Abstract
Many drugs are amphiphiles that, in addition to binding to a particular target protein, adsorb to cell membrane lipid bilayers and alter intrinsic bilayer physical properties (e.g., bilayer thickness, monolayer curvature, and elastic moduli). Such changes can modulate membrane protein function by altering the energetic cost (ΔGbilayer) of bilayer deformations associated with protein conformational changes that involve the protein-bilayer interface. But amphiphiles have complex effects on the physical properties of lipid bilayers, meaning that the net change in ΔGbilayer cannot be predicted from measurements of isolated changes in such properties. Thus, the bilayer contribution to the promiscuous regulation of membrane proteins by drugs and other amphiphiles remains unknown. To overcome this problem, we use gramicidin A (gA) channels as molecular force probes to measure the net effect of amphiphiles, at concentrations often used in biological research, on the bilayer elastic response to a change in the hydrophobic length of an embedded protein. The effects of structurally diverse amphiphiles can be described by changes in a phenomenological bilayer spring constant (HB) that summarizes the bilayer elastic properties, as sensed by a bilayer-spanning protein. Amphiphile-induced changes in HB, measured using gA channels of a particular length, quantitatively predict changes in lifetime for channels of a different length—as well as changes in the inactivation of voltage-dependent sodium channels in living cells. The use of gA channels as molecular force probes provides a tool for quantitative, predictive studies of bilayer-mediated regulation of membrane protein function by amphiphiles.
Keywords: bilayer elasticity, hydrophobic coupling, hydrophobic matching
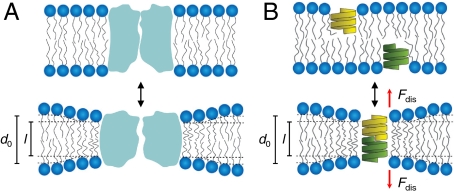
It long has been suspected that “membrane-active” or “membrane-stabilizing” drugs could regulate membrane protein function by partitioning into the host lipid bilayer and thereby alter its physical properties (1, 2). Indeed, numerous studies have shown that amphiphiles, including many drugs, alter lipid bilayer physical properties (for a recent review, see ref. 3). Moreover, membrane protein function involves conformational changes at the protein-bilayer boundary (4), which due to hydrophobic coupling will perturb the surrounding bilayer (Fig. 1A). The associated bilayer deformation energy (ΔGbilayer) contributes to the free energy of a protein conformational change (ΔGprot), meaning that changes in bilayer physical properties can alter protein function by altering ΔGbilayer, cf. refs. 4–8. The promiscuous regulation of membrane proteins by amphiphiles therefore may be due to amphiphile-induced changes in ΔGbilayer. This mechanism would provide a rationale for the observed correlations between the increased hydrophobicity (or lipophilicity) of drugs and the likelihood of adverse events (9) or attrition during drug development (10, 11).
Fig. 1.
Hydrophobic coupling between a bilayer-embedded protein and its host lipid bilayer. (A) A protein conformational change causes a local bilayer deformation. (B) Formation of a gA channel involves local bilayer thinning. Modified from ref. 4.
Amphiphilic molecules alter many different bilayer properties [including intrinsic curvature (12), thickness (13), and elastic moduli (14, 15)], some of which may have opposing effects on the bilayer deformation energy (4). This complicates attempts to predict even the sign of the changes in ΔGbilayer (12). The problem can be overcome, however, by using gramicidin A (gA) channels as probes to sense net changes in bilayer properties as experienced by a bilayer-spanning protein.
gA channels are dimers (D) formed by the transbilayer association of monomeric subunits (M) from each bilayer leaflet (Fig. 1B), and channel gating is described by
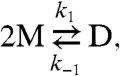 |
where k1 and k-1 are the association and dissociation rate constants for the monomer↔dimer equilibrium. Channel formation in a bilayer with a hydrophobic thickness (d0) that exceeds the channel hydrophobic length (l) involves a local bilayer deformation (16–19) with an associated deformation energy. The bilayer, in response, exerts a disjoining force (Fdis) on the channel, the magnitude of which is determined by the bilayer elastic properties (Fig. 1B). Amphiphiles that decrease Fdis will increase k1, which is reported as an increase in channel appearance frequency (f), and decrease k-1, which is reported as an increase in channel lifetime (τ = 1/k-1) (4, 12).
