Abstract
Macrofossils (mostly leaves) and sporomorphs (pollen and spores) preserve conflicting records of plant biodiversity during the end-Permian (P-Tr), Triassic–Jurassic (Tr-J), and end-Cretaceous (K-T) mass extinctions. Estimates of diversity loss based on macrofossils are typically much higher than estimates of diversity loss based on sporomorphs. Macrofossils from the Tr-J of East Greenland indicate that standing species richness declined by as much as 85% in the Late Triassic, whereas sporomorph records from the same region, and from elsewhere in Europe, reveal little evidence of such catastrophic diversity loss. To understand this major discrepancy, we have used a new high-resolution dataset of sporomorph assemblages from Astartekløft, East Greenland, to directly compare the macrofossil and sporomorph records of Tr-J plant biodiversity. Our results show that sporomorph assemblages from the Tr-J boundary interval are 10–12% less taxonomically diverse than sporomorph assemblages from the Late Triassic, and that vegetation composition changed rapidly in the boundary interval as a result of emigration and/or extirpation of taxa rather than immigration and/or origination of taxa. An analysis of the representation of different plant groups in the macrofossil and sporomorph records at Astartekløft reveals that reproductively specialized plants, including cycads, bennettites and the seed-fern Lepidopteris are almost absent from the sporomorph record. These results provide a means of reconciling the macrofossil and sporomorph records of Tr-J vegetation change, and may help to understand vegetation change during the P-Tr and K-T mass extinctions and around the Paleocene–Eocene Thermal Maximum.
Keywords: extinction, palaeobotany, palynology, taphonomy, Triassic–Jurassic
Compilations of stratigraphic ranges of land plants through geological time do not show abrupt declines in taxonomic diversity (1–3). This contrasts sharply with the history of animal life, which is marked by five geologically rapid decreases in global taxonomic diversity, known as mass extinctions (4, 5). This fundamental difference between the evolutionary histories of plants and animals may be due to the persistence of higher plant taxa, and has led to the suggestion that plants are more resistant to mass extinction than animals (1, 6–9). Despite this, studies of fossil plants during times of faunal mass extinction have revealed extensive ecological disruption and decreased plant genus/species diversity on local and regional scales (8, 9), suggesting that plants are not immune to the myriad environmental changes accompanying mass extinctions.
However, plant fossils preserve conflicting records of diversity loss during these critical intervals in Earth history. Estimates of diversity loss based on macrofossils (mostly leaves) are typically much higher than estimates of diversity loss based on sporomorphs (pollen and spores). The end-Permian mass extinction [P-Tr; ∼251 million years ago (Ma)] in Australia saw a 97% regional diversity loss of macrofossils but a 19% loss of sporomorph diversity (8, 10), and the end-Cretaceous mass extinction (K-T; ∼65 Ma) in North America resulted in a 70–90% diversity loss of macrofossils but a 25–30% loss of sporomorph diversity (11, 12). These discrepancies present a barrier to understanding floral change during episodes of faunal mass extinction. This hampers efforts to decipher the causes of mass extinctions and also questions the extent to which plants are more persistent than animals in the face of global change. Additionally, these observations raise doubts about the potential of the fossil record to provide accurate and consistent data on the response of terrestrial vegetation to episodes of major environmental change, which raises doubts about the utility of the fossil record as a source of information from which we can augment our understanding and management of the current climate and biodiversity crises. To explore this issue, we have investigated the macrofossil and sporomorph records of vegetation change during the Triassic–Jurassic mass extinction (Tr-J; ∼200 Ma).
The Tr-J coincided with massive volcanism associated with the opening of the Atlantic Ocean (13–15), which led to a four-fold increase in atmospheric CO2 levels (16) and a consequent rise in global temperatures of between 3 and 6 °C (16–18). Compilations of stratigraphic ranges of animal taxa indicate that 23% of marine families (5), 63% of marine invertebrate genera (19), and 22% of terrestrial families suffered extinction at this time (5). In contrast, family-level compilations of plant diversity indicate that only the Peltaspermaceae, a clade of seed-ferns, was lost from the Earth’s biota at the Tr-J (e.g., ref. 9). Investigations of macrofossils in Jameson Land, East Greenland, have shown a genus-level extinction rate of ∼17% and have revealed that standing species richness across the region declined by ∼85% at the Tr-J (20, 21). Plants with specialized reproductive biology (insect pollinated) were among those taxa at greatest risk of extinction or extirpation (21), and relative abundance distributions of macrofossil genera have shown that the pace of biodiversity loss in this region was abrupt rather than gradual (22). The Tr-J in the Newark Basin, North America, records a regional sporomorph diversity loss of ∼60% (23), but existing sporomorph records spanning the Tr-J in East Greenland, although qualitative, provide little evidence of such catastrophic diversity loss (24, 25). There is also little evidence for abrupt biodiversity loss in sporomorph records from nearby sections in Europe (e.g., ref. 26), where the Tr-J is characterized by compositional change (e.g., refs. 26 and 27).
Using a new high-resolution dataset of sporomorph assemblages from a Tr-J section at Astartekløft, East Greenland, we present a case study that offers broad insights into the taphonomic processes causing discrepancies between the macrofossil and sporomorph records of plants. Specifically, this study aims to provide (i) quantitative estimates of Tr-J terrestrial plant diversity at within- and among-sample scales; (ii) an assessment of the nature and timing of compositional change in the source vegetation; and (iii) an analysis of the agreement between the macrofossil and sporomorph records of the source vegetation. Our results show that the sporomorph record does not preserve an abrupt loss of terrestrial plant biodiversity across the Tr-J in East Greenland, and that the vegetation changed composition rapidly in the boundary interval. Reproductively specialized plants, which include cycads (28), bennettites (29, 30), and the seed-fern Lepidopteris (21), are underrepresented in the sporomorph fossil record, and this provides one explanation for the discrepancy between macrofossil and sporomorph records of Tr-J vegetation change.
