Abstract
The M2 protein is a small, single-span transmembrane (TM) protein from the influenza A virus. This virus enters cells via endosomes; as the endosomes mature and become more acidic M2 facilitates proton transport into the viral interior, thereby disrupting matrix protein/RNA interactions required for infectivity. A mystery has been how protons can accumulate in the viral interior without developing a large electrical potential that impedes further inward proton translocation. Progress in addressing this question has been limited by the availability of robust methods of unidirectional insertion of the protein into virus-like vesicles. Using an optimized procedure for reconstitution, we show that M2 has antiporter-like activity, facilitating K+ or Na+ efflux when protons flow down a concentration gradient into the vesicles. Cation efflux is very small except under conditions mimicking those encountered by the endosomally entrapped virus, in which protons are flowing through the channel. This proton/cation exchange function is consistent with the known high proton selectivity of the channel. Thus, M2 acts as a proton uniporter that occasionally allows K+ to flow to maintain electrical neutrality. Remarkably, as the pH inside M2-containing vesicles (pHin) decreases, the proton channel activity of M2 is inhibited, but its cation transport activity is activated. This reciprocal inhibition of proton flux and activation of cation flux with decreasing pHin first allows accumulation of protons in the early stages of acidification, then trapping of protons within the virus when low pHin is achieved.
Keywords: antiporter, liposomes, M2 channel, protons, ion channel
The influenza A virus M2 protein is a tetrameric integral membrane protein containing a short N-terminal extracellular domain, a transmembrane (TM) helix, and a 54-residue cytoplasmic tail (1, 2). Four M2 TM domains associate into a highly selective proton channel (3, 4) whose activity is essential for viral replication (5, 6). Influenza virus enters cells via the endosomal pathway, and the M2 protein functions to equilibrate the pH of the virus interior with that of the acidic endosome. The lowering of the pH within the virus leads to disruption of the interactions between the viral ribonucleoprotein (RNP) complex and the M1 protein (5), an important step in viral uncoating (7). Additionally, for some subtypes of influenza A virus, the M2 proton channel activity helps maintain a neutral pH in the lumen of the trans-Golgi network to prevent premature triggering of the hemagglutinin to the low-pH form (8–11). A long-standing question has been how M2 can conduct a substantial number of protons into the very small-volume interior of the virus without developing a substantial electrical gradient. Diffusion of a few protons down their concentration gradient from the acid environment of the endosome into the viral interior would result in an electrical gradient that would abruptly halt further proton flow and thereby prevent acidification of the viral interior. Here we demonstrate that under physiological pH gradients M2 mediates a significant outward flux of K+ that is activated by acidification of the vesicle interior, abrogating the buildup of a large electrical potential. Moreover, while proton flux through M2 increases at low pHout we now show that it is inhibited by low pHin.
Given its very small size and modular structure, M2 has already captured the attention of biophysicists and biochemists as a pharmacologically relevant model system for understanding proton translocation and gating; the demonstration of the complex pH-dependence of cation and proton transport should heighten interest in this protein. Peptides spanning M2’s TM helix are both necessary and sufficient to reproduce its proton-translocating and drug-binding activity (12). M2 has a single ionizable group in its TM domain, His37, which acts as both a pH sensor (13, 14) and determinant of its proton selectivity; variants in which His37 is mutated to other polar residues lose their selectivity for protons (15). Trp41 acts in concert with His37 as a “gate,” helping to define the rate of proton flux and to kinetically trap protons within an acidified virus (16–18). Recent studies have shown that valine 27 at the entrance of the pore may act as a secondary gate of the A/M2 channel (17–19).
M2 is the target of the adamantane-containing antiinfluenza drugs as well as related hydrophobic amine-containing structures (3, 4, 20–23). Structures of the A/M2 TM domain in complex with amantadine have been determined by X-ray crystallography (17) and solid-state NMR (24). The X-ray structure of M2TM crystallized from micelles as well as the solid-state NMR structure in phospholipid bilayers showed that a single molecule of amantadine binds in the N-terminal half of the pore, surrounded by residues Val27, Ala30, Ser31, and Gly34 (24); a second low-affinity site on the outside of the protein has been observed (25), although this site is not pharmacologically relevant (26). These studies demonstrated the presence of a substantial pore, which is hydrated in the absence of drug (27).
The conductance of the M2 channel has been most thoroughly studied by measuring macroscopic whole-cell current in oocytes and mammalian cells at mildly acidic pH (3, 4, 6, 18, 20, 28, 29). Under these conditions, the channel is approximately 106 to 107-fold more permeable to protons relative to alkali metal cations such as Na+ and K+. Numerous investigations have shown that the channel is activated by low exterior pH (pHout), and its current/pH profile is sigmoidal with a midpoint near pH 6, reaching a limiting rate near pH 4.5 to 5. This behavior is reminiscent of transporters, and quite different from most channels, whose conductance increases with increasing permanent ion concentration, well into the high-mM range (30). The saturation of the rate at low pH has been hypothesized to be associated with additional kinetic barriers involving protonation/deprotonation of His37 (31) or the formation of significant energy barriers at low pH (32). The rate of proton flux through the M2 channel, which has been reported to range from 10 to 1,000 sec-1, depending on the electrical and chemical potential driving conduction (33), is also similar to that of transporters. Moreover, the electrophysiologically determined selectivity of 106 for protons vs. Na+/K+ suggests that cation flux might be on the same order of magnitude as that of proton flux, given that the physiologically relevant proton concentration is 10-6 M while that of K+ is 0.15 M.