Results and Discussion
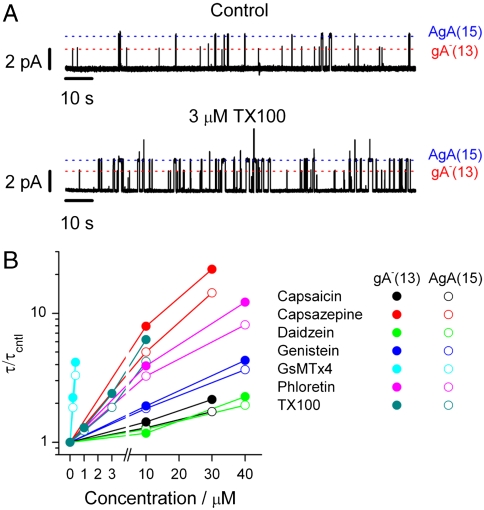
The amphiphile Triton X-100 (TX100) increases τ, meaning that it decreases Fdis (12). To quantify the changes in the bilayer elastic properties, we compare the changes in τ for gA channels formed by subunits of different length and chirality; the 13-residue [des-Val1-Gly2]gA- and the 15-residue [Ala1]gA (20) [designated gA-(13) and AgA(15), respectively]. Fig. 2A shows current traces that illustrate the effects of 3 μM TX100 on gA-(13) and AgA(15) channels in diphytanoylphosphatidylcholine (DPhPC)/n-decane bilayers. TX100 increases f and τ for both channel types, with the larger effects on the shorter channels.
Fig. 2.
Effects of amphiphiles on gA channels in DPhPC/n-decane bilayers. (A) Current traces before and after addition of 3 μM TX100 to both sides of a bilayer doped with gA-(13) and AgA(15). The red and blue lines denote the current levels for gA-(13) and AgA(15) channels. (B) Concentration-dependent effects of TX100, capsaicin, capsazepine, daidzein, genistein, phloretin, or GsMTx4 on lifetime of channels formed by monomeric subunits having 13 or 15 residues (except for TX100, based on results from refs. 12, 21, and 22).
Fig. 2B shows the concentration-dependent effects of TX100 and six other amphiphiles [capsaicin, capsazepine (12), daidzein, genistein, phloretin (21), and GsMTx4 (22)], on τ for gA channels formed by (left- or right-hand) subunits of 13 residues (τ13) or 15 residues (τ15). At concentrations where these amphiphiles alter membrane protein function (summarized in the original articles), they all increase τ for both channel types—and all with the larger effects on the shorter channels; cf. refs. 4, 12, 21, 22. [At the concentrations used in the gA channel experiments, the amphiphiles do not alter membrane capacitance (12, 21, 23). Thus changes in bilayer thickness cannot account for the effects.]
The changes in channel lifetimes reflect changes in activation energy for channel dissociation (ΔG‡):
| [1] |
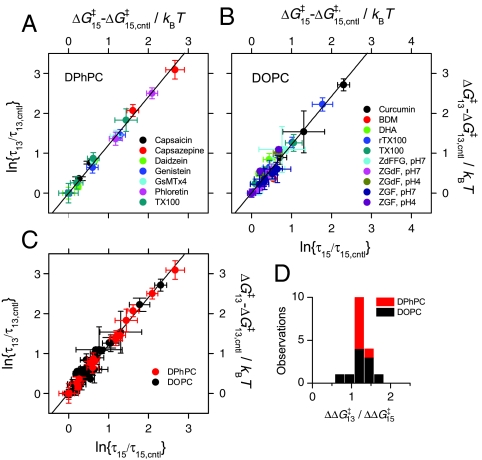
where kB and T are, respectively, Boltzmann’s constant and the temperature in Kelvin, and “cntl” denote results in the absence of amphiphile. The larger changes in lifetime for the shorter channels relative to the longer channels mean that the changes in ΔG‡ also are larger for the shorter channels than for the longer channels (Fig. 3). Moreover, because the changes in τ13 and τ15 result from the same underlying mechanism, the changes τ (in 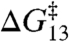 and
and 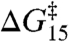 ) are likely to be correlated (5). Fig. 3A shows the changes in
) are likely to be correlated (5). Fig. 3A shows the changes in 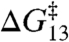 as a function of the changes in
as a function of the changes in 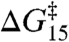 in experiments with DPhPC as the bilayer-forming lipid. Despite the very different structures of the amphiphiles, the changes are described by a shared linear relation with a slope:
