Abstract
One of the basic principles that nature uses in evolution is to recycle successful concepts and create new functions by modifying existing units. This conservatism in evolution has resulted in an astonishingly high sequence identity of genes, even between evolutionarily distant species such as the nematode Caenorhabditis elegans and Homo sapiens. The recycling of successful concepts in conjunction with gene duplication events has also led to the existence of highly homologous proteins within the genome of many species. Often, these homologous proteins show similar, yet distinct functions that, in combination with their individual tissue distribution, define their specific physiological role. One prominent example is the p53 protein family, which consists of p53, p63, and p73. Recent advances in understanding the specific biological functions of these members have shed some light onto the evolution of this crucial protein family, from a germ line-specific quality-control factor to a somatic tumor suppressor. Furthermore, structures of the oligomerization domains of the mammalian paralogs, p53 and p73, and invertebrate orthologs, CEP-1 and DMP53, have delineated evolutionary changes and revealed that the oligomerization domain of p53 lacks additional stabilizing structural elements present in all other p53 family members. This suggests that p53 is the most recent evolutionary member of this protein family and predicts a mechanism for p53 activation.
Keywords: p53, p63, p73, evolution, oligomerization
The step from single-cell organisms to multicellular ones and in particular, the invention of renewable tissue have not only laid the foundation for the evolution of a great variety of different species that consist of different, highly specialized tissues but also created threats, such as the development of tumors. To counter this threat, nature has developed tumor-suppressor proteins that monitor the genetic stability of cells and eliminate cells with genetic defects. Arguably, the most important and certainly the most famous of these tumor suppressors is p53 (1–3). Its importance for the suppression of tumor development in humans has led to its nickname of the guardian of the genome because of the fact that more than 50% of all human cancers show mutations in p53 (4) and that in many remaining cases, p53 is inactivated by other mechanisms. The discovery of two homologous proteins, named p73 (5) and p63 (6–9), with high sequence identity originally led to the suggestion that p63 and p73 must be tumor suppressors as well and that tumor suppression and genetic surveillance are maintained by an entire network of similar proteins (10, 11). This view was further supported by the observation that p73 is located on a chromosomal stretch that is often deleted in neuroblastomas where the location of one or more tumor suppressors was expected (5, 12–15).
Surprisingly, however, knockout mouse studies of all three family members resulted in very different phenotypes, showing that both p63 and p73 are involved in developmental processes. These studies created serious doubts about the classification of both proteins as tumor suppressors, despite their high sequence identity with p53. More recent studies, however, revealed links to tumor suppression for p63 and p73 by discovering their role in quality surveillance of female oocytes. In addition, functions for p53 in developmental processes and stem-cell maintenance were discovered, which further closed the gap in our understanding of the functional relationship between p63/p73 on the one hand and p53 on the other hand (Table S1). These results have made it now possible to describe the evolutionary pathway of this very important class of proteins. In this article, we will describe, in detail, the functional and structural characteristics of all three family members that have initially led to the difficulties in understanding the common origin of all three proteins and the discovery of similarities that now constitutes the basis for our current understanding. We will focus on the description of mouse studies and results of structure-determination projects. We have abstained from a detailed description of transcriptional networks and protein–protein interactions, because several excellent reviews in this area are available (16–25) and we believe that the evolutionary relationship can best be seen in the in vivo investigations and the structures of individual domains.
p53, p63, and p73 Are Expressed as Multiple Isoforms Created by the Combination of Different Promoters and Splicing Events
Originally, p53 was described as a single protein of 393 amino acids that consists of an N-terminal transactivation domain (amino acids 1–56), a proline-rich domain (57–101), a central DNA-binding domain (102–292), an oligomerization domain (327–355) that is essential to form the active tetrameric state, and a C-terminal regulatory domain (356–393) (26–28). In contrast, both p73 and p63 were discovered as several different isoforms (5, 6, 29, 30). These are created by combining two promoters that express either form containing the full-length N-terminal transactivation domain (TA isoforms) or truncated forms (ΔN) that lack the first ∼55 amino acids with several C-terminal splice variants (Fig. S1). Whereas the isoforms with the shortest C termini (TAp63γ,δ,ε and TAp73γ,δ) show a very similar domain organization as p53, the isoforms with the longest C termini (TAp63α and TAp73α) contain additional domains that are not present in p53. The sterile α motif (SAM) domain (31) is a folded, globular domain type that is present in many other proteins and is believed to be a domain that promotes protein–protein interactions. A second domain (417–508 in p63) that is rich in prolines and serines seems to be unstructured but can act as a transactivation domain when fused to the DNA-binding domain of Gal4 (32). Finally, both proteins contain, at their very C terminus, a transcriptional inhibitory domain (TID) that regulates their transcriptional activities (33–35).
