Abstract
Cross-talk between integrin receptors and activated growth factor receptors has been hypothesized to play a critical role in the initiation and progression of cancer. Despite in vitro evidence documenting the important role of integrin receptors in the regulation of cancer cell proliferation, the relative contribution of the integrin receptors to the initiation and progression of tumors remains unclear. Previous studies with a polyomavirus middle T mammary tumor model have indicated that targeted disruption of β1-integrin in the mammary glands of these mice completely blocks tumor induction. To further explore the general significance of these observations, we have crossed these conditional β1-integrin strains to a strain of mice carrying mouse mammary tumor virus/activated erbB2 (herein referred to as the NIC strain). In contrast to the tumor induction block in the polyomavirus middle T model, tumor onset in the β1-integrin–deficient NIC mice was delayed by only 30 d and was 100% penetrant. This modest effect on tumor induction was not a result of inefficient excision, as all tumors were confirmed as β1-integrin–null. Animals bearing β1-integrin–deficient ErbB2 tumors exhibited significantly reduced tumor volume, which was associated with increased tumor cell apoptosis and a reduction in tumor angiogenesis. In addition, β1-integrin–deficient tumors were compromised in their capacity to metastasize to the lung, a deficiency associated with abrogation of adhesion signaling. Taken together, these observations suggest that, although β1-integrin is dispensable for the initiation of ErbB2 tumor induction, it plays a critical role in metastatic phase of tumor progression.
Keywords: metastasis, tumorigenesis
The transmembrane integrin protein family constitutes the major cellular receptors for the various components of the ECM and have a preponderant role in modulating the interaction between cells and their environment. The active participation of integrin proteins in tumorigenesis is hence a longstanding concept that has only recently been formally validated in mouse models of human cancer. These studies have underscored the multifaceted importance of these proteins during tumorigenesis, especially their impact on the regulation of tumor cell proliferation (1–4) and in tumor cell survival (5). Accordingly, genetic disruption of integrin coupled signaling partners such as ILK, FAK, and c-Src have a profound impact on tumor induction (6–11).
Although these studies suggest that integrin-coupled signaling plays a critical role in mammary tumor induction, the relative contribution of this signaling pathway in ErbB2 tumor progression remains to be addressed. One experimental challenge posed by Cre-mediated conditional mammary epithelial ablation of β1-integrin is that mammary epithelial cells that fail to express Cre, and thus continue to express β1-integrin, have a strong proliferative advantage over those that successfully deleted the protein. Indeed, mammary tumors and metastatic cells that emerge in polyomavirus middle T (PyV mT) transgenic mice bearing both conditional β1 alleles remain proficient for β1-integrin because of a lack of Cre expression (4). To avoid the positive selection for this Cre-negative “escapee” epithelial population, we recently derived transgenic mice that express mouse mammary tumor virus (MMTV)/activated erbB2 with an internal ribosome entry site driving expression of the Cre recombinase (hereafter referred to as the NIC strain). With this system, expression of activated erbB2 is directly coupled to the Cre-mediated excision of any LOXP1 flanked conditional allele. We have previously demonstrated that this Cre transgenic system couples the expression of ErbB2 with conditional deletion of a targeted allele, thus avoiding the generation of “escapee” populations (12, 13).
To evaluate the role of β1-integrin in ErbB2 tumor progression, we have crossed the conditional β1-integrin strain to the NIC strain. By contrast to the complete block in PyV mT–induced tumorigenesis, tumor onset was extended by only 30 d and was 100% penetrant. Examination of the metastatic potential of these tumors also revealed that β1-integrin–deficient tumors were compromised in their capacity to metastasize to the lung. The dramatic differences in metastatic potential in the lung were further correlated with differences in the levels of tyrosine phosphorylation of p130Cas and paxillin. Thus, unlike the PyV mT model in which β1-integrin is required for tumor initiation, β1-integrin function is primarily involved in the metastatic phase of tumor progression in the MMTV/ErbB2 model.
Results
Deletion of β1-Integrin Impacts on Metastasis of ErbB2 Mammary Tumors.
