Abstract
Disregulated Wnt/β-catenin signaling has been linked to various human diseases, including cancers. Inhibitors of oncogenic Wnt signaling are likely to have a therapeutic effect in cancers. LRP5 and LRP6 are closely related membrane coreceptors for Wnt proteins. Using a phage-display library, we identified anti-LRP6 antibodies that either inhibit or enhance Wnt signaling. Two classes of LRP6 antagonistic antibodies were discovered: one class specifically inhibits Wnt proteins represented by Wnt1, whereas the second class specifically inhibits Wnt proteins represented by Wnt3a. Epitope-mapping experiments indicated that Wnt1 class-specific antibodies bind to the first propeller and Wnt3a class-specific antibodies bind to the third propeller of LRP6, suggesting that Wnt1- and Wnt3a-class proteins interact with distinct LRP6 propeller domains. This conclusion is further supported by the structural functional analysis of LRP5/6 and the finding that the Wnt antagonist Sclerostin interacts with the first propeller of LRP5/6 and preferentially inhibits the Wnt1-class proteins. We also show that Wnt1 or Wnt3a class-specific anti-LRP6 antibodies specifically block growth of MMTV-Wnt1 or MMTV-Wnt3 xenografts in vivo. Therapeutic application of these antibodies could be limited without knowing the type of Wnt proteins expressed in cancers. This is further complicated by our finding that bivalent LRP6 antibodies sensitize cells to the nonblocked class of Wnt proteins. The generation of a biparatopic LRP6 antibody blocks both Wnt1- and Wnt3a-mediated signaling without showing agonistic activity. Our studies provide insights into Wnt-induced LRP5/6 activation and show the potential utility of LRP6 antibodies in Wnt-driven cancer.
Keywords: antibody therapeutics, cancer
The Wnt/β-catenin pathway regulates diverse biological processes during development and tissue homeostasis by modulating the protein stability of β-catenin (1–3). In the absence of extracellular Wnt proteins, cytoplasmic β-catenin is associated with the β-catenin destruction complex and degraded by ubiquitin-mediated proteolysis. Wnt signals are transduced by two distinct receptors, the serpentine receptor Frizzled (Frz) and the single-span transmembrane proteins LRP5 or LRP6. Wnt proteins promote the assembly of the Frz–LRP5/6 signaling complex and induce phosphorylation of LRP5 or LRP6. Phosphorylated LRP5 or LRP6 inactivates the β-catenin degradation complex, allowing stabilized β-catenin to enter the nucleus, bind to the TCF transcription factors, and act as a transcriptional coactivator.
The extracellular domain of LRP5 or LRP6 contains four YWTD-type β-propeller domains each followed by an EGF-like domain and an LDLR domain. Each propeller contains six YWTD motifs that form a six-bladed β-propeller structure (4). Biochemical studies suggest that Wnt proteins physically interact with both Frz and LRP6 and induce the formation of an Frz–LRP6 signaling complex (5, 6). Experimentally induced proximity of Frz and LRP6 is sufficient to activate Wnt signaling (7–9). In addition to Wnt proteins, the large extracellular domain of LRP5 or LRP6 binds to multiple secreted Wnt modulators, including the Wnt antagonists DKK1 and Sclerostin (SOST), and the Wnt agonists R-Spondins. Existence of natural secreted modulators of LRP5/6 and mutations of LRP5/6 in various human diseases points to the possibility of using LRP5/6 antibodies to modulate Wnt signaling in disease settings (10–12).
Disregulation of the Wnt-signaling pathway has been linked to cancer. Mutations in pathway components such as APC and β-catenin have been associated with a number of human cancers, and recent studies suggest that overexpression of Wnt proteins and/or silencing of Wnt antagonists such as DKK1, WISP, and sFRPs may promote cancer development and progression (13–16). In addition, Wnt signaling plays a critical role in the homeostatic regulation of tissue stem cells and hence, is implicated in the maintenance of cancer stem cells (17, 18). These data suggest that antagonists of Wnt signaling might be used in the treatment of Wnt-dependent cancers.
Wnt signaling also plays important roles in tissue homeostasis and regeneration. Wnt signaling promotes bone formation by increasing the growth and differentiation of osteoblasts (19), and in humans, gain-of-function mutations of LRP5 (10, 20) and loss-of-function mutations of Wnt antagonist SOST (21, 22) lead to high bone-mass diseases. Wnt signaling is also critical for the homeostasis of the intestinal epithelium by maintaining the proliferative status of stem cells in the intestinal crypt (23). Agents that sensitize cells to Wnt signaling might be used to treat bone or intestinal disorders such as osteoporosis and mucositis.
