infection (36). This indicates that S. Typhimurium and S. Typhi have qualitatively different interactions with innate and adaptive immunity, and the murine S. Typhimurium infection model fails to recapitulate essential aspects of human typhoid.
S. Typhi is highly adapted to humans and fails to cause progressive infection in normal mice. Only the coadministration of hog gastric mucin to overwhelm host phagocytes or the use of massive bacterial inocula renders S. Typhi lethal for mice (37, 38); however, such models provide limited insights into typhoid pathogenesis and have not been found to correlate well with human typhoid (39), because they are drastically removed from physiological host–pathogen interactions. The lack of a small-animal model has been a major impediment in understanding mechanisms of S. Typhi virulence. Here, we report the development of a humanized small-animal model for the study of human typhoid fever.
Immunocompromised mouse strains, including mice lacking a respiratory burst and inducible nitric oxide synthase (31), or sublethally irradiated mice, with impaired cell-mediated immunity (37), fail to support productive infection of S. Typhi, suggesting that S. Typhi replication in mice is not restricted by murine innate immunity but rather, that infection of human immune cells is required for S. Typhi replication in vivo. This is consistent with studies indicating that human macrophage cell lines are better able than murine cells to support S. Typhi replication in vitro (40), although more substantial differences in intracellular survival of S. Typhimurium and S. Typhi are observed when mouse macrophages are infected in vivo (41).
Recent technological advances have permitted the development of humanized mice engrafted with human hematopoietic stem cells that generate human immune systems. This has been facilitated by lineages of immunodeficient mice, most notably NOD (nonobese diabetic)-scid IL2rγnull mice that lack the IL-2 receptor common γ-chain (42). These mice exhibit multiple defects in innate immunity, lack adaptive immune function, and support heightened human hematolymphoid engraftment. The IL-2r γ-chain is required not only for IL-2 high-affinity ligand binding and intracellular signaling but also for IL-4, IL-7, IL-9, IL-15, and IL-21 binding and signaling (42), and its absence results in a complete block of mature T cell, B cell, and NK (natural killer) cell development. Sublethally irradiated newborn NOD-scid IL2rγnull mice engrafted with CD34+ hematopoietic stem cells (HSC) from T cell-depleted human umbilical cord blood (hu-SRC-SCID or human-SCID repopulating cell-SCID mice) develop into mice with chimeric hematopoietic systems containing human immune cells in the immunodeficient mouse environment (42), including human B cells, CD4+ and CD8+ T cells, NK cells, monocytes, and myeloid and plasmacytoid dendritic cells (43, 44).
Results
Course of Salmonella Typhi Infection in hu-SRC-SCID Mice.
I.p. injection of ∼105 cfu of S. Typhi strain Ty2 (Vi-antigen positive) resulted in progressive lethal infection of hu-SRC-SCID mice within 2–3 d (Fig. 1A). NOD immunocompetent and nonengrafted NOD-scid IL2rγnull animals infected in parallel as controls survived and seemed well throughout the course of the experiment, confirming that even severely immunocompromised NOD-scid IL2rγnull mice are resistant to S. Typhi. At necropsy, the organism burden in livers and spleens of hu-SRC-SCID mice exceeded the initial inoculum by more than 10-fold (Fig. 1B), indicating the occurrence of net S. Typhi replication in vivo. This contrasts with the absence of net S. Typhi replication observed in immunocompromised mice treated with inhibitors of inducible nitric oxide synthase or the NADPH phagocyte oxidase (Fig. S1) or in nonengrafted NOD-scid IL2rγnull animals (Fig. 1B). Infected hu-SRC-SCID mice contained 10- to 100-fold higher organism burdens in the liver and spleen compared with nonengrafted NOD-scid IL2rγnull animals, with statistically significant differences observed in the livers (P = 0.0317) and a nonsignificant trend observed in the spleens (P = 0.1905).
Fig. 1.