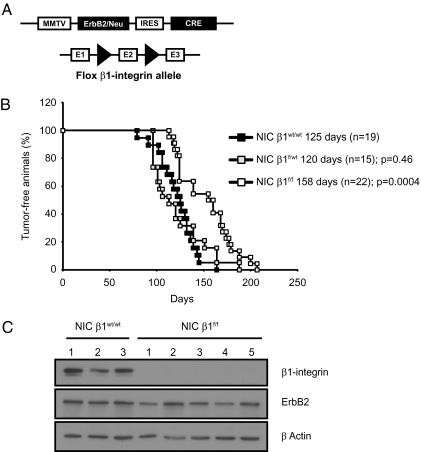
To evaluate the relative contribution of β1-integrin to ErbB2-dependent mammary tumor progression, we interbred mice bearing both β1-integrin conditional alleles with mice expressing activated ErbB2 oncogene and Cre recombinase under the transcriptional control of the MMTV promoter (NIC mice; Fig. 1A) (13). Mammary epithelial disruption of β1-integrin in these NIC female mice resulted in a significant 33-d delay in the induction of mammary tumors (Fig. 1B), but the tumors were histologically similar to their WT counterparts (Fig. S1B). The delayed tumor onset was correlated with complete ablation of β1-integrin protein (Fig. 1C and Fig. S1A) and was not a result of differences in ErbB2 expression as the β1-integrin deficient tumors expressed equivalent levels of ErbB2 protein (Fig. 1C).
Fig. 1.
β1-Integrin deletion delays tumor onset. (A) Schematic representation of the MMTV/activated erbB2 transgenic mice (NIC strain) and of the β1-integrinflox allele. (B) Kaplan-Meier analysis of tumor onsets. Control NIC β1wt/wt mice versus NIC β1f/wt versus NIC β1f/f (P = 0.0004). Median tumor onset values are indicated. The P values were calculated from a two-tailed Student t test. (C) β1-Integrin expression in the mammary breast tumor was assessed for NIC β1wt/wt or NIC β1f/f mice by immunoblotting.
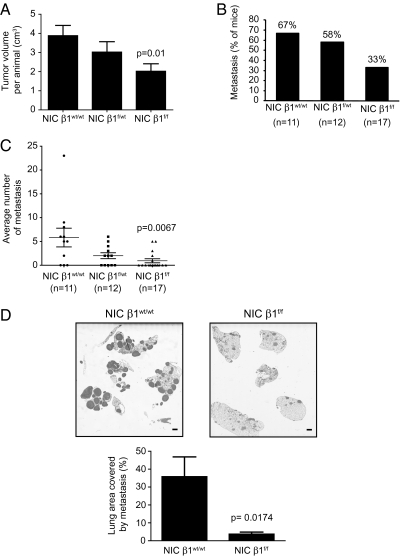
To further characterize the delay in tumor induction, 8 wk after tumor detection by manual palpation, we measured tumor volume in both β1-integrin–proficient and β1-integrin–deficient genetic backgrounds. Consistent with the initial delay in tumor induction, the β1-integrin–deficient mice had a significantly reduced overall tumor burden (Fig. 2A). To evaluate whether loss of β1-integrin also impacted on the capacity of mammary tumors to metastasize to the lung, we quantified both the penetrance and the extent of metastasis in the β1-integrin–deficient mice by counting metastatic lesions in H&E-stained step sections of lungs (Materials and Methods). Loss of β1-integrin was associated with a twofold reduction in the number of mice that developed metastatic lesions (Fig. 2B). In addition, quantification of the lung metastatic lesions observed for each metastasis-bearing animal cohort shows a sixfold decrease in the amount of β1-integrin NIC tumor cells able to efficiently invade the lung (Fig. 2C). Consistent with these impaired metastatic properties, injection of equal numbers of β1-integrin–deficient and β1-integrin–proficient tumor cells into the tail veins of immunodeficient animals revealed that β1-integrin function had a major impact on the ability of the tumor cells to efficiently colonize the lung (Fig. 2D). Taken together, these results suggest that, although loss of β1-integrin has a relatively modest effect on ErbB2 mammary tumor induction, it plays a role in the subsequent metastatic phase of tumor progression.
Fig. 2.