To discover antibody modulators of Wnt signaling, we generated both antagonistic and agonistic antibodies directed against LRP6. Using these antibodies, we find that distinct propellers of LRP6 are differentially required for the signaling activities of different Wnt proteins. These findings provide insights into the mechanism of Wnt-induced LRP5/6 activation and show the utility of LRP6 antibodies that inhibit Wnt signaling in cancer.
Results
Identification of Anti-LRP6 Fab Fragments That Specifically Inhibit Wnt1- or Wnt3a-Induced β-Catenin Signaling.
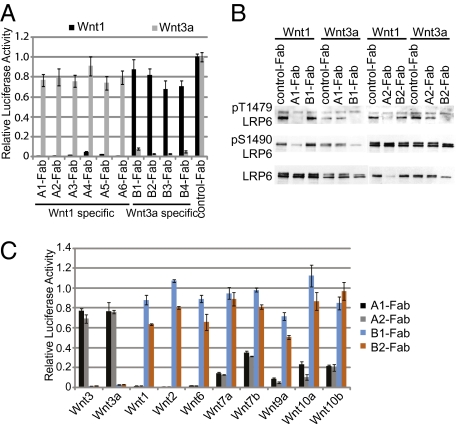
To identify LRP6 antibodies modulating Wnt signaling, HEK293 cells overexpressing a GFP-tagged intracellular domain-truncated LRP6 (LRP6ΔC-GFP) or recombinant proteins of the extracellular domain of LRP6 were used for panning with the HuCAL GOLD phagemid library. Fab fragments with activity in LRP6-binding assays were tested for the ability to modulate Wnt1- or Wnt3a-induced SuperTopFlash (STF) reporter. Surprisingly, all antagonistic anti-LRP6 Fab fragments could be grouped into two distinct classes, class A and class B. Fab fragments of class A, represented by A1, A2, A3, A4, A5, and A6, strongly inhibited Wnt1-induced STF without affecting Wnt3a-induced STF (Fig. 1A). Fab fragments of class B, represented by B1, B2, B3, and B4, strongly inhibited Wnt3a-induced STF without affecting Wnt1-induced STF (Fig. 1A). In addition, Fab fragments of each class displayed the same differential inhibition of Wnt1- or Wnt3a-induced LRP6 phosphorylation in HEK293 cells (Fig. 1B).
Fig. 1.
Anti-LRP6 Fab fragments differentially inhibit signaling activity of Wnt1 and Wnt3a. (A) Anti-LRP6 Fab fragments specifically inhibit Wnt1- or Wnt3a-induced STF activation. HEK293 cells were transfected with Wnt1- or Wnt3a-expression plasmid and STF reporter and treated with various anti-LRP6 Fab fragments at 10 μg/mL. (B) Anti-LRP6 Fab fragments specifically inhibit Wnt1- or Wnt3a-induced phosphorylation of LRP6. HEK293 cells stably expressing Wnt1 or Wnt3a were treated with indicated antibodies at 10 μg/mL for 24 h. Cell lysates were fractionated and blotted with indicated antibodies. (C) Anti-LRP6 Fab fragments differentially affect STF activation induced by various Wnt proteins. HEK293 cells were transfected with the STF reporter and indicated Wnt expression constructs, and they were treated with indicated antibodies at 10 μg/mL for 24 h. Luciferase reporter activities were normalized against cells treated with control antibody.
We next sought to determine whether β-catenin signaling induced by diverse Wnt proteins was differentially affected by Wnt1- or Wnt3a-specific anti-LRP6 Fab fragments. There are 19 Wnt proteins in the human, and at least 10 of them can activate β-catenin signaling and the STF reporter in HEK293 cells with or without coexpression of Frz receptors. In these Wnt-driven STF assays, Wnt proteins can be grouped into two classes (Fig. 1C). One class of Wnt proteins, including Wnt1, Wnt2, Wnt6, Wnt7a, Wnt7b, Wnt9a, Wnt10a, and Wnt10b, was specifically inhibited by Wnt1-specific anti-LRP6 Fab fragments (A1-Fab and A2-Fab). The other class of Wnt proteins, including Wnt3 and Wnt3a, was specifically inhibited by Wnt3a-specific anti-LRP6 Fab fragments (B2-Fab and B3-Fab). Multiple lines of evidence suggest that Wnt proteins trigger downstream signaling by physically interacting with both Frz and LRP5/6. Hence, these data strongly suggest that different Wnt proteins bind to distinct regions of LRP6.