Full-length M2 and various variants of M2TM have been reconstituted in large unilamellar vesicles (LUVs) and shown to have a conductance similar to that measured in whole cells in terms of selectivity and rate of proton conduction (33–39). However, electrophysiological studies of M2 show that its conductance is asymmetric with regard to the direction of the proton gradient; by contrast the current/voltage curves from M2 in vesicle assays are highly linear, probably because the channel is randomly inserted in the bilayer in two orientations with the N terminus oriented either inward or outward (34, 35). Here, we describe conditions for unidirectional insertion of full-length M2 into LUVs. Under these conditions, the conduction properties depend on voltage and pHout in a similar manner to that observed in M2-expressing cells. Unidirectional insertion allowed examination of asymmetric ion conduction under controlled conditions. We demonstrate that M2 has proton/cation exchange activity; diffusion of external protons (Hout) into vesicles activates the protein for outward conduction of K+ ions (Kin) as the concentration of Hin increases. Thus, the high inner concentration of K+ provides an adequate reservoir of cations to exchange for protons until the buffering capacity of the viral interior has been overcome and equilibration of protons between the endosome and virus is reached.
Results
Reconstitution of M2 in Proteoliposomes (M2PLs).
The M2 protein used in these studies is a full-length construct that has been extensively studied for a variety of biophysical and proton-transport experiments (37). Full-length M2 protein corresponding to the sequence of WT Udorn strain (GenBank accession no. CAD22815) was expressed with a C-terminal His tag and four extra mutations (W15F, C17S, C19S, C50S). These mutations significantly improve the protein expression yield and do not affect M2 function (40). Final M2 protein was solubilized with octyl glucoside (OG) and eluted from superflow Ni-NTA column (Qiagen) with 300 mM imidazole. The purity of the M2 protein was verified by both gel electrophoresis and HPLC to be > 98%. The M2 protein was reconstituted in liposomes by solubilization of preformed vesicles in the presence of M2 followed by slow detergent removal by hydrophobic beads. Using this method, we obtained a good yield for reconstitution between pH 6.5 and 7.5, but poorer reconstitution below pH 6.5. To establish the directionality of insertion, proteoliposomes were exhaustively proteolyzed and the released fragments were analyzed by MS. Although both the N-terminal extodomain and C-terminal cytoplasmic domain of the protein are highly susceptible to proteolysis (41), the only fragments detected in our M2PLs were derived from the N-terminal domain, which faces the outside of the virus. Moreover, the vesicle size was approximately 200 nm, which represents a compromise between the curvature encountered in spherical influenza particles (approximately 100 nm) and M2-expressing cells for which electrophysiological data are available. Thus, M2 proteoliposomes are excellent mimics in terms of size and protein orientation.
ΔpH-Driven Flux.
The physiological role of M2 is to transfer protons from the acidifying endosome into the viral interior whose primary cationic electrolyte is K+ ( serves as a membrane-impermeant counterion). We therefore examined proton flux under conditions of pHin (initial) = 7, while varying pHout (Fig. 1A). These experiments were performed in the presence of K+, and valinomycin was included to allow K+ to diffuse rapidly out of the vesicles as protons diffused in, thereby maintaining electrical neutrality. The salt composition was the same inside and out (symmetrical K+; [Kin] = [Kout] = 0.13 M), and the buffering capacity inside the vesicles was significantly smaller than [Kin] to prevent the formation of a large change in the chemical potential of K+ as it flowed outward in response to proton influx (42).
serves as a membrane-impermeant counterion). We therefore examined proton flux under conditions of pHin (initial) = 7, while varying pHout (Fig. 1A). These experiments were performed in the presence of K+, and valinomycin was included to allow K+ to diffuse rapidly out of the vesicles as protons diffused in, thereby maintaining electrical neutrality. The salt composition was the same inside and out (symmetrical K+; [Kin] = [Kout] = 0.13 M), and the buffering capacity inside the vesicles was significantly smaller than [Kin] to prevent the formation of a large change in the chemical potential of K+ as it flowed outward in response to proton influx (42).
Fig. 1.
pH change of the aqueous contents of M2PLs in response to the induction of a pH gradient induced at time = 0. Initial interior pH = 7 in all traces; external pH from top to bottom: pHout = 6.8, 6.5, 6.0, 5.5, 5.0. (A) Symmetrical [K+]in = [K+]out = 0.13 M, valinomycin present. (B) Symmetrical [K+]in = [K+]out = 0.13 M, no valinomycin. (C) Symmetrical [Na+]in = [Na+]out = 0.13 M, no valinomycin. These data are displayed as proton flux per tetramer in Fig. S1. Dotted (top) line of 1 B: Response of protein-free liposomes containing the protonophore CCCP but no valinomycin to a pH gradient of 1 pH unit induced at time = 0. (Trace offset -0.1 pH unit for clarity. The lower noise level of this trace is due to higher Glu3 concentration).
As observed in electrophysiological investigations, the rate of proton flux increases with decreasing pHout (i.e., increasing proton activity, Fig. 1A). Under these conditions, 90% of the time course followed a single-exponential decay. No significant flux on this time scale was observed in control liposomes lacking M2, and proton flux was > 90% blocked by amantadine (see below).