The discovery of the p63/p73 isoforms led to reinvestigations of p53 and the identification of different isoforms for p53 as well (36–39) (Fig. S1). Just like p63 and p73, p53 uses a second promoter to create a Δ133p53 isoform that lacks the TA-binding and parts of the DNA-binding domains and that acts as a dominant negative form for wild-type p53. An additional N-terminal variant, Δ40, is created by a splicing event that results in a different N terminus. At the C terminus, β- and γ-isoforms exist that lack the oligomerization domain, and the variant called Δp53 misses the C-terminal part of the DNA-binding domain. For some of these isoforms, their specific biological functions have started to emerge. It was shown that Δ133p53 and p53β are involved in regulating p53-mediated replicative senescence, with p53β promoting and Δ133p53 inhibiting senescence (36). Interestingly, different isoforms are not only found in mammalian members of the p53 protein family. Proteins with sequence similarity to p53 have also been identified in many different invertebrate species (40–42), including the two important model organisms Drosophila melanogaster and Caenorhabditis elegans (43–45) (Table S2). For the p53-like gene of Drosophila, dmp53, the existence of an internal promoter has been shown (38), and for the C. elegans gene, cep1, it has been predicted (www.wormbase.org), showing that this internal promoter is conserved throughout evolution.
Inactivation of p53, p63, or p73 in Mice Results in Very Different Phenotypes
Despite the high sequence identity between all three proteins, serious doubts about the classification of p73 and p63 as classical tumor-suppressor proteins were cast originally by the analysis of the p63 and p73 knockout mice. Although p53 knockout mice are mainly characterized by their high frequency of developing tumors at very early age (46, 47), both the p63 and p73 knockout mice show severe developmental defects (48–50). Inactivation of all p73 isoforms by destroying the DNA-binding domain results in hippocampal dysgenesis, hydrocephalus, chronic infections, and inflammation as well as abnormalities in pheromone-sensory pathways. An increased susceptibility to the development of tumors, however, was not observed (49), and mutations of p73 in human tumors are rare (51, 52).
The p63 knockout mice show even more pronounced developmental defects, such as severe limb truncations and lack of a multilayered skin and other epithelial structures (48, 50). The p63 knockout-mouse studies led to the identification of mutations in the human p63 gene as the cause for six different human syndromes that are characterized by limb truncations, cleft lip and palates, and/or skin abnormalities (53–56). The combination of these patient data, knockout-mice studies, and observations that p63 is highly expressed in the basal layer of stratified epithelial tissues, including skin, resulted in the model that p63 plays a major role in maintaining stem cells in the basal layer of epithelia tissues (48, 57). A different model of the role of p63 in skin development was based on results obtained with a different knockout mouse (50, 58). According to this model, p63 is important for the commitment of cells to the epithelial lineage. Both models, however, ascribe p63 an essential role for the development of the skin, other stratified epithelial tissues, and their appendages. In contrast to this clear developmental function of p63, thus far, there is no direct evidence that p63 also acts as a tumor suppressor. Although the early lethality of the newborn p63−/− mice prevents a detailed study of the development of spontaneous tumors in these mice, analysis of tumor cells from human patients revealed only a few mutations, disfavoring the classification of p63 as a tumor suppressor, despite its high sequence identity with p53. This view was further supported by the absence of an elevated number of tumors in human patients bearing heterozygote p63 mutations (52). According to the Knudson two-hit model (59), these patients with only one functional p63 allele should show a higher susceptibility to develop malignancies. A higher risk to develop cancer has been shown in mice with only one functional p53 allele (60). The significance of the absence of tumors in human patients with p63 mutations is, however, limited by the small number of patients with p63-related syndromes.