Characterization of Monovalent Agonistic LRP6 Antibodies.
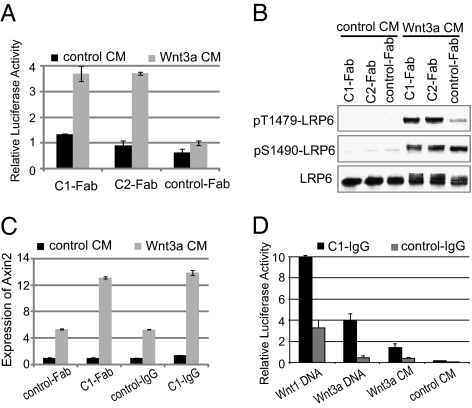
In addition to the two classes of antagonistic LRP6 antibodies described above, we also identified a class of agonistic LRP6 antibodies (class C). Two anti-LRP6 Fab fragments (C1-Fab and C2-Fab) did not affect STF reporter without exogenous Wnt proteins but increased Wnt3a-induced STF activation (Fig. 2A) and Wnt3a-induced LRP6 phosphorylation (Fig. 2B). Using analytical size-exclusion chromatography, we found that both C1-Fab and C2-Fab were over 99% monomer (Fig. S1). Therefore, it is unlikely that these Fab fragments form protein aggregates and sensitize cells to Wnt signaling through dimerization of LRP6. Indeed, C1 in the IgG format had a similar, not stronger, stimulating activity than in the Fab format on Wnt3a-induced expression of Axin2, a β-catenin target gene (Fig. 2C). Furthermore, this class of antibodies increased both Wnt1- and Wnt3a-mediated signaling (Fig. 2D). Because C1 and C2 bind to the same region of LRP6 (Discussion), they likely increase Wnt signaling through a specific mechanism, possibly by relieving an inhibitory conformation of LRP6.
Fig. 2.
Characterization of monovalent agonistic LRP6 antibodies. (A) Monovalent agonistic anti-LRP6 Fab fragments increase Wnt3a-induced STF in HEK293 cells. (B) Monovalent agonistic anti-LRP6 Fab fragments increase Wnt3a-induced phosphorylation of LRP6 in HEK293 cells. (C) Agonistic anti-LRP6 antibodies in both the Fab and IgG formats increase Wnt3a-induced Axin2 mRNA expression. Rat2 cells were treated overnight with control- or Wnt3a-conditioned medium together with indicated antibodies, and Axin2 expression was analyzed by qPCR. (D) Agonistic anti-LRP6 antibodies increase Wnt1- and Wnt3a-induced STF in HEK293 cells.
Propeller 1 and Propeller 3 of LRP5/6 Are Differentially Required for Wnt1- and Wnt3a-Mediated Signaling.
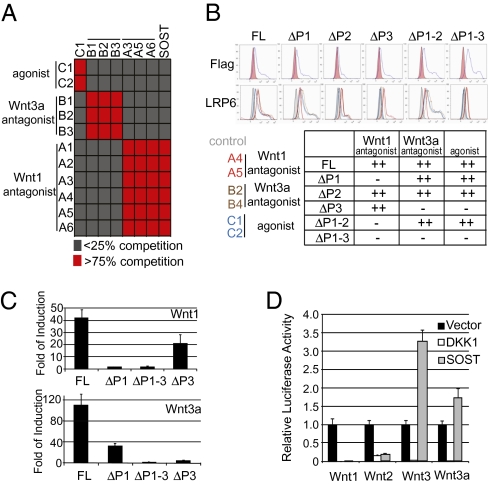
To characterize the binding domains of the three classes of LRP6 antibodies identified in this study, we performed FACS-based cross-competition experiments by testing the binding of biotinylated Fab fragments to ectopically expressed LRP6 in an excess of unbiotinylated competing Fab fragments. We found that antibodies in these three classes competed with antibodies in the same class but not antibodies in different classes (Fig. 3A), suggesting that antibodies in the same class bind to the same or overlapping site of LRP6. To further map the binding region of these antibodies, N-terminal Flag-tagged LRP6 constructs were generated, with each propeller deleted individually or in combination. HEK293 cells coexpressing Flag-tagged LRP6 deletion mutants and the chaperone protein MESD (24) were stained with anti-Flag antibody or the relevant test Fab fragments. Binding of various antibodies to LRP6-overexpressing cells was determined by FACS. Membrane expression of various LRP6 truncation mutants was indicated by anti-Flag antibody staining. Results from this experiment suggest that Wnt1 class-specific LRP6 antibodies (A4 and A5) require propeller 1 of LRP6 for binding, whereas Wnt3a class-specific LRP6 antibodies (B2 and B4) and agonistic LRP6 antibodies (C1 and C2) require propeller 3 of LRP6 for binding (Fig. 3B).