β1-Integrin–deleted tumors have reduced metastasis. (A) Average total tumor burden per animal (in cm3) for NIC β1wt/wt, NIC β1f/wt, or NIC β1f/f mice that have developed tumors. The error bars represent the SE for 19 NIC β1wt/wt, 14 NIC β1f/wt, or 17 NIC β1f/f mice. The indicated P value was calculated from a two-tailed Student t test. (B) Percentage of mice from FVB primary genotype (as indicated) that developed lung metastatic lesions. (C) Average number of metastatic lesions per lung in mice from B. The indicated P value was calculated from a two-tailed Student t test. Error bars represent SE for 11 NIC β1wt/wt, 12 NIC β1f/wt, or 17 NIC β1f/f mice. (D) Cells (0.5 million) from dissociated NIC β1wt/wt or NIC β1f/f primary tumors were injected in the tail vein of Ncr mice. (Upper) H&E-stained sections of the lungs collected 8 wk after injection. (Scale bar: 1 mm.) (Lower) Average lung area that is covered by metastatic lesions in percentage. The indicated P value was calculated from a two-tailed Student t test. Error bars represent SE for each genotype (n = 4).
Loss of β1-Integrin Results in a Decrease Tumor Epithelial Cell Survival and Tumor Angiogenesis.
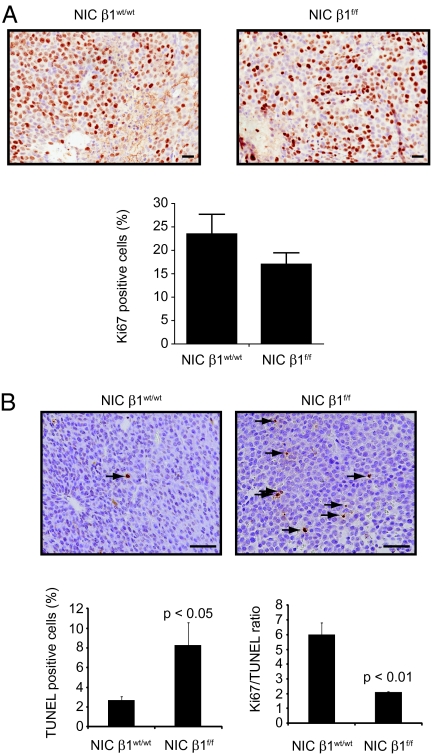
To elucidate the cellular basis for the observed defects in tumor burden in the β1-integrin–deficient ErbB2 tumors, we evaluated the effects of β1-integrin deletion on apoptosis, proliferation, and angiogenesis of ErbB2-induced mammary tumors by performing immunohistochemistry on paraffin-embedded mammary tumor sections with the appropriate markers. Although β1-integrin–deficient tumors exhibited a mild defect in proliferative status, as reflected by the quantification of the cellular Ki67 expression levels (Fig. 3A), they display a fourfold increase in the number of apoptotic cells as evaluated by TUNEL analysis (Fig. 3B).
Fig. 3.
β1-Integrin deletion decreases tumor growth by impairing cell survival. (A) Proliferation was assessed by immunohistochemistry staining on sections of paraffin-embedded mammary tumors with Ki67 antibody. (Scale bar: 50 μm.) The bar graph (Lower) represents the percentage of Ki67-positive cells. Error bars represent the SE of cells counted in at least three mammary tumors. (B) TUNEL-stained sections of paraffin-embedded mammary tumors of the indicated genotype. (Scale bar: 50 μm.) Apoptotic cells appear brown (arrows). The bar graph (Lower) represents the percentage of TUNEL-positive cells (Left) and the Ki67/TUNEL ratio (Right). Error bars represent SE of cells counted in at least three mammary tumors. The indicated P values were calculated from a two-tailed Student t test.