S. Typhi virulence in hu-SRC-SCID Mice. (A) Survival of engrafted hu-SRC-SCID mice after i.p. injection of 5.5 × 104–3.2 × 105 cfu (low, n = 11) or 3 × 106 cfu (high, n = 8). S. Typhi Ty2 is shown as a Kaplan–Meier plot. Survival is compared with that of parental nonengrafted NOD-scid IL2rγnull (n = 6) or immunocompetent NOD+/+ (n = 6) mice receiving i.p. injection of 5.5 × 104–1.8 × 105 cfu. Aggregate data include mice receiving Ty2 and Ty2-derived transposon pools. (B) Organism burden was quantified in livers and spleens of nonengrafted NOD-scid IL2rγnull and engrafted hu-SRC-SCID mice 48 h after i.p. inoculation of 1–3 × 105 cfu S. Typhi Ty2. Horizontal lines indicate medians. Asterisk denotes significant difference by Wilcoxon rank-sum test.
Pathology of the hu-SRC-SCID Model.
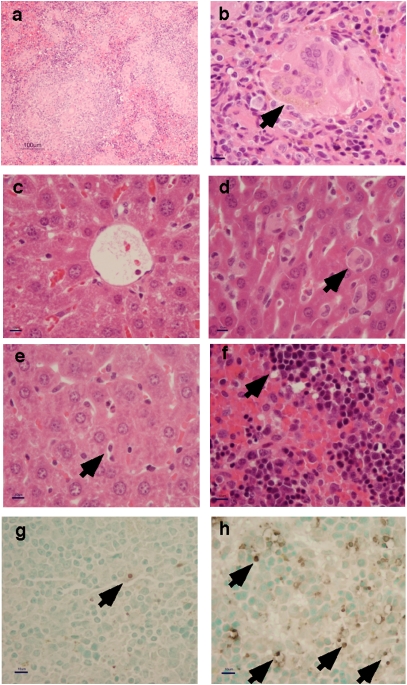
Pathological changes in infected hu-SRC-SCID mice included evidence of central lobular hepatocellular injury with vesiculation and cytoplasmic hyaline changes, Kupffer cell swelling, effacement of normal splenic architecture with lymphocyte depletion, and the presence of large splenic granulomas with palisading epithelioid macrophages and multinucleated giant cells (Fig. 2). Human mononuclear cells could be visualized in the livers and spleens of infected hu-SRC-SCID mice by immunohistochemical staining (Fig. 3). Cell death in the spleens of infected animals was confirmed by TUNEL staining (Fig. 2 G and H). In contrast, few inflammatory changes were observed in infected but nonengrafted NOD-scid IL2rγnull control animals.
Fig. 2.
Pathology of typhoid in hu-SRC-SCID mice. (A) Granulomatous inflammation with mononuclear cell infiltration in the spleen of an infected hu-SRC-SCID mouse after 48–72 h. (B) Multinucleated giant cell (arrow) in the spleen of an infected hu-SRC-SCID mouse. (C) Central lobular hepatocellular changes in the liver of an infected hu-SRC-SCID mouse. (D) Kupffer cell swelling (arrow) in the liver of an infected hu-SRC-SCID mouse. (E) Mild hepatocellular changes with normal-appearing Kupffer cells (arrow) in the liver of an infected control NOD-scid IL2rγnull mouse. (F) Pyknotic lymphocytes (arrow) with cytoplasmic shrinkage in the spleen of an infected hu-SRC-SCID mouse. (G) Low background levels of cell death (arrow) visualized by TUNEL straining in the spleen of an infected control NOD-scid IL2rγnull mouse. (H) Increased cell death (arrows) visualized by TUNEL staining in the spleen of an infected hu-SRC-SCID mouse. (Magnification: A, 100×; B–H, 400×; scale bar: A, 100 μm; B–H, 10 μm.)
Fig. 3.