To determine whether M2 might allow K+ efflux concomitant with proton influx, pHout/flux curves were measured under the same conditions, but in the absence of valinomycin (Fig. 1B). As expected from the work of Busath et al. (33), the rates were slower, but they were not decreased to the background rate of proton flux for M2PLs inhibited by amantadine. Single-exponential kinetics were again observed; however, the rate was approximately threefold slower than in the presence of valinomycin. Together with previous studies (33), these findings suggest that, in the absence of a K+-carrier, the inward flux of protons is balanced by outward flux of K+ through the M2 channel.
Several experiments were conducted to confirm that M2 was a proton-selective channel that occasionally allowed counterconduction of cations, as well as to determine the range of size of cations that can pass through the channel. To establish that the K+ flux in Fig. 1B was not due to nonspecific diffusion of the ion through the bilayer, M2-free liposomes containing the specific protonophore CCCP but no valinomycin were subjected to a pH gradient of one pH unit (Fig. 1B, top trace). Virtually no protons were admitted into the liposomes under these conditions, showing impermeability of the bilayer with respect to K+. To determine the size-selectivity of M2’s counterconduction of cations, we compared the proton flux with symmetrical Na+ or N-methyl glucose-ammonium (NMG) to the results with K+. As in Fig. 1B, the flux was measured in the absence of valinomycin, and a proton gradient favoring inner proton flux. With the large cation NMG, no significant proton flux was observed (Fig. S2), indicating that M2 is unable to conduct large organic cations. By contrast, with symmetrical Na+, the kinetics of proton flux were similar (Fig. 1C) to those measured in the presence of symmetrical K+, and the rate was approximately 50% greater than the corresponding rate with K+ ions (Fig. 1B).
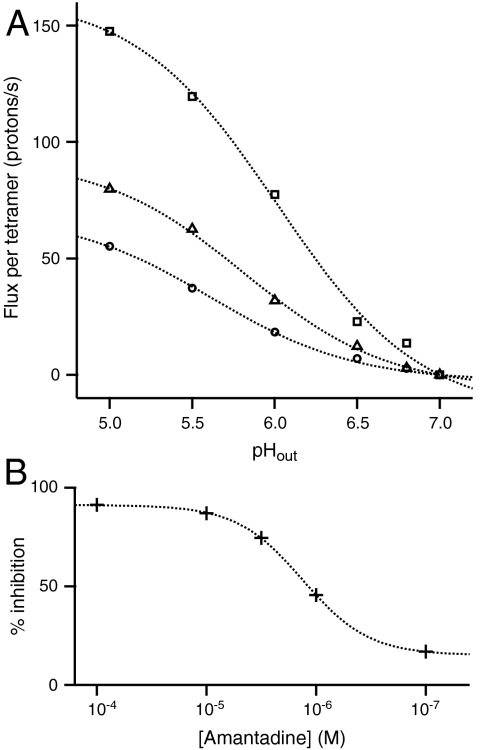
Fig. 2A illustrates the dependence of the initial rate of proton flux on pHout with constant initial pHin = 7.0 in the presence of symmetrical Na+ or K+ with and without valinomycin. Similar to whole-cell recordings (3, 4, 6, 18, 20, 28, 29), all three curves can be described by a simple conductance scheme with an effective pKa of 5.9 ± 0.2 (see SI Text). Confirming the qualitative features in Fig. 1A vs. Fig. 1B, the rate of conduction at pH 6 and symmetrical K+ = 0.13 M was threefold greater in the presence of valinomycin than in its absence. Finally, the proton flux was inhibited by up to approximately 90% in a dose-dependent manner by amantadine (Fig. 2B). These findings demonstrate that M2 mediates outward conduction of potassium ions in response to an inward proton flux. If the cation efflux were a simple consequence of passive diffusion, the rate of K+ efflux (in the absence of valinomycin) would be significantly greater than that of Na+, because K+ is a larger and much more easily dehydrated cation.
Fig. 2.
(A) The effect of varying pHout (initial pHin = 7.0) on the magnitude of the net inward flux of protons into M2PL. The inner pH at the start of each experiment was 7, and the rates are quoted as protons per M2 tetramer and sec, extrapolated to time = 0. Squares: K M2PLs with valinomycin (Fig. 1A); circles: K M2PLs without valinomycin (Fig. 1B); triangles: Na M2PLs (Fig. 1C). The smooth curves through the data were generated using a kinetic scheme based on a proton relay mechanism to fit a conducting pKa and maximal rate to the data (see SI Text). Other mechanisms such as a gated channel also provided a reasonable fit to the data. (B) Inhibition of the initial rate of proton conduction driven by a K+/valinomycin-induced potential (62 mV), as a function of amantadine concentration. The data were fit with a sigmoidal binding isotherm with Kdiss = 1.3 μM.
Finally, we wished to determine whether alkali metal ion flux occurred as a simple response to the buildup of an electrical potential as protons flowed down their concentration gradient through M2, or whether the conductance of cations was tightly coupled to that of protons as in a classical antiporter mechanism. To address this question, NMG was used as an impermeable cation, and a proton gradient was imposed by decreasing the outer pH one unit (Fig. S2). As described above, no significant proton flux is observed under these conditions. However, addition of 20 mM SCN- to the bathing solution results in rapid M2-mediated proton flux by balancing the charge of protons. This finding confirms that proton movement into M2PL requires charge equilibration across the membrane, which is equally satisfied by a membrane-permeable anion or M2-permeable Na+ or K+ cations.