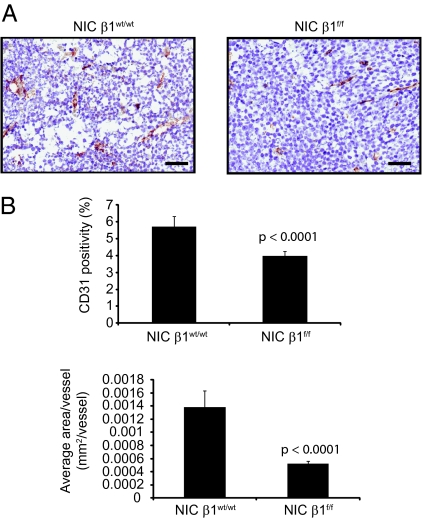
Another possible explanation for the observed growth defects exhibited by the β1-integrin–deficient tumors may be an inability to efficiently recruit tumor vasculature, as β1-integrin has been previously implicated in promoting tumor angiogenesis (14). To test this possibility, we performed immunohistochemical analyses on the tumors by using an anti-CD31 antibody (Fig. 4A). Interestingly, β1-integrin–deficient tumors exhibited a different pattern of CD31 staining compared with their proficient counterparts (Fig. 4). Although the number of CD31-positive vessels was only slightly reduced between the two genotypes (Fig. 4B Upper), the number of CD31-positive pixels observed for each independent vessel within β1-integrin–deficient tumors was reduced by an average of threefold (Fig. 4B Lower). These results reflect a diminution in the average diameter of the tumor-infiltrated vessels observed in NIC tumors and suggest an overall impaired blood supply to these tumors.
Fig. 4.
β1-Integrin–deleted tumors exhibit impaired vascularization. (A) Immunohistochemistry staining of paraffin-embedded mammary tumors with an antibody directed against the endothelial-cell marker CD31. (Scale bar: 50 μm.) (B) (Upper) Percentage of CD31-positive cells for the indicated genotype. (Lower) Average area per vessel in square millimeters. Error bars represent SE of cells counted for at least three mammary tumors. The indicated P values were obtained from a two-tailed Student t test.
Given that β1-integrin has been implicated in the ability of endothelial cells to migrate during various events of neovascularization during development (15), we assessed whether this effect was cell autonomous. To accomplish this, we enumerated the extent of CD31 infiltration in tumors that had been transplanted into the fat pads of immunodeficient mice that retain a functional β1-integrin in the endothelial compartment. The results confirmed that the observed vascular defect in the β1-integrin–deficient ErbB2 tumors is cell autonomous. Collectively, these observations indicate that the defects in tumor growth and progression exhibited by β1-integrin–deficient ErbB2 tumors are correlated with reduced tumor cell survival and angiogenesis.
β1-Integrin–Deficient Tumors Exhibit a Dramatic Defect in Adhesion-Dependent Signaling.
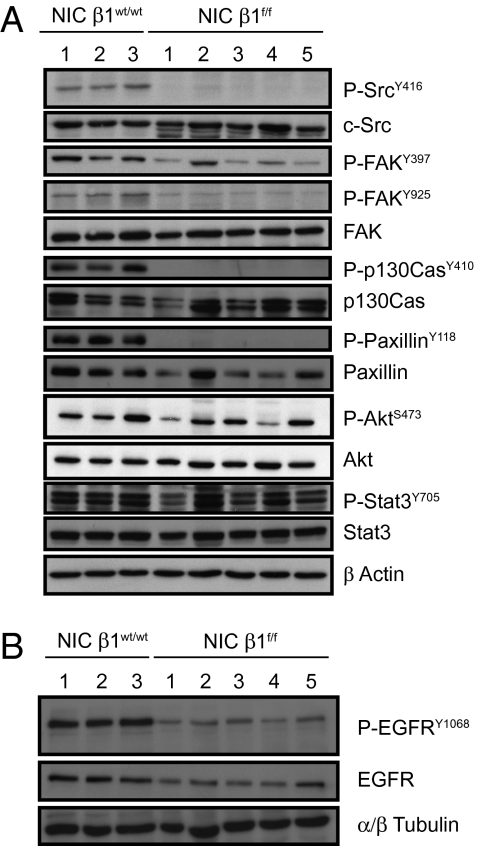
To investigate the molecular basis for the defect in tumor metastasis in the β1-integrin–deficient cells, we measured the activation of numerous integrin-coupled signaling partners using phospho-tyrosine–specific antibodies directed against FAK, c-Src, p130Cas, paxillin, Akt, and Stat3 (Fig. 5A). The results showed that β1-integrin–deficient tumors exhibited a decrease in c-Src, p130Cas, and paxillin tyrosine phosphorylation levels compared with their WT counterparts (Fig. 5A). The dramatic differences in the tyrosine phosphorylation status of these signaling molecules in tumors was not a result of levels of protein, as both β1-integrin–deficient and β1-integrin–proficient tumors possessed equal levels of total protein. Interestingly, the loss of tyrosine 416 phosphorylation in c-Src was further correlated with the degradation of c-Src (Fig. 5A). Taken together, these observations suggest that poorly metastatic β1-integrin–deficient cells possess major defects in the activation status of all the β1-integrin partners classically associated with adhesion and survival signaling.