Results
The macrofossils and sporomorphs analyzed here were collected from eight horizons rich in plant macrofossils at Astartekløft in Jameson Land, East Greenland (Fig. 1 and ref. 21). The Tr-J boundary at Astartekløft is approximated by the first occurrence of the pollen morphospecies Cerebropollenites thiergartii in plant bed 5 (following recommendations in ref. 32). Accordingly, plant beds 1–4 are of Late Triassic (Rhaetian) age, plant bed 5 represents the topmost upper Rhaetian and plant beds 6 and 7 are of Early Jurassic (Hettangian) age (Fig. 1). A total of 2,898 macrofossils were recorded in situ from plant beds 1–7 (SI Text, Dataset S1). Rock samples for sporomorph analysis were taken at 10 cm intervals within each plant bed and a total of 14,579 sporomorphs were recorded from 40 productive samples (Table 1 and Dataset S2).
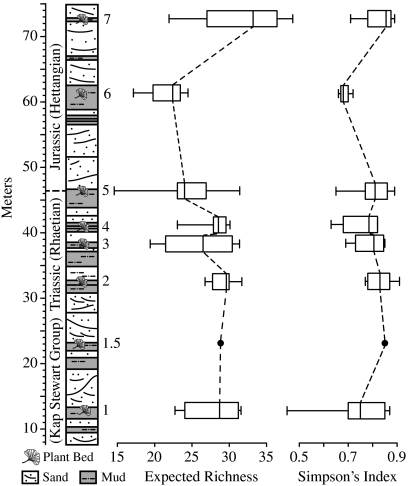
Fig. 1.
Schematic sedimentary log of the Astartekløft section (adapted from ref. 31). Box and whisker plot of rarefied within-sample sporomorph richness at 301 counted sporomorphs, and Simpson’s diversity index. Boxes show median and interquartile range. Whiskers represent maximum and minimum.
Floral Diversity and Composition.
Within-sample richness and Simpson’s diversity index values (D) fluctuate through the section (Fig. 1). Median richness declines through the Triassic from plant bed 1 to plant bed 5 (Fig. 1). Plant bed 6 (earliest Jurassic) records the lowest median richness in the section and plant bed 7 records the highest median richness in the entire section (Fig. 1). Median D is lowest in plant bed 6 and highest in plant bed 7 (Fig. 1). The lowest D values are recorded in samples from plant bed 1 (Fig. 1). The interquartile ranges of within-sample richness and D are large, demonstrating considerable variation within-samples from each plant bed. Using a Kruskal–Wallis test, there is no statistically significant difference in median within-sample richness (KW = 12.3196,1,4,7,6,9,3,4 p = 0.0905) or median D values (KW = 10.326,1,4,7,6,9,3,4 p = 0.1712) between plant beds (Fig. 1).
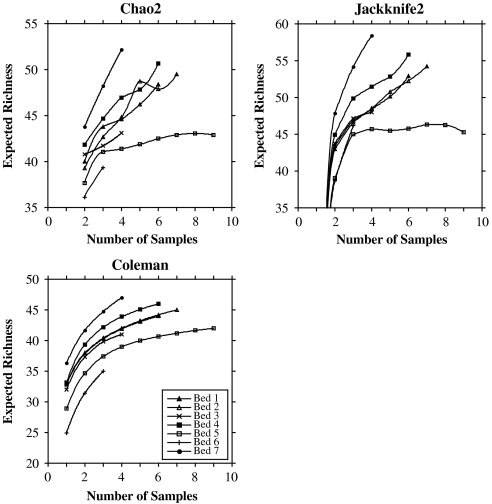
Estimates of among-sample sporomorph richness using the Chao2 and Jackknife2 metrics indicate that plant bed 5 is the least taxonomically rich plant bed of Triassic age, and that plant bed 7 is the most taxonomically rich plant bed at Astartekløft (Fig. 2). The Chao2 and Jackknife2 metrics estimate total species richness (33), and the trends in sporomorph species richness using these metrics are largely confirmed by Coleman rarefaction, which estimates species richness for a subsample of the pooled total species richness (33). This lends support to the trends in expected among-sample sporomorph richness through the section. The Jackknife2 estimator presents the only discrepancy, calculating the same estimate of maximum richness for plant beds 5 and 6 (Fig. 2 and Table 1).
Fig. 2.
Chao2 and Jackknife2 richness estimates and Coleman rarefaction curves for plant beds 1–7 (excluding plant bed 1.5). Curves show mean number of expected taxa (y-axis) for a given number of pooled samples (x-axis).
Table 1.