Electrically Induced Proton Flux.
To investigate further the possibility of cation transport through M2, we performed experiments in which a transmembrane electrical potential was generated by rapidly diluting vesicles prepared in K+ buffer into Na+ buffer containing valinomycin. Valinomycin is very selective for transport of K+ relative to Na+ and facilitates very rapid diffusion of K+ out of the vesicle, which generates an electrical potential that drives inward proton flux up a concentration gradient. Fig. 3 illustrates data for M2PLs diluted into buffer with Na+ and K+ concentrations adjusted to induce a 62 mV potential (initial pHin = pHout). Under these conditions there was no significant proton flux (per-tetramer flux < 1 - 2 protons/ sec) in the absence of valinomycin. However, addition of valinomycin allowed rapid efflux of K+, establishing an electrical potential and inducing rapid acidification of the vesicle interior.
Fig. 3.
pH change of the aqueous contents of M2PLs in response to the induction of a 62 mV electrical potential, positive outside (A) or inside (B). The initial pH was symmetrical, and the [K+] gradient was induced at time = 0, with initial concentrations [K+]in = 0.13 M, [Na+]in = 0 M, [K+]out = 0.011 M, [Na+]out = 0.119 M (A) and [K+]in = 0.01 M, [Na+]in = 0.12 M, [K+]out = 0.12 M, [Na+]out = 0.01 M (B).
The observed time courses for vesicle acidification of M2PLs are biphasic for initial symmetrical pH (pHin = pHout) between 5.5 and 7 (Fig. 3A). The first phase requires approximately 1 to 5 min, and results in acidification of the interior of the vesicle. In marked contrast to the experiment in which only the initial pHout was varied while holding pHin constant, the rate of proton flux actually decreased with low pH when pHin and pHout were the same. This behavior has been seen in an earlier study of reconstituted M2 protein (42) and does not contradict electrophysiological studies, which did not examine proton flux at pHin < 6. When considered in relation to the experiment with variable pHout these data suggest that proton flux is activated by low pHout but inhibited by low pHin; indeed, a plot of the initial rate of proton flux vs. pHin is well described by a single-site isotherm with a pKa = 6.0 ± 0.16 (Fig. 4A). The same pKa was also observed when the potential was reversed (Figs. 3B and 4A), consistent with previous findings of small to no effect of voltage on the conducting pKa (29).
Fig. 4.
(A) The effect of symmetrical pH on the magnitude of the initial rate of flux through the M2 channel in response to a K+/valinomycin-induced potential of 62 mV. Circles: K-containing M2PLs (Fig. 3A); triangles: Na-containing M2PLs (Fig. 3B). (B) The chord conductance computed for the proton flux (open symbols) shown in A and the K+/valinomycin-induced potential of 62 mV at t = 0. (Note that the valinomycin allows distribution of potassium ions at a rate much greater than proton flux so that the full potential is essentially formed at time = 0). Also shown in B is the value of the chord conductance for Na+ ions vs. pHin (closed symbols—circles: K-containing M2PLs; triangles: Na-containing M2PLs). The Na+ conductance was computed between t = 6 min and 10 min during which the pH changed relatively slowly, allowing computation of the mean V and ENa+ over this interval (see SI Text).
Each time course of vesicle acidification also shows a second phase, which occurs at a much slower time scale and results in a gradual increase of the interior pH toward the initial value (Fig. 3A). We ascribe this slow increase in pHin to a loss in the electrical potential (42) due to slow M2-dependent equilibration of the sodium and potassium ion concentrations across the bilayer. With high 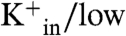
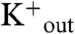 (and low
(and low 
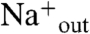 ) M2PLs containing valinomycin, the carrier allows rapid outward flux of K+ generating the electrical potential. Under these conditions, M2 promotes not only inward flux of protons but also Na+, ultimately leading to equilibration of the K+ and Na+ across the membrane (42). Our data now show that the magnitude of this inward Na+ flux is strongly pH-dependent, displaying opposite dependence on pH from the corresponding proton flux: The Na+ flux increases markedly with decreasing pHin (= pHout), while the proton flux decreases (Figs. 3 and 4).
) M2PLs containing valinomycin, the carrier allows rapid outward flux of K+ generating the electrical potential. Under these conditions, M2 promotes not only inward flux of protons but also Na+, ultimately leading to equilibration of the K+ and Na+ across the membrane (42). Our data now show that the magnitude of this inward Na+ flux is strongly pH-dependent, displaying opposite dependence on pH from the corresponding proton flux: The Na+ flux increases markedly with decreasing pHin (= pHout), while the proton flux decreases (Figs. 3 and 4).
The apparent Na+ flux during the slow phase can be computed from the rate of the depolarization as described previously (42). The per-channel flux increases from 1 to 10 ions per sec as the pH is lowered from 7.0 to 5.5. These values are smaller in magnitude and in reverse order from the initial rate of proton flux, which decreases from 40 to 5 protons/ sec over the same pH range (Fig. 4A). To allow a better comparison of the rates, the Na+ and H+ fluxes were converted to chord conductance (gi = flux/(V - Ei), in which V is the electrical potential created by the rapid valinomycin-dependent diffusion of K+ ions, and Ei is the chemical potential difference of Na+ or H+ ions). The same trends and magnitudes are observed as when the raw fluxes were compared; both curves conform to a classical binding isotherm with a pKa of 6.0 ± 0.15 for proton conductance and 6.0 ± 0.4 for Na+ conductance (Fig. 4B).