Fig. 5.
Loss of β1-integrin impairs signaling. (A) Activation levels of protein of the c-Src pathway (as indicated) in primary tumor lysate from the indicated genotype addressed by immunoblotting. (B) Activation and total levels of EGFR in primary tumor lysate of the indicated genotype tested by immnublotting.
Another important function of β1-integrin signaling is its capacity to transphosphorylate the EGFR during the endocytic recycling of integrin and growth factor receptors. Specifically, the enhanced invasion of tumor cells has been shown to involve a Rab25/RCP-dependent recycling and activation the β1-integrin and EGFR complex to plasma membrane of cells (16). To explore whether loss of β1-integrin impacts on EGFR autophosphorylation, we measured the state of activation of EGFR in β1-integrin–deficient tumors using the appropriate antibodies. Loss of β1-integrin was associated with a significant reduction in EGFR phosphorylation levels, suggesting decreased EGFR activity in β1-integrin–deficient tumors (Fig. 5B).
In addition to impaired transactivation of EGFR, immunoblot analyses revealed that the α1-, α6-, and β4-integrin subunits were down-regulated upon disruption of β1-integrin (Fig. S1C). However, the levels of α5-, β3-, and β5-integrins were increased (Fig. S1C). Taken together, these molecular analyses indicate that impaired metastatic phenotype exhibited by β1-integrin–deficient ErbB2 tumors is most likely a result of associated defects in integrin signaling and transphorylation of the EGFR.
Discussion
Integrins are thought to play a critical role in tumor progression, in part because of cross-talk with growth factor receptor pathways. Several previous studies, including our own (4), have emphasized the key role played by β1-integrin during tumor growth and metastasis (2–5, 17–23). Here, we show that targeted disruption of β1-integrin in ErbB2-induced mammary tumors is associated with impaired tumor progression. Molecular and biochemical characterization of β1-integrin–deficient tumors revealed that observed defects in tumor growth and metastasis is correlated with enhanced apoptotic cell death and impaired angiogenic infiltration. Examination of a number of integrin-coupled signaling pathways has revealed that the β1-integrin–deficient tumors exhibit a dramatic reduction in activation of c-Src, FAK, p130Cas, and paxillin. Similarly, we recently demonstrated that targeted disruption of ILK in an ErbB2 model is associated with complete loss of p130Cas and paxillin tyrosine phosphorylation that is associated with impaired ErbB2 tumor progression (11). In addition to the reduction of these downstream effectors of integrin signaling, we also demonstrate that β1-integrin–deficient tumors are impaired in capacity to transphoshorylate the EGFR. Taken together, these data indicate that β1-integrin function plays a critical role in ErbB2 tumor induction.
In addition to the demonstrated importance of integrins and their coupled signaling pathways in mammary tumor progression, there is an increasing body of evidence to suggest that endocytic recycling of integrin and growth factors receptors also may be involved in cross-talk between β1-integrin and EGFR. Consistent with this observation, examination of tyrosine phosphorylation status of EGFR in β1-integrin–deficient tumors is reduced compared with β1-integrin–proficient tumors (Fig. 5B). Recent studies suggest that activation of EGFR by β1-integrin may be mediated by the Rab-associated protein known as RCP (16). The association of β1-integrin and EGFR through Rab/RCP complex has also been correlated with enhanced invasion in 3D matrices (16). Thus, the profound defect in metastasis observed in β1-integrin–deficient ErbB2 may be the result of altered recycling of β1-integrin. This is correlated with the decreased level of α1-, α6-, and β4-integrin receptors in the β1-integrin–deleted tumors (Fig. S1). The importance of this integrin/EGFR recycling in mammary tumor progression is highlighted by the fact that Rab25 (also known as Rab11c) and its effector, Rab coupling protein (also known as Rab11FIP1), are frequently expressed and amplified in primary human breast cancers (24, 25). However, whether elevated expression of Rab/RCP complex is involved in this aspect of ErbB2 tumor progression in vivo awaits further experimental validation.