Diversity of Tr-J sporomorph and macrofossil assemblages at Astartekløft
| Sporomorphs | Macrofossils | |||||||||||||||
| Plant bed | 1 | 1.5 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 1.5 | 2 | 3 | 4 | 5 | 6 | 7 |
| No. samples | 6 | 1 | 7 | 4 | 6 | 9 | 3 | 4 | 4 | 2 | 4 | 4 | 3 | 4 | 1 | 4 |
| No. individuals | 2379 | 361 | 2528 | 1475 | 2139 | 3206 | 1083 | 1408 | 153 | 55 | 248 | 556 | 547 | 361 | 126 | 821 |
| S(obs) | 44 | 30 | 45 | 41 | 46 | 42 | 35 | 47 | 11 | 7 | 11 | 9 | 11 | 15 | 11 | 8 |
| Chao2(max ) | 48 | — | 50 | 43 | 51 | 43 | 39 | 52 | — | — | — | — | — | — | — | — |
| Jackknife2(max ) | 53 | — | 54 | 48 | 56 | 46 | 46 | 58 | — | — | — | — | — | — | — | — |
| Coleman(n=3) | 40 | — | 40 | 40 | 42 | 37 | 35 | 45 | — | — | — | — | — | — | — | — |
| Coleman(n=4) | 42 | — | 42 | 41 | 44 | 39 | — | 47 | — | — | — | — | — | — | — | — |
| Mean Salpha | 29 | 30 | 31 | 27 | 29 | 25 | 23 | 33 | 7.78 | 6.27 | 6.73 | 4.84 | 8.18 | 7.99 | 11.00 | 5.51 |
| Sbeta | 15 | 0 | 14 | 14 | 17 | 17 | 12 | 14 | 3.22 | 0.73 | 4.27 | 4.16 | 2.82 | 7.01 | 0.00 | 2.49 |
| Proportion Salpha | 0.67 | 1.00 | 0.68 | 0.66 | 0.63 | 0.60 | 0.65 | 0.70 | 0.71 | 0.90 | 0.61 | 0.54 | 0.74 | 0.53 | 1.00 | 0.69 |
| Proportion Sbeta | 0.33 | 0.00 | 0.32 | 0.34 | 0.37 | 0.40 | 0.35 | 0.30 | 0.29 | 0.10 | 0.39 | 0.46 | 0.26 | 0.47 | 0.00 | 0.31 |
| Dtotal | 0.73 | 0.85 | 0.85 | 0.88 | 0.81 | 0.84 | 0.70 | 0.84 | 0.83 | 0.76 | 0.81 | 0.22 | 0.66 | 0.63 | 0.74 | 0.64 |
| Mean Dalpha | 0.70 | 0.85 | 0.83 | 0.79 | 0.75 | 0.80 | 0.69 | 0.83 | 0.77 | 0.73 | 0.74 | 0.21 | 0.65 | 0.60 | 0.74 | 0.60 |
| Dbeta | 0.03 | 0.00 | 0.02 | 0.09 | 0.05 | 0.04 | 0.01 | 0.01 | 0.05 | 0.03 | 0.07 | 0.00 | 0.02 | 0.03 | 0.00 | 0.04 |
| Proportion Dalpha | 0.96 | 1.00 | 0.98 | 0.89 | 0.94 | 0.95 | 0.98 | 0.99 | 0.93 | 0.96 | 0.91 | 0.98 | 0.97 | 0.96 | 1.00 | 0.94 |
| Proportion Dbeta | 0.04 | 0.00 | 0.02 | 0.11 | 0.06 | 0.05 | 0.02 | 0.01 | 0.07 | 0.04 | 0.09 | 0.02 | 0.03 | 0.04 | 0.00 | 0.06 |
S(obs), number of taxa in all samples; Salpha, number of taxa within samples; Sbeta, among-sample diversity; Dtotal, Simpson’s diversity index for all samples; Dalpha, Simpson’s diversity index within samples; Dbeta, among-sample Simpson’s diversity index.
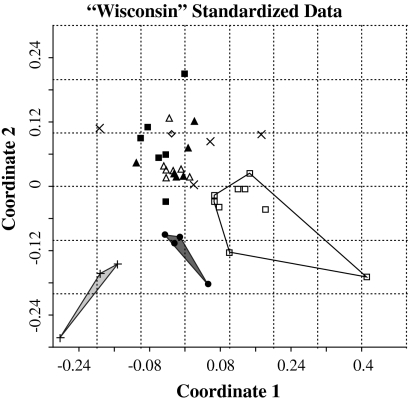
Ordination of “Wisconsin” standardized (34, 35) sporomorph data using nonmetric multidimensional scaling (NMDS) shows that samples from plant beds 1 to 4 overlap in empirical space, indicating that there are no discernable differences in the composition of samples from these horizons (Fig. 3). Samples from plant beds 5, 6, and 7 form discreet groups in the ordination space, demonstrating that they are compositionally distinct both from samples of Triassic age, and from each other (Fig. 3). The differences in median NMDS Coordinate 1 and Coordinate 2 sample scores for these four sample groups are statistically significant. Wisconsin standardized data: NMDS Coordinate 1 (KW = 23.0324,9,3,4 p < 0.0001); NMDS Coordinate 2 (KW = 26.5924,9,3,4 p < 0.0001). These compositional differences are also evident in relative abundance data (SI Text and Fig. S1).
Fig. 3.
Nonmetric multidimensional scaling plot of Wisconsin standardized data. Samples from plant bed 5 represented by open squares and enclosed within an unshaded envelope. Samples from plant bed 6 represented by vertical crosses and enclosed within a pale gray envelope, and samples from plant bed 7 represented by closed circles and enclosed within a dark gray envelope.
Comparison of Macrofossil and Sporomorph Records.