The partial depolarization associated with the slow inward Na+ flux is also responsible for the fact that the maximal pH change, ΔpHmax, observed in the first phase does not reach the limit of 1.0 expected from the 62 mV potential associated with the initial potassium gradient (Fig. 3A). The value of ΔpHmax at pH 7 is near 0.8, and decreases progressively with decreasing initial pH to a value of < 0.5 at pH 5.5. At ΔpHmax the electrical potential should approximately equal the proton-motive force (PMF), allowing calculation of the inner and outer ionic composition from which the mean inward Na+ flux can be estimated (as described in SI Text). A plot of the inner proton flux vs. inner Na+ flux has a shape similar to that of the initial pH flux but trends upward rather than downward at low pH.
To determine the effect of reversing the potential, M2PLs were prepared with the salt concentrations reversed to drive outward flux of H+ and Na+ (Fig. 3B). The resulting initial outward proton flux is of similar shape and magnitude to the inner proton flux under otherwise identical conditions. These findings are consistent with electrophysiological studies, which showed symmetrical current/voltage curves between approximately -60 and 60 mV when the proton concentration is the same on both sides of the bilayer. Similar results were observed for the inward Na+ flux, again increasing with decreasing pHin.
M2 Proton Flux Is Fully Inhibited by Amantadine.
The M2-mediated current induced by an electrical gradient was inhibited by amantadine in a dose-dependent manner, and the sigmoidal inhibition curve is well described by a classical single-site binding isotherm with Kdiss = 1.3 μM (Fig. 2B). This value compares well with IC50 values of 0.3 to 10 μM reported in the literature (20, 37). Previous studies of M2 proteoliposomes often showed only partial inhibition (33), which was ascribed to incomplete accessibility of the drug to randomly inserted protein. In contrast, our preparations show full inhibition at high amantadine concentrations.
Discussion
The results of this investigation, while unexpected, are consonant with the biological function of the protein and generally in good agreement with previous biophysical studies. The biological function of the protein is to pass protons down a pH gradient toward the interior of the virus. The experiments in which pHout was varied (while maintaining the initial pHin at 7.0; Fig. 2A) mimic this situation, and the results are in good agreement with previous electrophysiological studies (33, 43). Previously, there has been a lack of agreement between the rate of conduction measured by electrophysiology, which was on the order of 103 protons/ sec (31), and vesicle measurements, which tended to provide a maximal conductance rate of 1 to 10 protons/ sec. The improved reconstitution methods used herein gave M2PLs with the protein unidirectionally inserted as in the native virus. Cholesterol is important for maximal activity and efficient reconstitution, which is consistent with its known interaction with cholesterol (44) (45). The proper insertion might also be related to the curvature of the vesicles, because a cytoplasmic helix immediately C-terminal to the TM helix of M2 plays an important role in cholesterol-binding and determining the morphology of the virus (46).
Using our optimized reconstitution method, proton flux was too rapid to be easily measured in our system at 37 °C. Therefore, the experiments were conducted at 18 °C, at which a flux of 80 protons/ sec was observed with pHin = 7 and pHout = 6 (0.013 fA; 0.2 fS). The corresponding conductance inferred from electrophysiological experiments at 37 °C is approximately 4 fS (31). Given that M2-mediated proton flux M2 in vesicles increases about 5 to 10-fold between 18 °C and 37° (35), the expected conductance of M2 in vesicles at 37 °C (1 to 4 fS) is the same in M2PLs and vesicles within experimental error.
What has been less clear from electrophysiological studies has been how protons can accumulate within a virus without developing a large electrical gradient. The buffering capacity of the cytoplasm and organelles is substantial (approximately 50 to 100 mM pH-1) at mildly low pH (30). The extremely high effective concentration of RNA in the virus interior would further increase the buffering capacity because this biopolymer has excellent buffering capacity near pH 5 (47). Thus, to achieve a change in internal pH, a very large number of protons must flow into the virus to overcome this high internal buffering capacity. This would result in a large increase of the electrical potential if cations were unable to flow outward in response to inward proton flux. We show that the channel is in fact capable of outward K+ flux to counterbalance the inward flux. With symmetrical K+, the rate of proton inward flux is threefold slower in the absence of valinomycin than in its presence; thus, proton flux is attenuated due to the slower rate of potassium ion efflux in the absence of a K+ carrier. From the rate of proton flux in the presence and absence of valinomycin, and the concentration of protons and potassium ions, the selectivity of M2 at pHin 7.0, pHout = 6.5 is approximately 106, within the range obtained from electrophysiology (4, 48). The implication of this proton-dependent cation flux is that under these conditions M2 is functionally akin to an antiporter, exchanging protons for potassium ions. However, experiments with the permeable anion, SCN-, showed that protons and cations are not cotransported in a concerted manner as in classical antiporters.
Until now, it has been much more difficult to study the later stages of proton equilibration, in which the inside of the viral compartment becomes acidified. Electrophysiological measurements of M2 have been largely limited to whole-cell recordings, because the small conductance of the channel has precluded single-channel measurements of isolated membrane patches. Although this has limited the extent to which it has been possible to vary the interior pH, previous studies of proton and ion flux versus pHin are in very good qualitative agreement with the present study (4, 48). Of particular interest is our current finding that as the pHin was lowered, the rate of proton flux decreased and the rate of outward cation flux increased.