Materials and Methods
Antibodies.
The primary antibodies used in the different experiments performed in this study have been purchased from the following companies: anti-Ki67 from Novocastra; anti–β-actin from Sigma; anti–β1-integrin, antipaxillin, anti-p130Cas, and anti-CD31 from BD Biosciences; anti–α/β-tubulin, anti–phospho-SrcY416, anti–c-Src, anti–phospho-FAKY925, anti–phospho-p130CasY410, anti–phospho-paxillinY118, anti–phospho-AKTS473, anti-AKT, anti–phospho-Stat3Y705, anti-Stat3 and anti–phospho-EGFRY1068 from Cell Signaling; anti-ErbB2, anti–β2-integrin, anti–β3-integrin, anti–β4-integrin, anti–β5-integrin, anti–α1-integrin, anti–α5-integrin, anti–αv-integrin, and anti–α6-integrin from Santa Cruz Biotechnology; anti–phospho-FAKY397 from Millipore; anti-FAK from Upstate; and anti-EGFR from R&D Systems.
Transgenic Mice.
MMTV-Neu–internal ribosome entry site–CRE (NIC) and β1-integrinflox mice were generated and characterized previously (4, 13). To preclude the possible involvement of genetic background variability, all transgenic mice used were derived from the inbred FVB/N strain. The monitoring of mammary tumor formation was performed weekly by manual palpation and initiated when virgin mice reached 80 d of age. Animals were killed 8 wk after tumor onset except when the tumor burden reached the maximal volume allowed by our protocol. This occurred in some of the β1-integrin–proficient NIC mice that were accordingly killed 6 wk after tumor onset. All animal studies were approved by the Animal Resources Centre at McGill University and complied with the guidelines set by the Canadian Council of Animal Care.
PCR.
PCR analysis of CRE-mediated excision was performed on total genomic DNA prepared from tail, mammary gland, or tumor tissues collected from mice by using a standard protocol. PCR primer sequences and amplification parameters were described elsewhere (26, 27).
Histological and Immunohistochemical Analysis.
Following necropsy, tumors and lungs were fixed in 10% neutral buffered formalin (Surgipath) and transferred to 70% ethanol the next day. Samples were then paraffin-embedded and sectioned at 4 μm. Mammary gland tumors and lung metastases were identified by microscopic analyses of H&E-stained sections of 4 μm. For lung metastasis analysis, five step sections of all five lobes separated by 50 μm were observed and extra- or intravascular metastases were counted. For immunohistochemistry analysis, endogenous peroxidase activity was blocked with 3% peroxide hydrogen in methanol. Antigen retrieval was accomplished in citrate buffer by using a pressure cooker (Cuisinart). Sections were then blocked with Power Block universal blocking agent (Biogenex) and incubated in primary antibody as described previously (4). For classical immunohistochemistry, slides were scanned using a ScanScope XT digital slide scanner (Aperio) and numerical data were analyzed by using a positive nuclear algorithm (in the case of Ki67 staining and TUNEL assay; as detailed later) or a positive pixel analysis (in the case of CD31 staining). In the latter case, we determined the average area of the tumor-infiltrated CD31 vessels by normalizing the average CD31-positive pixels counted using the Aperio software algorithm by the number of vessels observed for a given area of the tumor observed. Statistical analysis was then performed.
Tail Vein Injection.
Five hundred thousand cells dissociated from NIC β1wt/wt or NIC β1f/f primary tumors were injected in the tail vein of Ncr mice. Eight weeks after injection, the animals were killed and lungs were collected, fixed in 10% neutral buffered formalin (Surgipath), and transferred to 70% ethanol the next day. Samples were then paraffin-embedded and five step sections of all five lobes separated by 50 μm were stained by H&E. Slides were scanned using a ScanScope XT digital slide scanner (Aperio) and the lung area covered by metastatic lesions was measured using Aperio software.