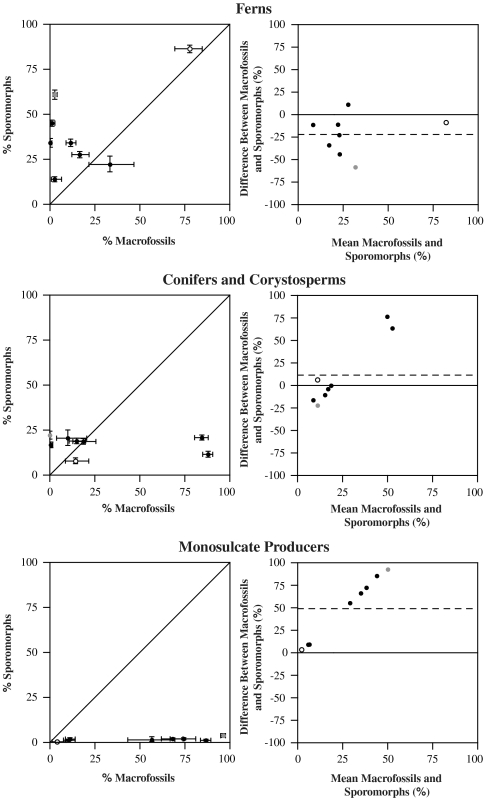
Plant macrofossils and sporomorphs are both used to track past vegetation change, but the degree to which the two groups of fossils give the same picture of the vegetation is often unclear (36). We have investigated this issue at Astartekløft by using scatter diagrams and mean-difference plots (37) to compare the relative representation of three plant groups in the macrofossil and sporomorph records. These groups are as follows: Ferns (1); Conifers and Corystosperms (2); Monosulcate Producers (comprising cycads, bennettites, ginkgos and the seed-fern Lepidopteris) (Fig. 4). See SI Text and Tables S1, S2, and S3 for details of all plant groups at Astartekløft).
Fig. 4.
Scatter diagrams and mean-difference plots illustrating the agreement between the macrofossil and sporomorph records of the Astartekløft vegetation. Each plant bed represented by a single data point (n = 8). Closed black circles = plant beds 1–5 (overbank deposits), open circle = plant bed 6 (poorly developed coal swamp) and closed grey circle = plant bed 7 (abandoned channel). Scatter diagrams: solid diagonal line of equality, error bars represent 95% binomial confidence intervals. Mean-difference plots: solid horizontal line of equality, dashed horizontal line showing mean difference between macrofossil and sporomorph records.
Ferns.
The scatter diagram indicates that this group of plants is generally a greater component of the sporomorph record than the macrofossil record and the mean difference between the macrofossil and sporomorph records of this plant group is -22.33% (Fig. 4). The difference between fossil groups does not tend to get larger or smaller as the mean increases (Fig. 4).
Conifers and Corystosperms.
The scatter diagram indicates that this plant group generally constitutes less than ∼25% of the total plant assemblage in both the macrofossil and sporomorph records (Fig. 4). There is evidence of a major disagreement between the two fossil groups in plant beds 3 and 5, where the relative abundance of this plant group increases dramatically in the macrofossil record, but not in the sporomorph record (Fig. 4). The mean difference between the macrofossil and sporomorph records of this plant group is 11.69% (Fig. 4). The agreement between the two fossil groups improves as mean values approach ∼25%, but then worsens as the mean increases further (Fig. 4). The disagreement in plant bed 3 is caused by high abundance of the broad-leaved conifer Podozamites and in plant bed 5 by high abundances of Podozamites and the “swamp conifer” Stachyotaxus (see Dataset 1).
Monosulcate Producers.
The scatter diagram indicates that this group of plants is almost entirely absent from the sporomorph record at Astartekløft, but is a major component of the macrofossil record (Fig. 4). The mean difference between the macrofossil and sporomorph records of this plant group is 49.25% (Fig. 4). The difference between the macrofossil and sporomorph records of this plant group increases as the mean increases and that there is reasonable agreement between the two fossil groups only when relative abundance is very low (< 10%). These data indicate that reproductively specialized plants such as cycads (28), bennettites (29, 30), and Lepidopteris (21) are effectively silent in the sporomorph record at Astartekløft.
Taphonomic Selection and Taxonomic Resolution.
Macrofossils and sporomorphs record different parts of the source vegetation at Astartekløft. Macrofossil assemblages are weighted toward woody taxa such as conifers, cycads, bennettites and ginkgos, whereas sporomorph assemblages are weighted to ferns and other spore-producing plants such as lycopods (Fig. 4 and Table S1). This pattern is consistent with the observation that woody plants are dominant in leaf litter and fossil leaf accumulations (38–40), and is congruent with the notion that fern spores have high preservation potential in fluvial/deltaic systems (41). The sporomorph morphotypes at Astartekløft are likely to have been produced by a number of different parent plants. This imposes taxonomic smoothing onto the sporomorph record and this effect is distributed unevenly across plant clades. For example, the monosulcate pollen grain Cycadopites was produced by at least four orders of plants in three classes (Table S3), whereas the trilete spore Baculatisporites was produced by ferns of the Osmundaceae family (42). Both macrofossils and sporomorphs fail to accurately census certain plant groups at Astartekløft, and therefore likely underestimate the diversity of the source vegetation.
Discussion
A Catastrophic Diversity Decline?
Our results indicate that sporomorph diversity within-samples, as measured by species richness and D, did not decline catastrophically across the Tr-J at Astartekløft. Sporomorph richness among-samples is slightly lower in plant bed 5 according to both richness estimators and Coleman rarefaction (Fig. 2). Depending on the estimator, expected richness in plant bed 5 is 10% (Chao2) or 12% (Jackknife2) lower than mean richness from a preboundary bin comprising plant beds 1–4 (Table 1), and we interpret this as a result of detrimental environmental change at the Tr-J. This richness decline is less pronounced than that recorded in the Newark Basin, North America (∼60%) (23) and agrees with previous work on Tr-J sporomorph records in East Greenland (24, 25) and elsewhere in Europe (26), which also provide little evidence of catastrophic richness loss.
The shapes of the expected richness curves indicate that plant bed 5 is fully sampled whereas all other plant beds are undersampled (Fig. 2 and ref. 43). In the case of undersampling “a rising Chao estimator can [be] regarded as a valid estimator of minimum richness, given the available data” (p. 692 in ref. 44). Consequently, differences in maximum estimated richness between plant bed 5 and plant beds with higher richness (Table 1) should be viewed as minimum differences. Plant bed 5 saturates more quickly than other plant beds of Triassic age because it is less rich taxonomically and because of the distribution of species within the sample set. Both Chao2 and Jackknife2 provide increasingly high richness estimates as the number of taxa that occur in only one sample and exactly two samples increases (45). This indicates that there are more such rare taxa in plant beds 1–4, and fewer in plant bed 5 (43, 46), in agreement with the trend in macrofossil relative abundance distributions at Astartekløft, which increase in slope up-section as a result of the loss of rare taxa (22).