Fig. 3.
The propeller 1 and propeller 3 of LRP5/6 are differentially required for the signaling activity of Wnt1- and Wnt3a-class proteins. (A) FACS-based competition assay of three classes of LRP6 antibodies. Biotinylated anti-LRP6 Fab fragments were bound to HEK293 cells overexpressing LRP6 and competed away with an excess of unbiotinylated Fab fragments. (B) Mapping the binding region of various anti-LRP6 Fab fragments. HEK293 cells were cotransfected with MESD-expression construct and N-terminal Flag-tagged full-length (FL) or propeller-deleted LRP6 expression constructs, stained with anti-Flag or anti-LRP6 antibodies, and subjected to FACS analysis. (Upper) Cells were stained with anti-Flag antibodies, and cells transfected with MESD alone were used as negative control. Note that the LRP6 construct with the propeller 2 deleted (LRP6ΔP2) had lower membrane expression compared with other LRP6 constructs. (Lower) Cells were stained with indicated anti-LRP6 Fabs and control Fab. Bindings of anti-LRP6 Fab fragments to ectopically expressed LRP6 constructs are summarized in the table. (C) The propeller 1 and propeller 3 of LRP5 are differentially required for the signaling activity of Wnt1 and Wnt3a. HEK293 cells treated with LRP6 siRNA were cotransfected with STF reporter, Wnt1 plasmid, or Wnt3a plasmid together with empty vector or indicated LRP5-deletion constructs. Fold of induction measures the ratio of STF activity induced by LRP5-deletion mutant with Wnt-expression plasmid over STF activity induced by LRP5-deletion mutant and empty vector. (D) Coexpression of SOST preferentially inhibits the signaling activity of Wnt1 class of proteins. HEK293 cells were cotransfected with Wnt-expression construct and empty vector, DKK1-, or SOST-expression construct.
One possible explanation of the results is that Wnt1-specific LRP6 antibodies (class A) block the Wnt1–LRP6 interaction, Wnt3a-specific LRP6 antibodies (class B) block the Wnt3a–LRP6 interaction, and Wnt1 and Wnt3a bind to propeller 1 and propeller 3 of LRP6, respectively. To test this hypothesis, we sought to determine whether Wnt1 or Wnt3a could increase the signaling activity of exogenously expressed LRP6 lacking either propeller 1 or propeller 3. We have found that LRP6 plays a dominant role over LRP5 in HEK293 cells, because siRNA-mediated knockdown of LRP6 abolished Wnt-induced STF in HEK293 cells (Fig. S2). To eliminate the contribution of endogenous LRP6, HEK293 cells were cotransfected with siRNA to LRP6 along with the relevant LRP5 deletion mutants and empty vector, a Wnt1- or Wnt3a-expression plasmid. The synergy between Wnt and a given LRP5 deletion mutant was measured by taking the ratio of STF activity induced by LRP5 deletion mutant and Wnt-expression plasmid over the STF activity induced by LRP5 deletion mutant and the empty vector. As seen in Fig. 3C, deletion of propeller 1 or propellers 1–3 dramatically reduced the synergy between Wnt1 and LRP5, whereas deletion of propeller 3 only had a mild effect. In contrast, deletion of propeller 3 or propellers 1–3 severely reduced Wnt3a-mediated signaling, whereas deletion of propeller 1 had less of an effect. These results suggest that propeller 1 and propeller 3 are differentially required for the signaling activity of Wnt1 and Wnt3a.