TUNEL Assay.
TUNEL assay for apoptosis was performed according to the manufacturer's instructions for the Apoptag kit (Chemicon).
Tumor Cell Dissociation.
Tumors were chopped three times with a McIlwain tissue chopper (Mickle Laboratory Engineering) set to cut at 100-μm intervals, and the finely minced tissue was transferred to a digestion mix consisting of serum-free Dulbecco modified Eagle medium containing 4 mg/mL collagenase A, 4 mg/mL collagenase B, 4 mg/mL dispase, and 0.25 mg/mL hyaluronidase (all enzymes purchased from Roche) for 90 min at 37 °C under rocking. Cells were then pelleted by centrifugation and incubated for 4 min in 0.25% trypsin/EDTA (Wisent) at 37 °C and then with DNase for an additional 2 min at room temperature in PBS solution containing 2% bovine serum. Clumped cells were removed by filtering cells on a 70-μm cell strainer. Cells were then spun and resuspended in the appropriate volume of PBS solution containing 2% FBS.
Immunoblotting.
Flash-frozen tumor pieces were prepared on ice in Tris-HCl 50 mM, pH 7.4, sodium chloride 150 mM, Nonidet P-40 1%, sodium deoxycholate 1%, SDS 0.1%, EDTA 2 mM, PMSF 1 mM, sodium pyrophosphate 10 mM, sodium orthovanadate 1 mM, and sodium fluoride 10 mM. Protein amounts of the cleared lysates were quantified by Protein DC (Bio-Rad) and 20 μg of total protein were analyzed by immunoblot. Blots were incubated with HRP-conjugated secondary antibodies (Jackson Laboratories) and visualized by enhanced chemiluminescence (Amersham).
Statistical Analysis.
Statistical analysis were done using GraphPad software and two-tailed Student t tests were applied.
Supplementary Material
Acknowledgments
We thank Cynthia Lavoie and Vassilios Papavassiliou for their excellent technical help during the project and all members of the W.J.M. laboratory for their critical commentaries. S.M.P. received funding from a fellowship of the Terry Fox Foundation through an award from the National Cancer Institute of Canada. W.J.M. is supported by the Canada Research Chair in Molecular Oncology. This work was supported by grants from Cancer Research United Kingdom, the Canadian Institutes of Health Research/Canadian Breast Cancer Research Alliance, a Terry Fox Foundation team grant, the US Department of Defense, and National Institutes of Health Grant 5P01GA-099031-05.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.D.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003034107/-/DCSupplemental.
References
- 1.Grasso AW, et al. ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene. 1997;15:2705–2716. doi: 10.1038/sj.onc.1201447. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 3.Kren A, et al. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. EMBO J. 2007;26:2832–2842. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White DE, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahlou H, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean GW, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol. 2008;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pylayeva Y, et al. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Pontier SM, et al. Integrin-linked kinase has a critical role in ErbB2 mammary tumor progression: implications for human breast cancer. Oncogene. 2010;29:3374–3385. doi: 10.1038/onc.2010.86. [DOI] [PubMed] [Google Scholar]
- 12.Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ursini-Siegel J, et al. ShcA signalling is essential for tumour progression in mouse models of human breast cancer. EMBO J. 2008;27:910–920. doi: 10.1038/emboj.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch W, et al. Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–278. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senger DR, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caswell PT, et al. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira M, Fujiwara H, Morita K, Watt FM. An activating beta1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 2009;69:1334–1342. doi: 10.1158/0008-5472.CAN-08-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller PA, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Park CC, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam JM, Onodera Y, Bissell MJ, Park CC. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res. 2010;70:5238–5248. doi: 10.1158/0008-5472.CAN-09-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, et al. Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499–508. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills GB, Jurisica I, Yarden Y, Norma JC. Genomic amplicons target vesicle recycling in breast cancer. J Clin Invest. 2009 doi: 10.1172/JCI40256. 10.1172/JCI40256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009;19:524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troussard AA, et al. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- 27.Terpstra L, et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.