p63-, p73-, and p53-Like Proteins in Invertebrates Monitor the Genetic Stability of Female Germ Cells
This puzzling array of different functions of the individual p53 family members (Table S1) was difficult to reconcile in light of the very high sequence identity of all three family members. The first breakthrough in understanding the evolutionary relationship of all three proteins was the discovery of p53-like proteins in invertebrate species (40–42), particularly in the two important model organisms C. elegans (43, 61) and D. melanogaster (44, 45) (Table S2). The nematode C. elegans contains exactly 959 somatic cells as an adult worm with no renewable tissue, a short life span of 2–3 wk, and no threat from cancer. However, its genome contains one p53-like gene, called cep-1, which initiates apoptosis in female germ cells in response to DNA damage by inducing the two BH-3 (Bcl-2 homology 3)–only proteins, EGL-1 (egg laying abnormal-1) and CED-13 (cell death abnormality protein 13) (62). In addition, CEP-1 (C. elegans protein 1) is required for normal meiotic chromosome segregation in the germ cells (43). In contrast to p53, CEP-1 does not seem to induce cell-cycle arrest after DNA damage. These results suggested that cep-1 is not a tumor-suppressor gene but a quality-control factor for the female germ line that ensures that oocytes with genetic defects are eliminated. If one accepts that the invertebrate p53-like proteins are the evolutionary ancestors of the three mammalian p53 family members, functional similarities between CEP-1 and mammalian proteins should still exist. Isoform-specific knockout studies of p63 and p73 in mice in which only the TA isoforms that contain the full-length N-terminal transactivation domain were deleted indeed showed that these isoforms have functions in mammalian female germ cells similar to CEP-1 (63, 64). Staining of late-stage mouse embryos with TA isoform-specific monoclonal antibodies revealed that the TAp63α isoform is highly expressed in the nuclei of oocytes but not in the testes and male germ cells (65). Expression of TAp63α could be detected from embryonic day 18.5 forward, and at postnatal day 5, all oocytes showed strong expression. At that point, murine oocytes are arrested in prophase of meiosis I (dictyate arrest) waiting for recruitment for ovulation. After oocytes advance past this arrest and are recruited for ovulation, TAp63α expression is lost. The similar expression pattern in female germ cells of CEP-1 and TAp63α suggested that this p63 isoform might also play a decisive role in maintaining the genetic integrity of oocytes. Inducing DNA damage in mice with γ-irradiation indeed resulted in phosphorylation of p63, an ∼20-fold increase in its DNA-binding affinity, and elimination of all primordial oocytes, whereas the larger preantral oocytes were not affected (65). Specific deletion of TAp63 in mice revealed that the oocytes of these knockout mice were resistant to ionizing irradiation at all developmental stages, showing the link between TAp63α and DNA damage-induced cell death in female germ cells.
Isoform-specific knockout studies with p73 showed similar surprising results: deletion of TAp73 isoforms in mice resulted in their infertility. Although the complete knockout of all p73 isoforms also led to infertility because of abnormalities in the pheromone-sensing pathways (49), TAp73 knockout mice showed normal mating behavior and female mice exhibited normal cyclicity (63). More detailed analysis showed that oocytes taken from 3- to 4-wk-old TAp73 knockout mice showed a high percentage of spindle abnormalities, including multipolar spindles, spindle relaxation, and spindle scattering accompanied by varying degrees of misalignment (63, 64). Further in vitro investigations of fertilized oocytes revealed a significantly reduced rate of zygotes developing into blastocysts compared with wild-type mice. In addition, those blastocysts that developed showed abnormal cell numbers, leading to an arrest of embryonic development and suggesting a role of p73 in controlling chromosomal segregation (63).
Quality Control Is the Common Function of All Three Family Members
The knockout studies of the complete genes had revealed important roles for both p63 and p73 in development. The phenotypes of the p63 and p73 knockout mice were dominated by the developmental defects, which completely concealed the role of both proteins as quality-control factors in the female germ line. This function became observable only in the TA-specific knockout mice, also showing that the quality-control function is maintained by the TA isoforms. The developmental tasks, in the case of p63, seem to be mainly controlled by the ΔN isoforms. How can both the developmental tasks and the quality-control function be explained from an evolutionary perspective? At least in the case of p63, a functional link between its role as a quality-control factor in female germ cells and its function as a stem-cell factor in epithelial stem cells might be the stem-cell character of both cell types. With the appearance of renewable tissue, stem cells became an important and powerful new type of cell that, however, also introduced additional risks. Although the concept of cancer stem cells is still debated, it is evident that nature needed a control system to harness and regulate the power of these stem cells. Although the molecular mechanisms by which ΔNp63α maintains the stemness are still unknown, it seems likely that this control system developed based on the quality-control function of p63 in female germ cells. The assignment of specific biological functions to individual isoforms of p63 and p73 suggests an evolutionary path from a p63-like ancestor that acted as a quality-control factor in germ cells to the tumor suppressor p53.