SOST is a secreted Wnt antagonist that binds to the extracellular domain of LRP5/6 (25, 26). Missense mutations of LRP5, which are all clustered in propeller 1 of LRP5, are linked to high bone-mass (HBM) diseases. How SOST deficiency increases bone density is not well-understood, although SOST proteins show reduced binding to LRP5 HBM mutants (25, 27). We tested the ability of SOST to compete with various anti-LRP6 Fab fragments for binding to exogenously expressed LRP6. Here, we found that SOST blocked the binding of Wnt1 class-specific anti-LRP6 Fab fragments, but not other anti-LRP6 Fab fragments, to LRP6 (Fig. 3A). Together with our finding that Wnt1-specific anti-LRP6 antibodies bind to propeller 1 of LRP6, these results suggest that SOST binds to propeller 1 of LRP6 and blocks the binding of Wnt proteins, consistent with the observation that all HBM mutations are clustered in propeller 1 of LRP5.
Because SOST specifically competed with Wnt1 class-specific anti-LRP6 antibodies in the LRP6-binding assay, a prediction is that SOST would preferentially inhibit the signaling activity of the Wnt1-class proteins. Indeed, coexpression of SOST strongly inhibited Wnt1- and Wnt2-induced STF activation but slightly increased Wnt3- and Wnt3a-induced STF activation (Fig. 3D).The enhancement of Wnt3- and Wnt3a-mediated signaling could result from dimerization of LRP6 (Discussion). As a control, we showed that coexpression of DKK1 inhibited the signaling activity of all Wnt proteins (Fig. 3D). These data suggest that both Wnt1-class LRP6 antibodies and SOST bind to the first propeller of LRP6.
Bivalent LRP6 Antibodies Sensitize Cells to Wnt Signaling.
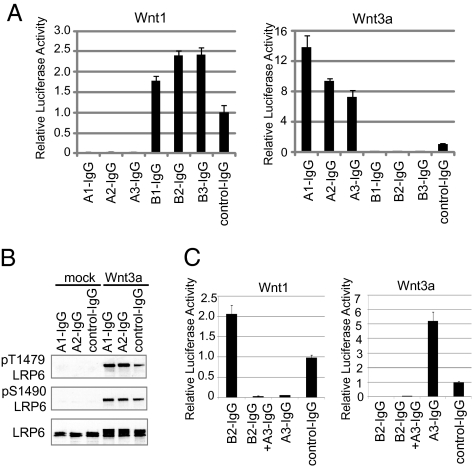
The signaling activity of many membrane receptors is affected by dimerization or oligomerization. How dimerization affects the signaling activity of LRP6 has been controversial (7, 28). The availability of antibodies capable of binding to endogenous LRP6 provided a unique opportunity to test whether dimerization of endogenous LRP6 affects Wnt/β-catenin signaling. We found that Wnt1-specific anti-LRP6 antibodies in the IgG format (A1-IgG, A2-IgG, and A3-IgG) strongly inhibited Wnt1-induced STF activity (Fig. 4A) compared with the Fab counterparts (Fig. 1A). In contrast, the Wnt1-specific anti-LRP6 antibodies markedly increased Wnt3a-induced STF activity (Fig. 4A) and Wnt3a-induced phosphorylation of LRP6 (Fig. 4B). Similarly, Wnt3a-specific anti-LRP6 antibodies in the IgG format (B1-IgG, B2-IgG, and B3-IgG) not only inhibited Wnt3a-induced STF activity but also increased Wnt1-induced STF activity (Fig. 4A). The synergy between bivalent anti-LRP6 antibodies and the alternative class of Wnt proteins is fully consistent with the hypothesis that dimerization of LRP6 enhances Wnt signaling (7). In the absence of Wnt3a- conditioned medium, bivalent anti-LRP6 antibodies did not activate the STF reporter (Fig. S3) or increase phosphorylation of LRP6 (Fig. 4B). Furthermore, combinations of Wnt1- and Wnt3a-specific anti-LRP6 IgGs completely blocked both Wnt1- and Wnt3a-induced STF activation (Fig. 4C). These results suggest that bivalent LRP6 antibodies do not activate signaling by themselves and they only sensitize cells to Wnt signaling, possibly through dimerization of endogenous LRP6.
Fig. 4.