Visualization of human CD45+ cells in infected hu-SRC-SCID mice. Sections were obtained from NOD-scid IL2rγnull or hu-SRC-SCID mice infected as in Fig. 2 and strained with H&E (Left). Engrafted hematopoietic cells expressing human CD45 are stained brown and counterstained with hematoxylin (Right).
Human and Murine Cytokine Production.
Blood samples obtained from S. Typhi-infected NOD-scid IL2rγnull and hu-SRC-SCID mice euthanized 56–72 h after inoculation were analyzed for cytokine levels using a commercial bead array capable of distinguishing cytokines of murine and human origin (BD Biosciences). Elevated levels of IL-6 and monocyte chemotactic protein-1 (MCP-1) of both human and murine origin were observed, along with IFNγ and TNFα of predominantly human origin and IL-10 of predominantly murine origin (Fig. 4). Only modest elevations in murine IL-6 and MCP-1 were observed in S. Typhi-infected nonengrafted NOD-scid IL2rγnull mice, indicating that the failure of S. Typhi to replicate in these mice is not a consequence of a heightened innate immune response. The elevated production of Th1 cytokines IFNγ and TNFα was more pronounced in S. Typhi-infected hu-SRC-SCID mice than in control C57BL/6 iNOS mice challenged with wild-type S. Typhimurium (Fig. S2), and elevated levels of MCP-1 were consistent with the observed infiltration of infected tissues with mononuclear inflammatory cells (Fig. 2).
Fig. 4.
Serum cytokine levels in S. Typhi-infected hu-SRC-SCID mice. Serum concentrations of murine (M) and human (H) IL-6, IL-10, MCP-1, IFNγ, TNFα, and IL12p70 were assayed by cytometric bead array in nonengrafted NOD-scid IL2rγnull or engrafted hu-SRC-SCID mice 56–72 h after infection with S. Typhi Ty2 (n = 5 per group, error bars = SEM).
Microarray-Based Screen to Identify S. Typhi Virulence Determinants in hu-SRC-SCID Mice.
A microarray-based strategy was used to determine the effects of transposon insertion mutations on the in vivo competitive fitness of S. Typhi in individual hu-SRC-SCID mice. Inocula of 3 × 106 cfu of S. Typhi Ty2 pools each carrying ∼1,500–2,000 random EZ-Tn5 transposon insertions (Epicentre Biotechnologies) were injected intraperitoneally into hu-SRC-SCID mice. After 30 h, livers and spleens were harvested and homogenized with aliquots removed to determine cfu per organ, with the remainder added to broth and grown overnight at 37 °C. DNA was extracted to represent the output DNA sample. DNA from input and output pools was hybridized to NimbleGen whole-genome tiling arrays (45) (Roche NimbleGen). From two independent transposon pools containing insertions in 1,953 loci (7,820 total) in the S. Typhi genome, 4.8% of loci contained transposon insertions that were underrepresented in at least one output pool (Tables S1 and S2).
Discussion
Humanized mice provide an unprecedented opportunity to investigate the pathogenesis of infections caused by microbes that are specifically adapted to humans. The present study shows that the presence of human immune cells allows the replication of the typhoid bacillus in mice. In contrast, neither immunocompetent NOD mice nor immunocompromised nonengrafted NOD-scid IL2rγnull mice were able to support S. Typhi replication. The hu-SRC-SCID mouse represents a tractable small-animal model that reproduces a number of important pathological and inflammatory features of human typhoid fever. The pathological abnormalities provide experimental correlates of the hepatocellular toxicity, splenic typhoid nodules, Kupffer cell swelling, and granulomatous inflammation with multinucleated giant cells observed in human typhoid (46–52). Hepatic centrilobular microvesicular changes and splenic lymphocyte apoptosis (53, 54), general systemic responses to lipopolysaccharide in both normal and humanized mice, were also evident. Elevated serum concentrations of IL-6, IFN-γ, and TNF-α, as measured in the hu-SRC-SCID mice, have also been observed in patients with typhoid fever (55, 56). The marked inflammatory response may be an important contributor to the mortality of S. Typhi infection in hu-SRC-SCID mice, because elevated IL-6 and TNF-α levels correlate with severity of illness in children with typhoid (57).