in experiments with DPhPC as the bilayer-forming lipid. Despite the very different structures of the amphiphiles, the changes are described by a shared linear relation with a slope: 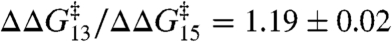 (± SE, r2 > 0.99). A similar relation is obtained using a number of other amphiphiles with dioleoylphosphatidylcholine (DOPC) as the bilayer-forming lipid. Fig. 3B shows the effects of TX100 and seven other amphiphiles [curcumin (24), docosahexaenoic acid (25), 2,3-butanedione monoxime (26), reduced Triton X-100 (27); and the viral antifusion peptides Z-Gly-D-Phe, Z-Gly-Phe and Z-D-Phe-Phe-Gly (28)]. Again the results can be described by a shared linear relation:
(± SE, r2 > 0.99). A similar relation is obtained using a number of other amphiphiles with dioleoylphosphatidylcholine (DOPC) as the bilayer-forming lipid. Fig. 3B shows the effects of TX100 and seven other amphiphiles [curcumin (24), docosahexaenoic acid (25), 2,3-butanedione monoxime (26), reduced Triton X-100 (27); and the viral antifusion peptides Z-Gly-D-Phe, Z-Gly-Phe and Z-D-Phe-Phe-Gly (28)]. Again the results can be described by a shared linear relation: 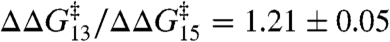 (± SE, r2 = 0.95). Indeed, the DPhPC and DOPC results can be superimposed (Fig. 3C), with the overall fit:
(± SE, r2 = 0.95). Indeed, the DPhPC and DOPC results can be superimposed (Fig. 3C), with the overall fit: 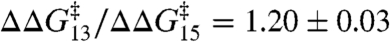 (± SE, r2 = 0.98). Fig. 3D shows the distribution of the slopes (values of
(± SE, r2 = 0.98). Fig. 3D shows the distribution of the slopes (values of 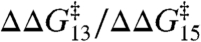 ) for the different amphiphiles.
) for the different amphiphiles.
Fig. 3.
Effects of amphiphiles on lifetimes of channels formed by 13-residue subunits (expressed as ln{τ/τcntl}, left axis, or activation energy, 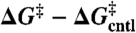 , right axis) vs. corresponding effects on channels formed by 15-residue subunits (bottom or top axis). (A) Effects of TX100, capsaicin, capsazepine, daidzein, genistein, phloretin, or GsMTx4 in DPhPC/n-decane bilayers (except for TX100, results from refs. 12, 21 and 22). A subset of the results was published previously (5). (B) Effects of TX100, curcumin, docosahexaenoic acid (DHA), 2,3-butanedione monoxime (BDM), reduced Triton X-100 (rTX100), Z-Gly-D-Phe (ZGdF), and Z-Gly-Phe (ZGF) (at pH 7 or pH 4) and Z-D-Phe-Phe-Gly (ZdFFG, at pH 7) in DOPC/n-decane bilayers (except for TX100, results from refs. 24–28). (C) Superimposition of results obtained using DPhPC (red) or DOPC (black). (D) Distribution of
, right axis) vs. corresponding effects on channels formed by 15-residue subunits (bottom or top axis). (A) Effects of TX100, capsaicin, capsazepine, daidzein, genistein, phloretin, or GsMTx4 in DPhPC/n-decane bilayers (except for TX100, results from refs. 12, 21 and 22). A subset of the results was published previously (5). (B) Effects of TX100, curcumin, docosahexaenoic acid (DHA), 2,3-butanedione monoxime (BDM), reduced Triton X-100 (rTX100), Z-Gly-D-Phe (ZGdF), and Z-Gly-Phe (ZGF) (at pH 7 or pH 4) and Z-D-Phe-Phe-Gly (ZdFFG, at pH 7) in DOPC/n-decane bilayers (except for TX100, results from refs. 24–28). (C) Superimposition of results obtained using DPhPC (red) or DOPC (black). (D) Distribution of 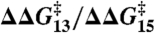 for individual amphiphiles using DPhPC (red) or DOPC (black). Mean ± SEM (n≥3) or ± range (n = 2).
for individual amphiphiles using DPhPC (red) or DOPC (black). Mean ± SEM (n≥3) or ± range (n = 2).