Bivalent anti-LRP6 antibodies sensitize cells to Wnt signaling. (A) Anti-LRP6 antibodies converted to the IgG format gain agonistic activity on the alternative class of Wnt proteins. HEK293 cells transfected with STF reporter and Wnt1- or Wnt3a-expression plasmid were treated with indicated anti-LRP6 IgGs at 10 μg/mL. (B) Wnt1-specific antagonistic anti-LRP6 IgGs increase Wnt3a-induced phosphorylation of LRP6. HEK293 cells were treated with control- or Wnt3a-conditioned medium together with indicated antibodies for 6 h. Phosphorylation of LRP6 was analyzed by Western blot assay. (C) The combination of Wnt1- and Wnt3a-specific anti-LRP6 IgGs inhibits both Wnt1- and Wnt3a-induced STF activation. HEK293 cells transfected with STF and Wnt1 or Wnt3a plasmids were treated with indicated antibodies at 3 μg/mL
Wnt1 or Wnt3a Class-Specific Anti-LRP6 Antibodies Specifically Inhibit the Growth of MMTV-Wnt1 or MMTV-Wnt3 Xenografts, Respectively.
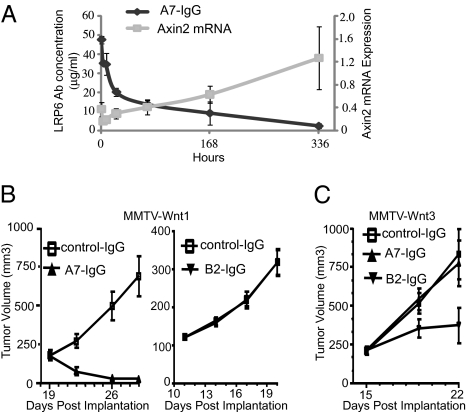
Because autocrine Wnt signaling has been implicated in tumorigenesis, we tested whether LRP6 antibodies in the IgG format can inhibit Wnt signaling and tumor growth in vivo. A xenograft model was established by passaging primary MMTV-Wnt1 tumor in nude mice. To measure the effect of LRP6 antibodies on Wnt signaling in MMTV-Wnt1 tumors, tumor-bearing mice were treated with a single dose of 5 mg/kg A7-IgG, which is an affinity matured Wnt1-specific anti-LRP6 antibody derived from A1. Serum concentrations of the antibody as well as the mRNA expression of β-catenin target gene Axin2 were analyzed over a period of 2 wk. As seen in Fig. 5A, the terminal β-phase half-life of A7-IgG is about 108 h. Corresponding to the antibody injection, we observed a significant decrease of Axin2 mRNA expression in tumors, and Axin2 expression gradually recovered 1 wk after the antibody injection when the antibody level in serum decreased (Fig. 5A and Fig. S4A). These results indicate that A7-IgG is able to suppress Wnt signaling in MMTV-Wnt1 xenografts and that this suppression is correlated with the antibody concentration in serum. To test the effect of anti-LRP6 antibodies on tumor growth, mice were treated with a Wnt1 class-specific LRP6 antibody (A7-IgG), Wnt3a class-specific LRP6 antibody (B2-IgG), or IgG control. We found that Wnt1 class-specific anti-LRP6 antibody induced tumor regression without causing body-weight loss, and Wnt3a class-specific anti-LRP6 antibody had no activity on MMTV-Wnt1 xenografts (Fig. 5B and Fig. S4B). We also tested these antibodies in an MMTV-Wnt3 xenograft model. Mice bearing established MMTV-Wnt3 tumor xenografts were treated with either IgG control or Wnt3a class-specific anti-LRP6 antibody (B2-IgG) or either Wnt1 class-specific anti-LRP6 antibody (A7-IgG) or IgG control. Wnt3a class-specific, but not Wnt1 class-specific, anti-LRP6 antibody inhibited the growth of MMTV-Wnt3 xenografts without inducing body-weight loss (Fig. 5C and Fig. S4C). Together, our results show that Wnt1 or Wnt3a class-specific anti-LRP6 antibody specifically inhibits the growth of MMTV-Wnt1 or MMTV-Wnt3 xenografts.
Fig. 5.