S. Typhi mutants carrying transposons in loci implicated in stress resistance, DNA repair, and iron acquisition as well as in known Salmonella virulence determinants exhibited reduced competitive fitness in hu-SRC-SCID mice (Table S1). The rseP protease identified in this screen has been recently shown to be required for Salmonella survival in macrophages (58). RcsC is part of a regulatory system shown to modulate the expression of invasion proteins, flagellin, and Vi capsular antigen in S. Typhi (59). The ampD locus was also identified, and this gene is required for S. Typhimurium virulence in mice (60). The entF locus encoding a serine-activating enzyme involved in siderophore synthesis is of interest, because S. Typhi mutants deficient in enterobactin-mediated iron uptake have been found to exhibit reduced growth in human mononuclear cells (61). Moreover, sera from patients with typhoid fever react with FepA, the enterobactin receptor (62), showing that S. Typhi uses enterobactin to obtain iron during human typhoid fever. MgtC is a known virulence determinant in S. Typhimurium encoded within the SPI-3 pathogenicity island (63). MgtC has been implicated in the ability of S. Typhimurium to survive in cultured macrophages, and recent studies have shown that MgtC also promotes S. Typhi survival in human cells (64). Several loci belonging to the SPI-1 and SPI-2 Pathogenicity Islands were also identified in our transposon screen (Table S1), and both SPI-1 and SPI-2 are expressed when S. Typhi is internalized by human macrophages (65). SPI-2 mutations have been incorporated in Ty2-based human typhoid vaccines to reduce virulence (66). It was of particular interest to obtain multiple insertions in SPI-6, which encodes a putative type VI secretion system (67). Type VI secretion has been implicated in the virulence of Pseudomonas aeruginosa and other pathogenic bacteria (68), and the present study provides evidence of a role in Salmonella virulence.
Some important limitations of this model should be recognized. The individual engraftment of newborn mice is an expensive and labor-intensive process. Significant subject to subject variation may be seen as a result of the genetic heterogeneity of donors and degrees of engraftment. The rapid progression to death observed in this model after i.p. inoculation may enable the recognition of host and bacterial factors involved in acute typhoid septicemia but cannot be used to study the pathogenesis of subacute or persistent typhoid infections or the complex interactions between S. Typhi and the host gastrointestinal tract. Finally, a chimeric immune system in which murine and human hematopoietic cells coexist may create artifactual interactions. However, new generation models under development will address these issues (69, 70). The limitations notwithstanding, the hu-SRC-SCID mouse model created by the engraftment of NOD-scid IL2rγnull mice with human umbilical cord-derived hematopoietic stem cells represents a small-animal model in which S. Typhi causes lethal infection and recapitulates important pathological features of human typhoid. This model promises to provide insights into typhoid pathogenesis, identify typhoid vaccine candidates, and lead to improved strategies for the prevention of S. Typhi infections.
Materials and Methods
Bacterial Strains and Growth Conditions.
The Ty2 strain of S. Typhi (JSG624) used in this study was provided by John Gunn (Ohio State University, Columbus, OH). Bacteria were cultivated in Terrific Broth (12 g Bacto Tryptone, 24 g yeast extract, and 4 mL 100% glycerol per 1 L with 100 mL 0.17 M KH2PO4 and 0.72 M K2HPO4 added after autoclaving). Liquid cultures were grown at 37 °C with vigorous shaking. Kanamycin 50 μg·mL−1 was used for selection as indicated. Cfus were enumerated by serial dilution in PBS and plating onto Terrific Broth agar followed by incubation at 37 °C overnight.
EZ-Tn5 <KAN-2> Transposon Library Construction.