Vegetation Heterogeneity.
We have examined Tr-J vegetation heterogeneity at Astartekløft through additive partitioning of species richness and D values into alpha (within-sample) and beta (among-sample) components of diversity (47, 48). Beta diversity is thought to increase in heterogeneous landscapes with patchy vegetation where few species are shared by samples, and will decrease in homogeneous landscapes where the species composition of each sampling unit is identical (45). Neither the beta component of richness, nor the beta component of D, show a sharp increase or decrease in any plant bed, and nowhere does beta diversity exceed alpha diversity (Table 1). This pattern is consistent in both the macrofossil and sporomorph records, and supports the idea that vegetation heterogeneity did not change dramatically across the Tr-J at Astartekløft (21). These results also suggest that vegetation heterogeneity was similar in the three depositional environments present at Astartekløft (Table 1). It should be noted, however, that sporomorph samples were collected vertically within each plant bed and therefore do not contain an explicitly spatial component, whereas macrofossils were collected from small quarries spaced laterally along each plant bed (ref. 21 and SI Text) and therefore do contain a spatial component.
Compositional Change.
Ten sporomorph taxa are absent from every sample in bed 5 but present in samples from plant beds 1–4 (Lycopodiumsporites semimuris, Cingulizonates rhaeticus, Concavisporites A, Triancoraesporites ancorae, Nevesisporites limatulus, Alisporites sp., Classopollis ?zwolinskai, Eucommiidites troedssonii, Ovalipollis ovalis, and O. breviformis), whereas just three sporomorph taxa have their first appearance in plant bed 5 (Trachysporites asper, Lycopodiacites rugulatus, and Cerebropollenites thiergartii) (Fig. S2). This suggests that local emigration and/or extirpation of plants played a greater role in changing the composition of the standing vegetation in plant bed 5 than immigration and/or origination (Fig. 3, cf. ref. 49). A similar pattern is recorded in the macrofossil record, where recovery of the vegetation in the Jurassic was achieved by recruitment of new dominant plants from within the Jameson Land region, rather than by origination or significant immigration of exotic taxa (21). The discreet groups formed by samples from beds 6 and 7 in the ordination space indicate that the vegetation did not return to a Triassic composition once it had changed in plant bed 5 (Fig. 3). Because plant beds 6 and 7 represent environments that were not present during the Triassic at Astartekløft (21) this may reflect the influence of depositional environment on sample composition. Nevertheless, this observation strongly suggests permanent compositional change in the East Greenland vegetation across the Tr-J, in common with the macrofossil record (21).
Macrofossil and Sporomorph Records of Tr-J Plant Diversity in East Greenland.
The macrofossil record indicates a complete regional turnover of dominant taxa and an 85% decline in species richness at the Tr-J in East Greenland (20, 21). A midcanopy habit was also eliminated at the Tr-J in this region (21). At Astartekløft, a ∼35% loss in macrofossil generic richness is recorded prior to the Tr-J boundary in plant beds 3 and 4 (21), and a decline in evenness is recorded in plant beds 3, 4, and 5 (ref. 12; see also D values in Table 1). None of these aspects of Tr-J vegetation change are clearly expressed in the sporomorph record at Astartekløft.
The analysis of the agreement between the macrofossil and sporomorph records at Astartekløft may help to explain this discrepancy (Fig. 4). Changes in the relative abundance of bennettites and cycads are a major factor in Tr-J vegetation change in East Greenland (21). For example, among bennettites Pterophyllum declines from 20% in the Triassic to 1.3% in the Jurassic and Anomozamites declines from 17% to 0.1% (12), and among cycads four form genera (Doratophyllum, Ctenis, Pseudoctenis, and Nilssonia) are present in Triassic rocks at Astartekløft, but are absent from Jurassic strata (21). It is clear that the sporomorph record fails to adequately sample the group of plants containing these taxa at Astartekløft (Fig. 4) and consequently, changes in their relative abundance in the source flora will be masked in the sporomorph record. Additionally, the only family level extinction among plants during the Tr-J, that of the Peltaspermaceae (9), is also obscured in the sporomorph record because the pollen grains produced by this family (Cycadopites, ref. 50) were also produced by several other plant groups (Table S3).
Reworking of sporomorphs at Astartekløft may also obscure changes in vegetation diversity and ecology that are expressed in the macrofossil record. The presence of channelized sandstone bodies laid down from river channels several meters deep (21, 51) suggest that reworking is possible. Although previous work at Astartekløft did not highlight pervasive reworking (24), certain sporomorph taxa occur in very low abundance in strata younger than their accepted stratigraphic range. Sporomorphs that are likely to have been reworked include single specimens of Rhaetipollis germanicus in plant bed 6 and 7, one specimen of Limbosporites lundbladii in a single sample from plant bed 7, and two specimens of Lunatisporites sp. and three specimens of Ovalipollis ovalis in a single sample from plant bed 7 (Dataset 2 and Fig. S2). Macrofossils with discontinuous stratigraphic ranges may be associated with sporomorphs with continuous ranges. One example is the macrofossil Cladophlebis, a fern of the Osmundaceae family, and the spore Baculatisporites comaumensis (ref. 42 and Fig. S2). Whether this is due to reworking or other factors remains unclear, and further work is required to understand the impact of reworking on the reconstruction of vegetation diversity and ecology using sporomorphs.