Wnt1 or Wnt3a class-specific LRP6 antibody specifically inhibits tumor growth of MMTV-Wnt1 or MMTV-Wnt3 xenografts. (A) Wnt1 class-specific anti-LRP6 antibody inhibits Wnt signaling in MMTV-Wnt1 xenografts. Nude mice implanted with MMTV-Wnt1 tumors were dosed i.v. with a single dose of 5 mg/kg A7-IgG. Serum concentrations of the antibody as well as the mRNA expression of β-catenin target gene Axin2 in tumors were analyzed over a period of 2 wk. The mRNA level of Axin2 was normalized to tumors from untreated mice. (B) Wnt1 class-specific LRP6 antibody inhibits the growth of MMTV-Wnt1 xenografts. Mice bearing established MMTV-Wnt1 xenografts were treated with either Wnt1 class-specific LRP6 antibody (A7-IgG) at 4 mg/kg every 7 d or Wnt3a class-specific LRP6 antibody (B2-IgG) at 10 mg/kg one time every 3 d. IgG served as negative control in both experiments. Tumor volume was measured every 3 d. (C) Wnt3a class-specific anti-LRP6 antibody inhibits the growth of MMTV-Wnt3 xenografts. Mice bearing established MMTV-Wnt3 tumor xenografts were treated with IgG control or Wnt3a class-specific anti-LRP6 antibody (B2-IgG) at 10 mg/kg two times a week or Wnt1 class-specific anti-LRP6 antibody (A7-IgG) at 3 mg/kg. IgG served as negative control. Tumor volume was measured two times a week.
Biparatopic LRP6 Antibody Inhibits both Wnt1- and Wnt3a-Induced Signaling.
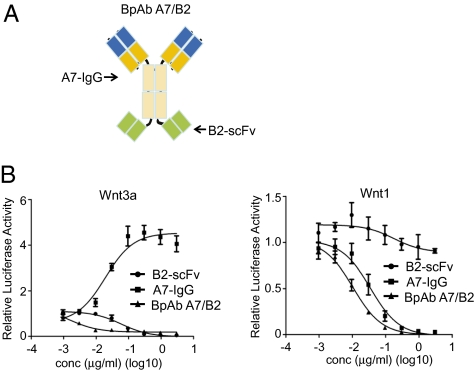
In this study, we have shown that different classes of Wnt proteins require different propellers of LRP6 for signaling and one LRP6 antibody can only inhibit one class of Wnt proteins. Furthermore, bivalent anti-LRP6 antibodies promote signaling mediated by the other class of Wnt proteins. These findings implicate the desire to inhibit with an anti-LRP6 antibody capable of blocking different Wnt proteins, because both classes of Wnt proteins have been implicated in tumorigenesis and it is not always clear which class of Wnt proteins is responsible for the growth or survival of a given tumor. To address this issue, we generated a biparatopic LRP6 antibody by fusing B2-scFv, which represents single-chain antibody fragments of B2, to A7-IgG (Fig. 6A). This biparatopic antibody (BpAb A7/B2) strongly inhibited both Wnt1- and Wnt3a-induced β-catenin signaling without showing any agonistic activity (Fig. 6B).
Fig. 6.
Biparatopic anti-LRP6 antibody blocks both Wnt1- and Wnt3a-mediated β-catenin signaling. (A) A schematic diagram of biparatopic anti-LRP6 antibody BpAb A7/B2. BpAb A7/B2 was generated by fusing B2 single-chain antibody (B2-scFv) to CH3 of A7-IgG. (B) BpAb A7/B2 inhibits both Wnt1- and Wnt3a-induced STF in HEK293 cells. A7-IgG and B2-scFv were used as control.
Discussion
Misregulation of Wnt signaling has been linked to various human diseases. To modulate Wnt signaling in diseases, we have generated humanized agonistic and antagonistic anti-LRP6 antibodies and shown that antagonistic anti-LRP6 antibodies block Wnt-driven tumor growth in vivo. Using these anti-LRP6 antibodies, we have made several findings of Wnt biology. We have provided multiple lines of evidence indicating that different Wnt proteins require different propellers of LRP5/6 for signaling. We have also identified two classes of Wnt-potentiating anti-LRP6 antibodies that act through different mechanisms.
Wnt proteins are thought to activate downstream signaling through binding to both Frz and LRP5/6 and promoting the formation of an active receptor-signaling complex. Using anti-LRP6 antibodies, we made the surprising finding that Wnt proteins can be divided into a Wnt1 class and a Wnt3a class, each requiring different propellers of LRP5/6 for signaling. This finding is further supported by a structural functional analysis of LRP5/6. During the preparation of this manuscript, it was published that Wnt3a binds to a fragment containing propeller 3 and 4 of LRP6, whereas Wnt9b binds to a fragment containing propeller 1 and 2 using an in vitro binding assay (29). These results are fully consistent with our findings, and our work further shows the functional significance of the differential binding between Wnt proteins and LRP6 both in vitro and in vivo.