The activation energy for gA channel dissociation is given by
| [2] |
where 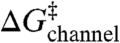 denotes an “intrinsic” energy cost due to loss of contributions such as hydrogen bonds that stabilize the channel dimer, whereas
denotes an “intrinsic” energy cost due to loss of contributions such as hydrogen bonds that stabilize the channel dimer, whereas 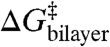 denotes the change in bilayer deformation energy associated with separating the channel subunits by δ [a distance of ∼0.16 nm (19, 29, 30)] to reach a transition state where the channel conductance is lost (Fig. 1B). A large body of work, summarized in ref. 4, has shown that the amphiphile-induced changes in τ primarily reflect changes in
denotes the change in bilayer deformation energy associated with separating the channel subunits by δ [a distance of ∼0.16 nm (19, 29, 30)] to reach a transition state where the channel conductance is lost (Fig. 1B). A large body of work, summarized in ref. 4, has shown that the amphiphile-induced changes in τ primarily reflect changes in 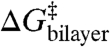 . This conclusion is reinforced by the results in Fig. 3 because the relation between
. This conclusion is reinforced by the results in Fig. 3 because the relation between 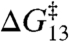 and
and 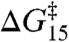 can be described by a shared function despite the different amphiphile structures, the different lipid structure, and the opposite chiralities of the 13- and 15-residue channel-forming subunits. Specific amphiphile-channel interactions thus do not contribute to the changes in τ, which means that the amphiphiles will have little effect on
can be described by a shared function despite the different amphiphile structures, the different lipid structure, and the opposite chiralities of the 13- and 15-residue channel-forming subunits. Specific amphiphile-channel interactions thus do not contribute to the changes in τ, which means that the amphiphiles will have little effect on 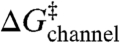 . The linear relation between
. The linear relation between 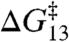 and
and 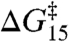 therefore reflects also a linear relation between
therefore reflects also a linear relation between 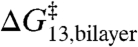 and
and 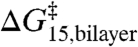 .
.
The shared linear relation between 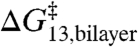 and
and 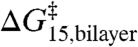 indicates that the effects of amphiphiles can be described by a single parameter that characterizes the changes in bilayer elastic properties. Because the same linear relation applies to compounds that cause positive [TX100 (12)] and negative [capsaicin (12), curcumin (31), and docosahexaenoic acid (25)] changes in intrinsic curvature, the curvature-dependent contributions to
indicates that the effects of amphiphiles can be described by a single parameter that characterizes the changes in bilayer elastic properties. Because the same linear relation applies to compounds that cause positive [TX100 (12)] and negative [capsaicin (12), curcumin (31), and docosahexaenoic acid (25)] changes in intrinsic curvature, the curvature-dependent contributions to 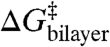 are likely to be small. In this case, one can approximate ΔGbilayer as
are likely to be small. In this case, one can approximate ΔGbilayer as
| [3] |
where the bilayer elastic properties are summarized by a single phenomenological spring constant, HB (e.g., ref. 4). [Our conclusion, that the curvature contributions to ΔGbilayer are small, does not depend on the use of planar bilayers formed using decane. Using a fluorescence quench-based method (32) that employs hydrocarbon-free large unilamellar vesicles to determine amphiphile-induced changes in lipid bilayer properties, as sensed by gA channels, we find that TX100 and capsaicin have similar effects—and that all compounds tested have similar effects when assayed in the planar bilayer and the lipid vesicle system.]