Conclusions
Quantitative analyses of sporomorph diversity do not support a catastrophic decline of terrestrial plant biodiversity in East Greenland. The Tr-J boundary interval is characterized by a modest decline in richness among-samples (Fig. 2) and rapid compositional change that was apparently driven by emigration and/or extirpation rather than immigration and/or origination of taxa (Fig. 3). This is in contrast to the macrofossil record of Tr-J vegetation in the same area, which preserves evidence of abrupt biodiversity loss and changes in forest structure (21, 22). This discrepancy may be explained by the poor representation of certain plant groups in the sporomorph record (Fig. 4). The clearest example is the effective absence of reproductively specialized plants that produced monosulcate pollen grains of the Monosulcites/Cycadopites and Chasmatosporites morphotypes [cycads (28), bennettites (29, 30) and Lepidopteris (21)] in the sporomorph record at Astartekløft (Fig. 4). This pattern apparently extends to Mesozoic sediments worldwide (52), and if Late Triassic diversity loss among reproductively specialized plants is characteristic of the Tr-J in a wider geographic area, this may explain why sporomorph records across the Tr-J in Europe also preserve little evidence of diversity loss (e.g., refs. 26, 27).
This Tr-J case study provides broader insights into the nature of the fossil plant record as it raises the possibility that the floral composition of a biome may affect the quality of its representation in the sporomorph record. Using the relationships shown in Fig. 4, biomes apparently rich in cycads, bennettites and ginkgos, such as the warm temperate biome (Fig. S3 and refs. 53 and 54), may be poorly reflected in Tr-J sporomorph assemblages. Those biomes with a lower proportion of these plants, and perhaps a higher proportion of conifers, which have the smallest mean difference between the macrofossil and sporomorph records at Astartekløft (Fig. 4), may be more accurately reflected in sporomorph assemblages. One example might be the summerwet tropical biome in which cycads were rare and ginkgos were absent (Fig. S3 and refs. 53 and 54). This suggestion may factor in to an explanation of the high regional extinction rates among sporomorphs in the Newark Basin (14), which was situated between 16–25° North during the Tr-J (55) and close to, or within, the summerwet tropical biome (53, 54).
The P-Tr, Tr-J, and K-T faunal mass extinctions were accompanied by diversity losses, ecological changes, and extinctions among plants that were locally/regionally severe and also selective (8, 9). Our results demonstrate that these local/regional patterns will be strongly influenced by the choice of fossil group used to study the vegetation. Our results provide a means of reconciling the contradictory macrofossil and sporomorph records of Tr-J vegetation change in East Greenland, and highlight a mechanism by which similar discrepancies at the P-Tr and K-T mass extinctions may be understood. Additionally, by demonstrating that changes in the diversity and ecology of reproductively specialized plants are not recorded faithfully by sporomorphs at the Tr-J, our results provide support for the idea that the ∼20% extinction among sporomorphs following the Paleocene–Eocene Thermal Maximum (PETM) in the paratropical Gulf Coast of North America is likely to be a considerable underestimate, and therefore may represent a major Cenozoic plant extinction event (43). The magnitude of regional sporomorph diversity loss following the PETM, which is not recognized as an episode of faunal mass extinction (4, 5), was apparently greater than sporomorph diversity loss at the Tr-J in East Greenland (e.g., Fig. 2).
Materials and Methods
Lithology and Palynological Processing.
Samples from plant bed 6 consist of coaly mudstone. Samples from all other plant beds consist of dark gray mudstone and siltstone. 15–20 g of each sample was washed and crushed, then dried for 24 h at 60 °C. Each sample was treated twice alternately with cold HCl (30%) and cold HF (38%). Residues were then sieved with 250 μm and 15 μm mesh. Organic and inorganic residues were separated using ZnCl2. At least 350 sporomorphs were counted per slide, but where one morphotype dominated the assemblage, counts were increased until at least 150 sporomorphs of the nondominant type were recorded.
Floral Diversity and Composition.
Within-sample richness was estimated using individual-based rarefaction using the program PAST (56). These analyses calculate expected richness at lower sample sizes, allowing richness in samples of different sizes to be compared. Simpson’s diversity index (D) calculations were performed using the R package Stratigraph (57, 58). D is weighted toward the most abundant species in a sample and is thus responsive to dominance (45). Among-sample richness was estimated using the Chao2 and Jackknife2 metrics (randomized 10,000 times), and Coleman rarefaction (without sample replacement) was used to compensate for differences in sampling intensity between plant beds (43, 45). These analyses were undertaken using the program EstimateS Version 8.2 (33). Additive diversity partitioning analyses were performed using the R package Stratigraph (results weighted by total number of individuals) (57, 58). NMDS ordination was performed in PAST (56). Wisconsin standardization scales the abundance of each taxon to its maximum value, and represents the abundance of each taxon by its proportion in the sample, which equalizes the effects of rare and abundant taxa and removes the influence of sample size on the analysis (34, 35). Ordination on Wisconsin standardized data is likely to be sensitive to the disappearance and appearance of individual taxa. Singletons (taxa that occurred in only one sample) were removed for the ordination. Kruskal–Wallis tests were performed in R (57).
Supplementary Material
Acknowledgments.
We thank Roy Plotnick for suggesting the use of mean-difference plots and Jan van Tongeren and Natasja Welters for laboratory assistance. Comments from three anonymous referees materially improved this work. L.M. and J.C.M. acknowledge a Marie Cure Excellence Grant (MEXT-CT-2006-042531). W.M.K. acknowledges the High Potential program of Utrecht University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004207107/-/DCSupplemental.