An S. Typhi Ty2 transposon library was constructed using the EZ-Tn5 <KAN-2> Promoter Insertion Kit from Epicentre. Log-phase S. Typhi Ty2 grown in 400 mL Terrific Broth (TB) broth was centrifuged, and the pellet was washed three times with cold 10% glycerol before concentration in 1.2 mL 10% glycerol. Two mixtures containing 1 μL TypeOne restriction inhibitor (Epicentre), 1 μL glycerol, 2 μL EZ-Tn5 transposase, and 2 μL EZ-Tn5 <KAN-2> were incubated for 3 h before electroporation of 1 μL into aliquots of 100 μL of S. Typhi suspended in 10% glycerol. After electroporation, 1 mL Luria-Bertani (LB) broth was added to each aliquot, and the cells were incubated at 37 °C with agitation for 1 h. After joining the electroporation mixtures, an aliquot was plated onto LB-kanamycin (50 μg·mL−1) agar, with the remainder used to inoculate 400 mL TB with 50 μg·mL−1 kanamycin, and incubated overnight, with aliquots subsequently frozen in glycerol at −80 °C.
Hu-SRC-SCID Mouse Construction.
A complete description of the construction of humanized mice used in this study is published elsewhere (43, 44). Briefly, newborn NOD-scid IL2rγnull pups from 24 to 48 h of age were irradiated with 100 cGy from a 137Cs source. After irradiation, pups were injected with a suspension of T cell-depleted human umbilical cord blood containing 3 × 104 CD34+ cells through intracardiac injection. The pups were returned to their mothers and weaned after 21–24 d. Successful engraftment was documented by assaying peripheral blood by flow cytometry (Fig. S3) to detect the presence of human CD45+, CD3+, and CD20+ cells. The mice used in this study contained an average of 28% ± 15% (range = 8–58%) CD45+ human hematopoietic cells in their peripheral blood 10 wk after transplantation, and an average of 26% ± 23% of these cells were CD3+ T cells by flow cytometry.
Mouse Infection and Histology.
NOD/LtJ (NOD), NOD.CB17-Prkdcscid (NOD-scid), NOD-scid IL2rγtm1Wjl/SzJ (NOD-scid IL2rγnull), and human hematopoietic stem cell-engrafted NOD-scid IL2rγnull (hu-SRC-SCID) mice were housed in a specific pathogen-free (SPF) vivarium and maintained on medicated water (0.168 mg·mL−1 trimethoprin-sulfamethoxazole, 0.015 mg·mL−1 voriconazole) until 3 d before infection. Overnight cultures of S. Typhi were grown in TB with kanamycin and diluted into PBS to desired concentrations for inoculation with cfu as indicated. S. Typhi was administered by i.p. injection with 500 μL bacterial suspension in PBS. Mice were closely monitored, and moribund animals were euthanized by CO2 asphyxiation at designated time points. Livers and spleens were removed aseptically from infected mice, and blood was collected by cardiac puncture. Blood was allowed to clot and then was centrifuged with removal of serum and storage at −80 °C. One half of each liver and spleen was placed in 20 mL 10% PBS-buffered formalin, and the other one-half was placed in 1 mL sterile PBS. Tissues were homogenized, and aliquots were serially diluted and plated onto TB agar to determine cfu per organ. Significance was determined by the Wilcoxon rank-sum (Mann–Whitney U) test. Organs were removed from formalin after 2 d and placed into 70% ethanol. Organs were embedded in paraffin, and sections were stained with H&E. TUNEL staining to detect cell death was performed using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore). Immunohistochemical staining was performed with mAb specific for human CD45 (clone 2B11 + PD7/26; Dako) using a DakoCytomation EnVision Dual Link system implemented on a Dako Autostainer Universal Staining System. Sections were counterstained with hematoxylin.
Measurement of Cytokine Production.