Our study has provided insights on the interplay of Wnt, LRP5/6, and SOST. The HBM mutations of LRP5 all reside in propeller 1 of LRP5. Recent studies suggest that SOST has reduced affinities to LRP5/6 HBM mutants (25, 27). Our observation that SOST competes with Wnt1 class-specific anti-LRP6 antibodies for LRP6 binding (Fig. 3A) suggests that SOST blocks Wnt signaling by binding to propeller 1 of LRP5/6 and directly competing with the Wnt1-class proteins. This is consistent with our finding indicating that Wnt10b and Wnt7b, two proteins implicated in bone formation (30, 31), belong to the Wnt1 class.
We have identified two anti-LRP6 antibodies that are agonistic in the monovalent Fab format. Epitope mapping and competition experiments suggest that both antibodies bind to a unique site within the propeller 3 of LRP6. Previous studies using LRP6 deletion mutants suggest that the extracellular domain might adopt an autoinhibitory conformation (28, 32, 33). In LDLR, the intramolecular interaction between the LDLR domain and the β-propeller domain controls LDL binding and release from LDLR (34). Whether an analogous intramolecular interaction exists in LRP5/6 and whether this interaction regulates Wnt signaling are currently unknown. It is possible that monovalent agonistic anti-LRP6 antibodies enhance Wnt signaling by relieving an inhibitory conformation of LRP6.
How the dimerization status of LRP5/6 regulates Wnt signaling has been controversial (7, 28). Our observation that bivalent anti-LRP6 antibodies inhibiting one class of Wnt proteins sensitize cells to the other class supports the hypothesis that dimerization of endogenous LRP6 promotes Wnt signaling, possibly by increasing the avidity of various interactions involving LRP6. This is consistent with data indicating that Wnt treatment induces the formation of plasma membrane-associated LRP6 aggregates, likely promoted by self-oligomerization of Dishevelled (33). Our discovery also raises the prospect of making even more potent Wnt sensitizers by generating multivalent anti-LRP5/6 antibodies.
Increased autocrine Wnt signaling has been associated with tumor development and progression, and therefore, antagonistic anti-LRP6 antibodies can potentially be used to treat cancer. Because the relevant Wnt proteins expressed in tumors are not well-defined, it would be advantageous to have a single molecule capable of inhibiting multiple Wnt ligands. This can be addressed with strategies to block signaling of all Wnt proteins without eliciting agonistic activities. In this study, we have generated a biparatopic anti-LRP6 antibody that blocks both Wnt1- and Wnt3a-mediated β-catenin signaling without showing agonistic activities (Fig. 6B). The therapeutic potential of this antibody in cancer treatment will be explored in future studies.
In summary, our study has provided insights on how LRP5 and LRP6 functionally interact with Wnt proteins and known secreted Wnt modulators. Anti-LRP6 antibodies generated in this study provide us unique opportunities to specifically modulate Wnt/β-catenin signaling mediated by different Wnt proteins in various disease settings.
Materials and Methods
The HuCAL GOLD antibody library was used for selection of LRP6-specific antibodies. HEK293 cells were cultured in DMEM supplemented with 10% FCS and antibiotics. Detailed methods are described in SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007428107/-/DCSupplemental.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 5.Semënov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 6.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 7.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 8.Tolwinski NS, et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 9.Holmen SL, Robertson SA, Zylstra CR, Williams BO. Wnt-independent activation of beta-catenin mediated by a Dkk1-Fz5 fusion protein. Biochem Biophys Res Commun. 2005;328:533–539. doi: 10.1016/j.bbrc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 12.Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H, et al. Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene. 2005;24:7946–7952. doi: 10.1038/sj.onc.1208910. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 16.Veeck J, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479–3488. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron R, Rawadi G, Roman-Roman S. Wnt signaling: A key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- 20.Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 23.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh JC, et al. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 25.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 26.Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 27.Semenov MV, He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281:38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourhis E, et al. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 33.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 34.Rudenko G, et al. Structure of the LDL receptor extracellular domain at endosomal pH. Science. 2002;298:2353–2358. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.