The bilayer contribution to ΔG‡ then is given by
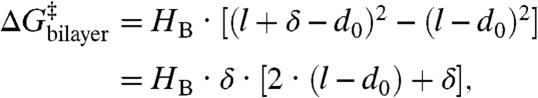 |
[4] |
and, if the amphiphile effect on  can be described by changes in HB:
can be described by changes in HB:
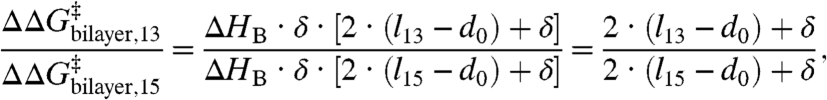 |
[5] |
which is independent of ΔHB. As a quantitative check of Eq. 5, we note that the hydrophobic length of the AgA(15) channel, l15, is ∼2.2 nm (16, 18, 33) and the channel length varies ∼0.08 nm/residue (e.g., ref. 4); therefore l13 ∼ 1.88 nm. Using these values and the estimates for  from Fig. 3C, we find that d0 ∼ 4.0 nm for bilayers formed using either DPhPC or DOPC. For comparison, the bilayer thickness at an applied potential of 200 mV, as estimated from capacitance measurements (assuming a dielectric constant of 2), is ∼4.2 nm for DPhPC/n decane (21) and between 3.7–4.3 nm for DOPC/n decane (24, 25). The agreement between the measured bilayer thickness and the prediction based on Eq. 5 is striking and supports the use of Eqs. 1–4 to describe the energetic coupling between gA channel function and lipid bilayer properties.
from Fig. 3C, we find that d0 ∼ 4.0 nm for bilayers formed using either DPhPC or DOPC. For comparison, the bilayer thickness at an applied potential of 200 mV, as estimated from capacitance measurements (assuming a dielectric constant of 2), is ∼4.2 nm for DPhPC/n decane (21) and between 3.7–4.3 nm for DOPC/n decane (24, 25). The agreement between the measured bilayer thickness and the prediction based on Eq. 5 is striking and supports the use of Eqs. 1–4 to describe the energetic coupling between gA channel function and lipid bilayer properties.
It is thus possible to evaluate the changes in HB from the changes in τ, using the relation
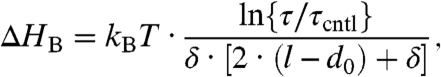 |
[6] |
which is obtained from Eqs. 1 and 4. For l - d0 = 1.8 nm and δ = 0.16 nm, either 200 nM GsMTx4 or 3 μM TX100 cause a 4 kJ/(mole·nm2) decrease in HB, which should be compared to HB in unmodified phospholipid bilayers, ∼56 kJ/(mole·nm2) (4). Even relatively modest changes in HB, thus, may cause measurable changes in channel (membrane protein) function.
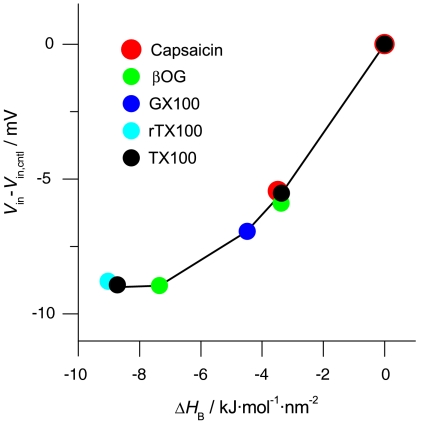
The amphiphile-induced changes in HB, as measured using gA channels formed by 15-residue subunits, are transferable in that they provide for quantitative predictions of changes in 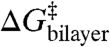 —and thus lifetime—for channels formed by 13-residue subunits in a bilayer of the same composition (and vice versa); cf. Fig. 3. They also are scalable, in that they provide for quantitative descriptions of the effects of amphiphiles on membrane protein function involving entirely different structural changes in plasma membranes. Fig. 4 shows the effects of five amphiphiles (TX100, reduced Triton X-100, β-octyl glucoside, Genapol X-100, and capsaicin) on the membrane potential for 50% inactivation (Vin) of voltage-dependent sodium channels in HEK293 cells, as function of changes in HB measured using gA channels in DOPC/n-decane bilayers (based on refs. 12 and 27). Despite the structural differences—and that capsaicin promotes opposite curvature from the other molecules—the Vin vs. HB relations vary little among the different amphiphiles.