The pH-dependent behavior of M2 is consistent with the biological restraints and function of the protein as the virus moves through various portions of its infectivity cycle. When present on the cell surface prior to packaging it is important to minimize cell toxicity; high pHin and pHout assures minimal K+ and Na+ flow, which would disrupt the ionic balance of the host cell. Moreover, the very low concentration of protons at pH 7 minimizes the degree of proton flux. Once encapsulated in an endosome, the high but not absolute proton selectivity assures rapid acidification of the interior of a virus at a rate limited only by the low conductance of K+ in the opposite direction. Finally, as the interior pH of the virus reaches the value required for disruption of viral RNA/M1 protein interactions the rate of proton flux decreases, essentially trapping protons in the virus. The minimization of reversed flow of protons might actually be advantageous if RNA uncoating leads to proton release from the M1 protein and/or RNA components due to changes in their substituents’ pKa values during the dissociation process.
Despite differences in their mechanisms, M2 resembles cotransporters in many ways (30) (49–51): (i) The rates of proton turnover in transporters (30) generally occurs at rates of 100 to 10,000 sec-1. At a pH of 6 (10-6 M proton activity) this turnover corresponds to an overall second-order rate constant of 108 to 1010 M-1 sec-1, approaching the diffusion-controlled rate and limited only by the architecture of typical proton-entry sites in proteins. Similarly, the rate of 100 to 1,000 sec-1 observed for M2 at pH 6 corresponds to a second-order rate of 108 to 109 M-1 sec-1, indicating that proton transport occurs at a rate approaching the “speed limit” for proton translocation. (ii) Proton-dependent transporters typically have proton-binding sites, which limit the turnover rate at low pH as saturation of the binding sites is approached. Along these lines, it is interesting to note that equilibrium studies show that one of the pKa values for His37 is 6.2 (13, 14)—identical within experimental error with the conducting pKa observed in this study. (iii) With each turnover, transporters couple proton-binding/dissociation events with conformational changes critical for cotransport. It is quite reasonable to expect that M2 might similarly become protonated by a proton diffusing through the channel, given that the side chains of His37 in the tetrameric channel are directly positioned along the proton-diffusion pathway and have pKa values ranging from 8 to 5 assuring the availability of the basic form over a wide pH range. Moreover, given a pKa of 6 for the “conducting pKa” (13) and the overall second-order rate constant of 108 to 109 sec-1 for proton diffusion through the channel, the rate of deprotonation is calculated to be 100 to 1,000 sec-1 (Ka = 10-pKa = koff/kon). Thus, either the chemical rate of deprotonation or a conformational change that enables deprotonation is expected to occur on precisely the same time scale as proton turnover and provides an attractive explanation for the saturation of the rate at low pH. (iv) The process of proton transport should couple to conformational changes that occur on the same time scale as translocation. M2 is known to undergo pH-dependent changes in dynamics, undergoing large-scale conformational changes that cause severe broadening of the main chain amides as the pH is lowered below 6.5 (12, 52), indicative of motions on the same time scale as the dispersion of chemical shifts in distinct conformational states of proteins (10 to 1,000 sec-1), again matching the time scale of proton turnover. (v) The rate of translocation of the transported species should be similar to the rate of proton transport in a directly or indirectly coupled system. The rate of Na+ transport through M2 is indeed most rapid (up to 100 protons/ sec) in the experiment with variable pHout and initial pHin = 7.0, which leads to rapid proton flux. By contrast, the rate of proton flux is slow during the second phase of the electrical-gradient experiment during which proton flux is minimal, despite the fact that the chemical potential of Na+ ions and the electrical gradient are both favorable to Na+translocation.
M2’s very low rate of passive cation transport is functionally similar to, although mechanistically distinct from, that in antiporters. In the absence of high proton flux its basal rate of Na+ conduction at 0.13 M salt is on the order of 1 sec-1, corresponding to a second-order rate constant of 10 M-1 sec-1 - 106-fold slower than a typical cation channel. Thus, cations experience large energy barriers during conduction through M2, preventing unregulated basal conduction that could be toxic to the host cell prior to virus packaging.
While the above discussion assumes net changes in protonation of His37 during proton translocation, it is also possible to devise gated-channel models in which protonation of His37 gates the channel into an open state that allows protons to transverse the pore region without formally protonating the His37 residues. Hybrid models are also possible in which protons are shared between clusters of water molecules and His37 residues as they pass this region of the channel. The small size of M2 makes it an ideal system to study such mechanistic questions.
In conclusion, the complex, functionally tuned pH-dependence of M2 is remarkable considering the very small size of the system. A recent electrophysiological study of a large number of single-site variants of M2 also found a remarkable degree of functional fine tuning underlying the surprising degree of conservation of the pore-lining residues in transmissible viruses. In this work, a large number of channel-lining mutants of M2 were found to be capable of forming proton-selective channels, but the pH dependence and/or the magnitude of their overall flux were altered relative to fit mutants. Here we uncover additional functional complexity, which might well contribute to fitness of the channel in transmissible viruses.
Materials and Methods
SI Text provides detailed materials, procedures for A/M2 SGC protein expression, purification, reconstitution in liposomes, trypsin digestion and MS analysis, and equations and models for the proton-flux data fitting. Proton-flux protocols and analysis using probe Glu3 were done according to SI Text and ref. 53.