References
- 1.Niklas KJ, Tiffney BH, Knoll AH. Apparent changes in the diversity of fossil plants. Evol Biol. 1980;12:1–89. [Google Scholar]
- 2.Niklas KJ, Tiffney BH, Knoll AH. In: Phanerozoic Diversity Patterns: Profiles in Macroevolution. Valentine JW, editor. Princeton: Princeton University Press; 1985. pp. 97–128. [Google Scholar]
- 3.Niklas KJ, Tiffney BH. The quantification of plant biodiversity through time. Philos T Roy Soc B. 1994;345:35–44. [Google Scholar]
- 4.Sepkoski JJ. Ten years in the library: new data confirm paleontological patterns. Paleobiology. 1993;19:43–51. doi: 10.1017/s0094837300012306. [DOI] [PubMed] [Google Scholar]
- 5.Benton MJ. Diversification and extinction in the history of life. Science. 1995;268:52–58. doi: 10.1126/science.7701342. [DOI] [PubMed] [Google Scholar]
- 6.Knoll AH. In: Exinctions. Nitecki MH, editor. Chicago: University of Chicago Press; 1984. pp. 21–68. [Google Scholar]
- 7.Traverse A. Plant evolution dances to a different beat: plant and animal evolutionary mechanisms compared. Hist Biol. 1988;1:277–301. [Google Scholar]
- 8.Wing SL. In: Extinctions in the History of Life. Taylor PD, editor. Cambridge, UK: Cambridge University Press; 2004. pp. 61–97. [Google Scholar]
- 9.McElwain JC, Punyasena SW. Mass extinction events and the plant fossil record. Trends Ecol Evol. 2007;22:548–557. doi: 10.1016/j.tree.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Retallack GJ. Permian—Triassic life crisis on land. Science. 1995;267:77–80. doi: 10.1126/science.267.5194.77. [DOI] [PubMed] [Google Scholar]
- 11.Hotton CL. The Hell Creek formation and the Cretaceous—Tertiary boundary in the northern Great Plains: An integrated continental record of the end of the Cretaceous. In: Hartman JH, Johnson KR, Nichols DJ, editors. Geol S Am S. 2002. pp. 473–501. (Geological Society of America Special Paper 361) [Google Scholar]
- 12.Nichols DJ, Johnson KR. Plants and the K-T Boundary. Cambridge, UK: Cambridge University Press; 2008. pp. 1–292. [Google Scholar]
- 13.Marzoli A, et al. Extensive 200-million-year-old continental flood basalts of the central Atlantic magmatic province. Science. 1999;284:616–618. doi: 10.1126/science.284.5414.616. [DOI] [PubMed] [Google Scholar]
- 14.Whiteside JH, Olsen PE, Eglinton T, Brookfield ME, Sambrotto RN. Compound-specific carbon isotopes from Earth’s largest flood basalt eruptions directly linked to the end-Triassic mass extinction. Proc Natl Acad Sci USA. 2010;107:6721–6725. doi: 10.1073/pnas.1001706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deenen MHL, et al. A new chronology for the end-Triassic mass extinction. Earth Planet Sc Lett. 2010;291:113–125. [Google Scholar]
- 16.McElwain JC, Beerling DJ, Woodward FI. Fossil plants and global warming at the Triassic-Jurassic boundary. Science. 1999;285:1386–1390. doi: 10.1126/science.285.5432.1386. [DOI] [PubMed] [Google Scholar]
- 17.Beerling DJ, Berner RA. Biogeochemical constraints on the Triassic–Jurassic boundary carbon cycle event. Global Biogeochem Cy. 2002;16:1–13. [Google Scholar]
- 18.Huynh TT, Poulsen CJ. Rising atmospheric CO2 as a possible trigger for the end-Triassic mass extinction. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;217:223–242. [Google Scholar]
- 19.Alroy J, et al. Phanerozoic trends in the global diversity of marine invertebrates. Science. 2008;321:97–100. doi: 10.1126/science.1156963. [DOI] [PubMed] [Google Scholar]
- 20.Harris TM. The fossil flora of Scoresby Sound East Greenland, Part 5. Stratigraphic relations of the plant beds. Meddelelser om Grønland. 1937;112:1–112. [Google Scholar]
- 21.McElwain JC, Popa ME, Hesselbo SP, Haworth M, Surlyk F. Macroecological responses of terrestrial vegetation to climatic and atmospheric change across the Triassic/Jurassic boundary in East Greenland. Paleobiology. 2007;33:547–573. [Google Scholar]
- 22.McElwain JC, Wagner PJ, Hesselbo SP. Fossil plant relative abundances indicate sudden loss of Late Triassic biodiversity in East Greenland. Science. 2009;324:1554–1556. doi: 10.1126/science.1171706. [DOI] [PubMed] [Google Scholar]
- 23.Fowell SJ, Olsen PE. Time calibration of Triassic/Jurassic microfloral turnover, eastern North America. Tectonophysics. 1993;222:361–369. [Google Scholar]
- 24.Pedersen KR, Lund JJ. Palynology of the plant-bearing Rhaetian to Hettangian Kap Stewart Formation, Scoresby Sund, East Greenland. Rev Palaeobot Palyno. 1980;31:1–69. [Google Scholar]
- 25.Koppelhus EB. Palynology of the lacustrine Kap Stewart Formation, Jameson Land, East Greenland. Danmark og Grønlands Geologiske Undersøgelse Rapport. 1996;Appendix 5:1–30. [Google Scholar]
- 26.Bonis NR, Kürschner WM, Krystyn L. A detailed palynological study of the Triassic–Jurassic transition in key sections of the Eiberg Basin (Northern Calcareous Alps, Austria) Rev Palaeobot Palyno. 2009;156:376–400. [Google Scholar]
- 27.van de Schootbrugge B, et al. Floral changes across the Triassic/Jurassic boundary linked to flood basalt volcanism. Nat Geosci. 2009;2:589–594. [Google Scholar]
- 28.Klavins SD, Kellogg DW, Krings M, Taylor EL, Taylor TN. Coprolites in a Middle Triassic cycad pollen cone: Evidence for insect pollination in early cycads? Evol Ecol Res. 2005;7:479–488. [Google Scholar]
- 29.Delevoryas T. Investigations of North American cycadeoids: Cones of Cycadeoidea. Am J Bot. 1963;50:45–52. [Google Scholar]
- 30.Harris TM. Williamsoniella lignieri: Its pollen and the compression of spherical pollen grains. Palaeontology. 1974;17:125–148. [Google Scholar]
- 31.Belcher CM, et al. Increased fire activity at the Triassic/Jurassic boundary in Greenland due to climate-driven floral change. Nat Geosci. 2010;3:426–429. [Google Scholar]
- 32.Kuerschner WM, Bonis NR, Krystyn L. Carbon-isotope stratigraphy and palynostratigraphy of the Triassic–Jurassic transition in the Tiefengraben section—Northern Calcareous Alps (Austria) Palaeogeogr Palaeoclimatol Palaeoecol. 2007;244:257–280. [Google Scholar]
- 33.Colwell RK. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples. 2005. Version 8.2 user’s guide and application published at http://purl.oclc.org/estimates.