Production of inflammatory cytokines IL-6, IL-10, MCP-1, IFNγ, TNFα, and IL-12p70 was measured using a Cytometric Bead Array (CBA-Flex Kit; BD Biosciences). Measurement of human and murine cytokine production was performed on samples from the hu-SRC-SCID or control nonengrafted NOD-scid IL2rγnull and C57BL/6 mice according to manufacturer's instructions. As a positive biological control for murine cytokines, sera were obtained from C57BL/6 iNOS−/− mice challenged 96 h earlier with 103 cfu wild-type S. Typhimurium 14028s. Reagents for cytokine measurements were provided with the kits. Standards for individual cytokines were purchased for the generation of standard curves. FACS analysis was performed using BD FACScan or BD FACSCanto, and data were analyzed with FlowJo (TreeStar) or FCAP Array (BD Biosciences) software at the University of Washington Department of Immunology Cell Analysis Facility.
Analysis of Transposon Pools for in Vivo Fitness.
Mice were injected intraperitoneally as above with 3 × 106 cfu S. Typhi transposon pools, each containing ∼1,500–2,000 unique EZ-Tn5 insertions. Mice were euthanized 30 h after infection. Liver and spleen homogenates were used to inoculate 20 mL TB in a 125-mL Erlenmeyer flask containing 50 μg·mL−1 kanamycin and incubated for 18 h at 37 °C with vigorous shaking. The culture-homogenate mixture was transferred to a 50-mL conical tube, and particulate matter was allowed to settle for 1 h. Supernatant was removed for DNA purification, and a portion was archived by the addition of sterile glycerol before storage at −80 °C. DNA representing the output pool was purified using MasterPure regents from Epicentre according to manufacturer's instructions. Total DNA representing the input pool was also purified from overnight cultures of S. Typhi used to infect mice. Samples were labeled and prepared for hybridization to NimbleGen Salmonella whole-genome tiling arrays (Roche NimbleGen) as described (45). Briefly, the DNA was fragmented by sonication and then, poly-A tailed; fragments containing Tn5-derived PT7 were subsequently amplified and in vitro transcribed (AmpliScribe T7 transcription kit; Epicentre). Cy-dye labels were incorporated during in vitro transcription, and purified RNA was hybridized to tiling arrays containing ∼387,000 oligos. Arrays were designed based on the S. Typhimurium LT2 genome, representing 4.14 Mb (86.4%) of the S. Typhi Ty2 genome at >95% accuracy, but not including Typhi-specific regions such as SPI-7. Normalized signal intensities were compared from input and output pools to identify transposon insertions counterselected during infection of hu-SRC-SCID mice. Transposon peaks were automatically detected by considering oligos exhibiting the top 2.5% of all intensities and comparing relative intensity with the next overlapping oligo. Analysis used one-half (background), median (within array), and quantile (between array) normalization, and transposon detection and analysis tools were implemented in WebarrayDB (www.webarraydb.org). Transposons were considered potentially changed in abundance if the median P value of the difference between input and output samples for oligonucleotides around the peak was P < 0.05. The detailed array platform and hybridization results are Miminal Information About a Microarray Experiment (MIAME)-compliantly deposited at www.webarraydb.org under MPMDB ID 168.
Supplementary Material
Acknowledgments
We thank Jean Leif and Joseph Laning for technical assistance. This work was supported by National Institutes of Health Grants AI48622 (to S.J.L.), AI46629 (to D.L.G. and L.D.S.), AI75093 and AI83646 (to M.M.), AI62859 (to K.D.S.), and AI039557, AI44486, and AI82785 (to F.C.F.). This work was also supported by Diabetes Endocrinology Research Center Grant DK32520 and grants from the Juvenile Diabetes Research Foundation International. R.C. was supported by a Beatriu de Pinos fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005566107/-/DCSupplemental.