Supplementary Material
Acknowledgments.
We thank Alexei L. Polishchuk and Chunlong Ma for helpful discussions and experimental assistance; Sergei A. Vinogradov for kindly supplying the Glu3 pH probe; and Chris Miller, Larry Pinto, and Huan-Xiang Zhou for insightful suggestions. S.P.Å. gratefully acknowledges support from the Crafoord Foundation and Carl Tesdorpf’s Foundation, and W.F.D. acknowledges support of grants GM56423 and AI74571 from the National Institutes of Health.
Note Added in Proof.
Liposomes containing the TM segment of M2 (amino acids 22–62) were recently shown to mediate both proton and potassium ion flux (54), in agreement with the current findings from full-length M2.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009997107/-/DCSupplemental.
References
- 1.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Zebedee SL, Richardson CD, Lamb RA. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol. 1985;56(2):502–511. doi: 10.1128/jvi.56.2.502-511.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 4.Chizhmakov IV, et al. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J Physiol. 1996;494:329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin K, Helenius A. Nuclear transport of influenza virus ribonucleoproteins: The viral matrix protein (M1) promotes export and inhibits import. Cell. 1991;67(1):117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- 6.Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol. 2002;76(3):1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhirnov OP. Solubilization of matrix protein M1/M from virions occurs at different pH for orthomyxo- and paramyxoviruses. Virology. 1990;176(1):274–279. doi: 10.1016/0042-6822(90)90253-n. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue RJ, et al. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO J. 1990;9:3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay AJ. The action of adamantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Semin Virol. 1992;3:21–30. [Google Scholar]
- 10.Grambas S, Hay AJ. Maturation of influenza A virus hemagglutinin—Estimates of the pH encountered during transport and its regulation by the M2 protein. Virology. 1992;190(1):11–18. doi: 10.1016/0042-6822(92)91187-y. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi T, Leser GP, Lamb RA. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol. 1996;133(4):733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cady SD, Luo W, Hu F, Hong M. Structure and function of the influenza A M2 proton channel. Biochemistry. 2009;48(31):7356–7364. doi: 10.1021/bi9008837. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, et al. Histidines, heart of the hydrogen ion channel from influenza A virus: Toward an understanding of conductance and proton selectivity. Proc Natl Acad Sci USA. 2006;103(18):6865–6870. doi: 10.1073/pnas.0601944103. (in English) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi H, Okada A, Miura T. Roles of the histidine and tryptophan side chains in the M2 proton channel from influenza A virus. FEBS Lett. 2003;552(1):35–38. doi: 10.1016/s0014-5793(03)00781-6. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman P, Lamb RA, Pinto LH. Chemical rescue of histidine selectivity filter mutants of the M2 ion channel of influenza A virus. J Biol Chem. 2005;280(22):21463–21472. doi: 10.1074/jbc.M412406200. (in eng) [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Zaitseva F, Lamb RA, Pinto LH. The gate of the influenza virus M2 proton channel is formed by a single tryptophan residue. J Biol Chem. 2002;277(42):39880–39886. doi: 10.1074/jbc.M206582200. [DOI] [PubMed] [Google Scholar]
- 17.Stouffer AL, et al. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451(7178):596–599. doi: 10.1038/nature06528. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balannik V, et al. Functional studies and modeling of pore-lining residue mutants of the influenza a virus M2 ion channel. Biochemistry. 2010;49(4):696–708. doi: 10.1021/bi901799k. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi M, Cross TA, Zhou HX. A secondary gate as a mechanism for inhibition of the m2 proton channel by amantadine. J Phys Chem-US. 2008;112(27):7977–7979. doi: 10.1021/jp800171m. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Takeuchi K, Pinto LH, Lamb RA. The ion channel activity of the influenza A virus M2 protein: Characterization of the amantadine block. J Virol. 1993;67(9):5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies WL, et al. Antiviral activity of 1-adamantanamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 22.Balannik V, et al. Design and pharmacological characterization of inhibitors of amantadine-resistant mutants of the M2 ion channel of influenza A virus. Biochemistry. 2009;48(50):11872–11882. doi: 10.1021/bi9014488. (in Eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, et al. Discovery of spiro-piperidine inhibitors and their modulation of the dynamics of the M2 proton channel from influenza A virus. J Am Chem Soc. 2009;131(23):8066–8076. doi: 10.1021/ja900063s. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cady SD, et al. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463(7281):689–692. doi: 10.1038/nature08722. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451(7178):591–595. doi: 10.1038/nature06531. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohigashi Y, et al. An amantadine-sensitive chimeric BM2 ion channel of influenza B virus has implications for the mechanism of drug inhibition. Proc Natl Acad Sci USA. 2009;106(44):18775–18779. doi: 10.1073/pnas.0910584106. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo WB, Hong M. Conformational changes of an ion channel detected through water-protein interactions using solid-state NMR spectroscopy. J Am Chem Soc. 2010;132(7):2378–2384. doi: 10.1021/ja9096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mould JA, et al. Permeation and activation of the M2 ion channel of influenza A virus. J Biol Chem. 2000;275(40):31038–31050. doi: 10.1074/jbc.M003663200. [DOI] [PubMed] [Google Scholar]