—and thus lifetime—for channels formed by 13-residue subunits in a bilayer of the same composition (and vice versa); cf. Fig. 3. They also are scalable, in that they provide for quantitative descriptions of the effects of amphiphiles on membrane protein function involving entirely different structural changes in plasma membranes. Fig. 4 shows the effects of five amphiphiles (TX100, reduced Triton X-100, β-octyl glucoside, Genapol X-100, and capsaicin) on the membrane potential for 50% inactivation (Vin) of voltage-dependent sodium channels in HEK293 cells, as function of changes in HB measured using gA channels in DOPC/n-decane bilayers (based on refs. 12 and 27). Despite the structural differences—and that capsaicin promotes opposite curvature from the other molecules—the Vin vs. HB relations vary little among the different amphiphiles.
Fig. 4.
Amphiphile-induced changes in inactivation of voltage-dependent sodium channels in HEK293 cells as a function of changes in HB in DOPC/n-decane bilayers. Shift in membrane potential for 50% inactivation (Vin-Vin,cntl) plotted vs. ΔHB (= HB-HB,cntl) in DOPC/n-decane bilayers. Amphiphiles: capsaicin, β-octyl puranoside (βOG), Genapol X-100 (GX100), reduced Triton X-100 (rTX100), and TX100. HEK293 cells were depolarized to +20 mV following 300-ms prepulses to potentials varying from -130 to +50 mV. Based on results from refs. 12 and 27.
Given the many different effects that amphiphilic compounds can have on the physical properties of lipid bilayers, it is perhaps surprising that the effects of structurally different amphiphiles on the bilayer elastic response can be characterized by changes in a single parameter, a phenomenological bilayer spring constant HB. The finding represents an important conceptual simplification of the bilayer-mediated effects of amphiphiles and, moreover, provides a quantitative measure (a number) that can be used in further mechanistic studies. That the amphiphiles decrease HB (and the energetic cost of deforming the bilayer) most likely reflects that water-soluble amphiphiles reversibly adsorb to lipid bilayers and thereby decrease the bilayer elastic moduli (14).
Though long recognized, the regulation of membrane protein function by membrane-active compounds has remained elusive because it has been difficult to obtain quantitative information about changes in the bilayer properties that are relevant for protein function. Nevertheless, studies on amphiphile regulation of membrane proteins often are done at concentrations that alter bilayer physical properties (see ref. 4 for a list of examples). The issue becomes particularly important in drug discovery and development—most orally available drugs are amphiphiles (34), and increasing drug lipophilicity has become a major and rising cause of nonspecific drug actions and attrition (10, 11). The gA channel-based approach provides the advantage of a direct readout of amphiphile-induced changes in bilayer properties, as experienced by an embedded protein. It provides a tool for quantitative and predictive explorations of the bilayer-mediated regulation of membrane protein function by drug candidates and other amphiphiles.
Materials and Methods
Gramicidin analogues [des-Val1-Gly2]gA- and [Ala1]gA were synthesized as described by ref. 20. Single-channel measurements using Triton X-100 were done at 25 °C in DPhPC or DOPC (Avanti Polar Lipids)/n-decane bilayers, separating 1.0 M NaCl, 10 mM Hepes, pH 7 solutions using the bilayer punch method, as described previously (4). The applied potential was ± 200 mV. The gA analogues and amphiphile were added to both sides of the bilayer. Survivor plots of channel lifetimes were fitted by a single exponential distribution, N(t)/N(0) = exp{-t/τ}, where N(t) is the number of channels with duration longer than time t, and τ is the average lifetime (12, 22, 24–28).
Acknowledgments.