References
- 1.Ivanoff B, Levine MM, Lambert PH. Vaccination against typhoid fever: Present status. Bull World Health Organ. 1994;72:957–971. [PMC free article] [PubMed] [Google Scholar]
- 2.Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis. 2008;21:531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- 3.Guzman CA, et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 4.Levine MM, et al. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–S27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 5.Corbel MJ. Reasons for instability of bacterial vaccines. Dev Biol Stand. 1996;87:113–124. [PubMed] [Google Scholar]
- 6.Andrews-Polymenis HL, Bäumler AJ, McCormick BA, Fang FC. Taming the elephant: Salmonella biology, pathogenesis and prevention. Infect Immun. 2010;78:2356–2369. doi: 10.1128/IAI.00096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galán JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensel M, et al. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 10.Uchiya K, et al. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez-Torres A, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 12.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150:436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RP, et al. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 15.Fang FC, et al. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libby SJ, et al. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci USA. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas RL, Lee CA. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol Microbiol. 2000;36:1024–1033. doi: 10.1046/j.1365-2958.2000.01961.x. [DOI] [PubMed] [Google Scholar]
- 18.Navarre WW, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 19.Mittrücker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 20.Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci USA. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickard D, et al. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J Bacteriol. 2003;185:5055–5065. doi: 10.1128/JB.185.17.5055-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt KE, et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet. 2008;40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 24.Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groisman EA, Chiao E, Lipps CJ, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacket CO, et al. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 27.Hohmann EL, Oletta CA, Killeen KP, Miller SI. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 28.Curtiss R, 3rd, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hone DM, et al. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988;56:1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: Major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 31.Mastroeni P, et al. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 33.Winkelstein JA, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ottenhoff TH, et al. Genetics, cytokines and human infectious disease: Lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet. 2002;32:97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 35.Gordon MA, et al. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: High mortality and frequent recrudescence. AIDS. 2002;16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 36.Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Collins FM, Carter PB. Growth of salmonellae in orally infected germfree mice. Infect Immun. 1978;21:41–47. doi: 10.1128/iai.21.1.41-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hone DM, Harris AM, Chatfield S, Dougan G, Levine MM. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine. 1991;9:810–816. doi: 10.1016/0264-410x(91)90218-u. [DOI] [PubMed] [Google Scholar]
- 39.Baker SJ, Daniels C, Morona R. PhoP/Q regulated genes in Salmonella typhi identification of melittin sensitive mutants. Microb Pathog. 1997;22:165–179. doi: 10.1006/mpat.1996.0099. [DOI] [PubMed] [Google Scholar]
- 40.Schwan WR, Huang XZ, Hu L, Kopecko DJ. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun. 2000;68:1005–1013. doi: 10.1128/iai.68.3.1005-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu T, Maloy S, McGuire KL. Macrophages influence Salmonella host-specificity in vivo. Microb Pathog. 2009;47:212–222. doi: 10.1016/j.micpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 43.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;15:21. doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brehm MA, et al. Parameters for establishing humanized mouse models to study human immunity: Analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santiviago CA, et al. Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 2009;5:e1000477. doi: 10.1371/journal.ppat.1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayhan A, Gokoz A, Karacadag S, Telatar H. The liver in typhoid fever. Am J Gastroenterol. 1973;59:141–146. [PubMed] [Google Scholar]