- 34.Bray JR, Curtis JT. An ordination of the forest upland communities of southern Wisconsin. Ecol Monogr. 1957;27:325–349. [Google Scholar]
- 35.Jardine PE, Harrinton GJ. The Red Hills Mine palynoflora: A diverse swamp assemblage from the Late Paleocene of Mississippi, USA. Palynology. 2008;32:183–204. [Google Scholar]
- 36.Chaloner WG. In: Evolution and Environment. Drake ET, editor. New Haven, CT: Yale University Press; 1968. pp. 125–138. [Google Scholar]
- 37.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- 38.Spicer RA. The formation and interpretation of plant fossil assemblages. Adv Bot Res. 1989;16:95–191. [Google Scholar]
- 39.Burnham RJ, Wing SL, Parker GG. The reflection of deciduous forest communities in leaf litter: Implications for autochthonous litter assemblages from the fossil record. Paleobiology. 1992;18:30–49. [Google Scholar]
- 40.Gastaldo RA. In: Paleobiology II. Briggs DEG, Crowther PR, editors. Oxford: Blackwell Science; 2001. pp. 314–317. [Google Scholar]
- 41.Hofmann C-C. Pollen distribution in sub-Recent sedimentary environments of the Orinoco Delta (Venezuela)—An actuo-palaeobotanical study. Rev Palaeobot Palyno. 2002;116:191–217. [Google Scholar]
- 42.van Konijnenburg-van Cittert JHA. In: Pollen and Spores: Morphology and Biology. Harley MM, Morton CM, Blackmore S, editors. Kew: Royal Botanic Gardens; 2000. pp. 435–449. [Google Scholar]
- 43.Harrington GJ, Jaramillo CA. Paratropical floral extinction in the Late Paleocene–Early Eocene. J Geol Soc London. 2007;164:323–332. [Google Scholar]
- 44.Longino JT, Coddington J, Colwell RK. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology. 2002;83:689–702. [Google Scholar]
- 45.Magurran AE. Measuring Biological Diversity. Oxford: Blackwell Publishing; 2004. pp. 1–256. [Google Scholar]
- 46.Harrington GJ. Comparisons between Paleocene–Eocene Paratropical swamp and marginal marine pollen floras from Alabama and Mississippi, USA. Palaeontology. 2008;51:611–622. [Google Scholar]
- 47.Lande R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos. 1996;76:5–13. [Google Scholar]
- 48.Wing SL, et al. Late Paleocene fossils from the Cerrejón Formation, Columbia, are the earliest record of Neotropical rainforest. Proc Natl Acad Sci USA. 2009;106:18627–18632. doi: 10.1073/pnas.0905130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wing SL, et al. Transient floral change and rapid global warming at the Paleocene–Eocene Boundary. Science. 2005;310:993–996. doi: 10.1126/science.1116913. [DOI] [PubMed] [Google Scholar]
- 50.Townrow JA. The Peltaspermaceae, a pteridosperm family of Permian and Triassic age. Palaeontology. 1960;3:333–361. [Google Scholar]
- 51.Dam G, Surlyk F. Posamentier HW, Summerhayes CP, Haq BU, Allen GP, editors. Sequence stratigraphy and facies association. Int As Sed. 1993;18:419–448. [Google Scholar]
- 52.Frederiksen NO. Significance of monosulcate pollen abundance in Mesozoic sediments. Lethaia. 1980;13:1–20. [Google Scholar]
- 53.Rees PM, Ziegler AM, Valdes PJ. In: Warm Climates in Earth History. Hueber FM, Macleod KG, Wing SL, editors. Cambridge, UK: Cambridge University Press; 2000. pp. 297–318. [Google Scholar]
- 54.Willis KJ, McElwain JC. The Evolution of Plants. Oxford: Oxford University Press; 2002. p. 152. [Google Scholar]
- 55.Kent DN, Tauxe L. Corrected Late Triassic latitudes for continents adjacent to the North Atlantic. Science. 2005;307:240–244. doi: 10.1126/science.1105826. [DOI] [PubMed] [Google Scholar]
- 56.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. 2001. Version 1.33 Available online at: http://folk.uio.no/ohammer/past.
- 57.R DCT. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 58.Green WA. R Package Stratigraph: Toolkit for the plotting and analysis of stratigraphic and palaeontological data. 2010. Version 0.6 Available online at: http://www.bricol.net.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.