We thank Denise V. Greathouse for the peptides and Kevin Lum for assistance with the single-channel measurements. This work was supported by National Institutes of Health Grants GM021342 and RR015569.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972;24:583–655. [PubMed] [Google Scholar]
- 2.Sackmann E. In: Biological Membranes. Chapman D, editor. London: Academic; 1984. pp. 105–143. [Google Scholar]
- 3.Seddon AM, et al. Drug interactions with lipid membranes. Chem Soc Rev. 2009;38:2509–2519. doi: 10.1039/b813853m. [DOI] [PubMed] [Google Scholar]
- 4.Lundbæk JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J R Soc Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundbæk JA. Regulation of membrane protein function by lipid bilayer elasticity: A single molecule technology to measure the bilayer properties experienced by an embedded protein. J Phys Condens Matt. 2006;18:S1305–S1344. doi: 10.1088/0953-8984/18/28/S13. [DOI] [PubMed] [Google Scholar]
- 6.Andersen OS, Koeppe RE., II Bilayer thickness and membrane protein function: An energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 7.Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim Biophys Acta. 2008;1778:1545–1575. doi: 10.1016/j.bbamem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes JD, et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg Med Chem Lett. 2008;18:4872–4875. doi: 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 11.Keserü GM, Makara GM. The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discov. 2009;8:203–212. doi: 10.1038/nrd2796. [DOI] [PubMed] [Google Scholar]
- 12.Lundbæk JA, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 13.Ebihara L, Hall JE, MacDonald RC, McIntosh TJ, Simon SA. Effect of benzyl alcohol on lipid bilayers. A comparisons of bilayer systems. Biophys J. 1979;28:185–196. doi: 10.1016/S0006-3495(79)85170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans E, Rawicz W, Hofmann AF. In: Bile Acids in Gastroenterology: Basic and Clinical Advances. Hofmann AF, Paumgartner G, Stiehl A, editors. Dordrecht: Kluwer Academic Publishers; 1995. pp. 59–68. [Google Scholar]
- 15.Ly HV, Longo ML. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys J. 2004;87:1013–1033. doi: 10.1529/biophysj.103.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HW. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986;50:1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen C, Goulian M, Andersen OS. Energetics of inclusion-induced bilayer deformations. Biophys J. 1998;74:1966–1983. doi: 10.1016/S0006-3495(98)77904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harroun TA, Heller WT, Weiss TM, Yang L, Huang HW. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys J. 1999;76:937–945. doi: 10.1016/S0006-3495(99)77257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundbæk JA, Andersen OS. Spring constants for channel-induced lipid bilayer deformations—estimates using gramicidin channels. Biophys J. 1999;76:889–895. doi: 10.1016/S0006-3495(99)77252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greathouse DV, Koeppe RE, II, Providence LL, Shobana S, Andersen OS. Design and characterization of gramicidin channels. Method Enzymol. 1999;294:525–550. doi: 10.1016/s0076-6879(99)94031-4. [DOI] [PubMed] [Google Scholar]
- 21.Hwang TC, Koeppe RE, II, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 22.Suchyna TM, et al. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 23.Lundbæk JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–3830. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- 24.Ingólfsson HI, Koeppe RE, II, Andersen OS. Curcumin is a modulator of bilayer material properties. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- 25.Bruno MJ, Koeppe RE, II, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci USA. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artigas P, et al. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: The role of bilayer material properties. Mol Pharmacol. 2006;70:2015–2026. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- 27.Lundbæk JA, et al. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling: Effects of micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashrafuzzaman Md, Lampson MA, Greathouse DV, Koeppe RE, II, Andersen OS. Manipulating lipid bilayer material properties using biologically active amphipathic molecules. J Phys Condens Matt. 2006;18:S1235–S1255. [Google Scholar]
- 29.Durkin JT, Koeppe RE, II, Andersen OS. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J Mol Biol. 1990;211:221–234. doi: 10.1016/0022-2836(90)90022-E. [DOI] [PubMed] [Google Scholar]
- 30.Miloshevsky GV, Jordan PC. Gating gramicidin channels in lipid bilayers: Reaction coordinates and the mechanism of dissociation. Biophys J. 2004;86:92–104. doi: 10.1016/S0006-3495(04)74087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry J, et al. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J Am Chem Soc. 2009;131:4490–4498. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingolfsson HI, Andersen OS. Screening for small molecule’s bilayer-modifying potential using a gramicidin-based fluorescence assay. Assay Drug Dev Techn. 2010 doi: 10.1089/adt.2009.0250. doi: 10.1089/adt.2009.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott JR, Needham D, Dilger JP, Haydon DA. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983;735:95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev. 1997;23:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]