- 47.Everest P, Wain J, Roberts M, Rook G, Dougan G. The molecular mechanisms of severe typhoid fever. Trends Microbiol. 2001;9:316–320. doi: 10.1016/s0966-842x(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 48.Grohe F. Beiträge zur pathologischen anatomie und physiologie. Virchows Arch Pathol Anat Physiol Klin Med. 1861;20:306–357. [Google Scholar]
- 49.Billroth T. Neue beobachtungen über die feinere structur pathologisch veränderter lymphdrüsen. Virchows Arch Pathol Anat Physiol Klin Med. 1861;21:423–443. [Google Scholar]
- 50.Mallory FB. A histological study of typhoid fever. J Exp Med. 1898;3:611–638. [PMC free article] [PubMed] [Google Scholar]
- 51.Jagadish K, et al. Hepatic manifestations in typhoid fever. Indian Pediatr. 1994;31:807–811. [PubMed] [Google Scholar]
- 52.Bharadwaj S, Anim JT, Ebrahim F, Aldahham A. Granulomatous inflammatory response in a case of typhoid fever. Med Princ Pract. 2009;18:239–241. doi: 10.1159/000204357. [DOI] [PubMed] [Google Scholar]
- 53.Norimatsu M, Ono T, Aoki A, Ohishi K, Tamura Y. In-vivo induction of apoptosis in murine lymphocytes by bacterial lipopolysaccharides. J Med Microbiol. 1995;43:251–257. doi: 10.1099/00222615-43-4-251. [DOI] [PubMed] [Google Scholar]
- 54.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol. 2009;86:219–227. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butler T, Ho M, Acharya G, Tiwari M, Gallati H. Interleukin-6, gamma interferon, and tumor necrosis factor receptors in typhoid fever related to outcome of antimicrobial therapy. Antimicrob Agents Chemother. 1993;37:2418–2421. doi: 10.1128/aac.37.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keuter M, et al. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis. 1994;169:1306–1311. doi: 10.1093/infdis/169.6.1306. [DOI] [PubMed] [Google Scholar]
- 57.Bhutta ZA, Mansoorali N, Hussain R. Plasma cytokines in paediatric typhoidal salmonellosis: Correlation with clinical course and outcome. J Infect. 1997;35:253–256. doi: 10.1016/s0163-4453(97)93004-8. [DOI] [PubMed] [Google Scholar]
- 58.Muller C, et al. Acid stress activation of the sigma(E) stress response in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2009;71:1228–1238. doi: 10.1111/j.1365-2958.2009.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arricau N, et al. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 60.Folkesson A, Eriksson S, Andersson M, Park JT, Normark S. Components of the peptidoglycan-recycling pathway modulate invasion and intracellular survival of Salmonella enterica serovar Typhimurium. Cell Microbiol. 2005;7:147–155. doi: 10.1111/j.1462-5822.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 61.Gorbacheva VY, Faundez G, Godfrey HP, Cabello FC. Restricted growth of ent(-) and tonB mutants of Salmonella enterica serovar Typhi in human Mono Mac 6 monocytic cells. FEMS Microbiol Lett. 2001;196:7–11. doi: 10.1111/j.1574-6968.2001.tb10532.x. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Beros ME, Gonzalez C, McIntosh MA, Cabello FC. Immune response to the iron-deprivation-induced proteins of Salmonella typhi in typhoid fever. Infect Immun. 1989;57:1271–1275. doi: 10.1128/iai.57.4.1271-1275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanc-Potard AB, Solomon F, Kayser J, Groisman EA. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Retamal P, Castillo-Ruiz M, Mora GC. Characterization of MgtC, a virulence factor of Salmonella enterica Serovar Typhi. PLoS ONE. 2009;4:e5551. doi: 10.1371/journal.pone.0005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faucher SP, Porwollik S, Dozois CM, McClelland M, Daigle F. Transcriptome of Salmonella enterica serovar Typhi within macrophages revealed through the selective capture of transcribed sequences. Proc Natl Acad Sci USA. 2006;103:1906–1911. doi: 10.1073/pnas.0509183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan S, et al. Ability of SPI2 mutant of S. typhi to effectively induce antibody responses to the mucosal antigen enterotoxigenic E. coli heat labile toxin B subunit after oral delivery to humans. Vaccine. 2007;25:4175–4182. doi: 10.1016/j.vaccine.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blondel CJ, Jiménez JC, Contreras I, Santiviago CA. Comparative genomic analysis uncovers 3 novel loci encoding type six secretion systems differentially distributed in Salmonella serotypes. BMC Genomics. 2009;10:354. doi: 10.1186/1471-2164-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mougous JD, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Legrand N, et al. Humanized mice for modeling human infectious disease: Challenges, progress, and outlook. Cell Host Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearson T, Greiner DL, Shultz LD. Humanized SCID mouse models for biomedical research. Curr Top Microbiol Immunol. 2008;324:25–51. doi: 10.1007/978-3-540-75647-7_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.