- 29.Chizhmakov IV, et al. Differences in conductance of M2 proton channels of two influenza viruses at low and high pH. J Physiol. 2003;546(Pt 2):427–438. doi: 10.1113/jphysiol.2002.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83(2):475–579. doi: 10.1152/physrev.00028.2002. (in eng) [DOI] [PubMed] [Google Scholar]
- 31.Mould JA, et al. Mechanism for proton conduction of the M2 ion channel of influenza A virus. J Biol Chem. 2000;275:8592–8599. doi: 10.1074/jbc.275.12.8592. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Wu Y, Voth GA. Proton transport behavior through the influenza A M2 channel: Insights from molecular simulation. Biophys J. 2007;93(10):3470–3479. doi: 10.1529/biophysj.107.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busath DD. Influenza A M2: Channel or transporter? In: Leitmannova Liu A, Iglič A, editors. Advances in Planar Lipid Bilayers and Liposomes. Vol 10. Burlington: Academic; 2009. pp. 161–201. [Google Scholar]
- 34.Lin T-I, Heider H, Schroeder C. Different modes of inhibition by adamantane amine derivatives and natural polyamines of the functionally reconstituted influenza virus M2 proton channel protein. J Gen Virol. 1997;78:767–774. doi: 10.1099/0022-1317-78-4-767. [DOI] [PubMed] [Google Scholar]
- 35.Lin TI, Schroeder C. Definitive assignment of proton selectivity and attoampere unitary current to the M2 ion channel protein of influenza A virus. J Virol. 2001;75(8):3647–3656. doi: 10.1128/JVI.75.8.3647-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroeder C, Ford CM, Wharton SA, Hay AJ. Functional reconstitution in lipid vesicles of influenza virus M2 protein expressed by baculovirus: Evidence for proton transfer activity. J Gen Virol. 1994;75(Pt 12):3477–3484. doi: 10.1099/0022-1317-75-12-3477. [DOI] [PubMed] [Google Scholar]
- 37.Ma C, et al. Identification of the functional core of the influenza A virus A/M2 proton-selective ion channel. Proc Natl Acad Sci USA. 106(30):12283–12288. doi: 10.1073/pnas.0905726106. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayvergiya V, et al. Proton conductance of influenza virus M2 protein in planar lipid bilayers. Biophys J. 2004;87(3):1697–1704. doi: 10.1529/biophysj.104.043018. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pielak RM, Schnell JR, Chou JJ. Mechanism of drug inhibition and drug resistance of influenza A M2 channel. Proc Natl Acad Sci USA. 2009;106(18):7379–7384. doi: 10.1073/pnas.0902548106. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen PA, et al. pH-induced conformational change of the influenza M2 protein C-terminal domain. Biochemistry. 2008;47(38):9934–9936. doi: 10.1021/bi801315m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochendoerfer GG, et al. Total chemical synthesis of the integral membrane protein influenza A virus M2: role of its C-terminal domain in tetramer assembly. Biochemistry. 1999;38(37):11905–11913. doi: 10.1021/bi990720m. [DOI] [PubMed] [Google Scholar]
- 42.Moffat JC, et al. Proton transport through influenza A virus M2 protein reconstituted in vesicles. Biophys J. 2008;94(2):434–445. doi: 10.1529/biophysj.107.109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto LH, Lamb RA. The M2 proton channels of influenza A and B viruses. J Biol Chem. 2006;281(14):8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell Biochem. 2010;51:77–108. doi: 10.1007/978-90-481-8622-8_3. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cristian L, Lear JD, DeGrado WF. Use of thiol-disulfide equilibria to measure the energetics of assembly of transmembrane helices in phospholipid bilayers. Proc Natl Acad Sci USA. 2003;100(25):14772–14777. doi: 10.1073/pnas.2536751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossman JS, et al. Influenza virus M2 ion channel protein is necessary for filamentous virion formation. J Virol. 2010;84(10):5078–5088. doi: 10.1128/JVI.00119-10. (in Eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoyanov AV, Righetti PG. Buffer properties of biopolymer solutions, as related to their behaviour in electrokinetic methodologies. J Chromatogr. 1999;838:11–18. [Google Scholar]
- 48.Shimbo K, Brassard DL, Lamb RA, Pinto LH. Ion selectivity and activation of the M(2) ion channel of influenza virus. Biophys J. 1996;70(3):1335–1346. doi: 10.1016/S0006-3495(96)79690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salom D, Hill BR, Lear JD, DeGrado WF. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 2000;39(46):14160–14170. doi: 10.1021/bi001799u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mould JA, et al. Mechanism for proton conduction of the M(2) ion channel of influenza A virus. J Biol Chem. 2000;275(12):8592–8599. doi: 10.1074/jbc.275.12.8592. [DOI] [PubMed] [Google Scholar]
- 51.Pinto LH, et al. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci USA. 1997;94(21):11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Qin H, Gao FP, Cross TA. Solid-state NMR characterization of conformational plasticity within the transmembrane domain of the influenza A M2 proton channel. Biochim Biophys Acta. 2007;1768(12):3162–3170. doi: 10.1016/j.bbamem.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leiding T, et al. Precise detection of pH inside large unilamellar vesicles using membrane-impermeable porphyrin-based nanoprobes. Anal Biochem. 2009;388:296–305. doi: 10.1016/j.ab.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson E, et al. Functional reconstitution of influenza A M2(22–62) Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2010.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.