This evolutionary theory is further supported by recent investigations that have revealed some tumor suppressor-like functions of the other p53 family members. In the case of p73, surveillance of genetic stability is not restricted to female germ cells but also occurs in somatic cells. Analysis of TAp73-specific knockout mice revealed that this mouse—in contrast to the complete knockout mouse—develops malignancies spontaneously, particularly lung adenocarcinomas (63). In addition, these mice show an increased susceptibility to cancer-inducing chemical agents. Further analysis of mouse embryonic fibroblasts (MEFs) obtained from TAp73−/− mice treated with nocodozole revealed a decrease in mitotic arrest and a concomitant increase in cells with an abnormal number of chromosomes. Furthermore, several studies have suggested a link between the tumor-suppressor potential of p53 and p73. Mice heterozygous for both proteins show a higher tumor burden and metastasis compared with heterozygous p53 mice (66). In addition, the tumor spectrum is different between p53−/−, p53−/− p73+/−, and p53−/− p73−/− mice (67). This additive effect of both proteins, however, seems to be tissue- and cell-type specific, because other studies revealed no additional effect of inactivating p73 (68, 69).
The role of p63 in tumorigenesis is more controversial. Mice heterozygous for p63 (p63+/−) were either found to be not tumor-prone and not sensitive to chemical-induced tumorigenesis (70) or to develop tumors spontaneously, albeit at older age (66). Using conditional knockout mice, it was shown that TAp63 isoforms inhibit tumorigenesis by promoting senescence in a p53-independent pathway (71, 72).
Interestingly, recent research has not only built a bridge between the developmental functions and potential tumor suppressor roles for p63 and p73, it has also shown that p53 is an important factor for stem cells, thus defining a new function of p53 that is more related to the other two family members. The reprogramming of somatic stem cells to induced pluripotent stem (iPS) cells is more efficient if p53 is either deleted or down-regulated, particularly in the presence of DNA damage (73–77). This suggests that p53 constitutes an important check point not only in tumorigenicity but also in iPS cell generation. A connection between p53 and the status of stem cells has been observed in hematopoietic, neuronal, and embryonic stem cells as well. In hematopoietic stem cells, p53 promotes quiescence by up-regulating two target genes, Gfi-1 and Necdin (78), whereas in adult neuronal stem cells, p53 suppresses self-renewal (79). In embryonic stem cells, it was shown that p53 regulates the Wnt signaling pathway (80) and binds to the promoter of Nanog, resulting in the inhibition of NANOG expression (81). This protein is needed for the self-renewal of these stem cells, and its down-regulation leads to the induction of differentiation. In addition, p53 seems to regulate the polarity of cell division in stem cells. Loss of p53 in mammary stem cells results in a significant increase in the number of these cells because of symmetric division (82). In addition, restoration of p53 activity in cancer stem cells correlates with the reinitiation of asymmetric divisions and reduction in tumor growth, again showing the connection between p53 and asymmetric stem-cell division (82). This asymmetric division might be regulated by interaction of p53 with NUMB, an antagonist of the NOTCH receptor family, which is known to be involved in asymmetric cell divisions (83). NUMB forms a complex with MDM2 and p53, preventing degradation of p53 (84). Furthermore, p53 is also involved in regulating maternal reproduction in mice by controlling the expression level of leukemia inhibitory factor (LIF), a factor that is essential for the implantation of blastocysts into the uterus (85). Inactivating p53 results in reduced pregnancy rates and reduced litter size in mice. Finally, p53 deficiency in certain mouse strains leads to failure of neural tube closure and overgrowth of the neural tissue in the mid-brain during embyogenesis (86, 87). All these data suggest a function for p53 in certain developmental processes and in particular, in regulating the self-renewal potential of stem cells, possibly one of the evolutionary older functions of this protein and closely related to the functions of the other p53 protein family members.
Structural Evolution
The evolutionary path described above is based on the investigation of biological functions. These results are, however, also supported by structure determination of individual domains of family members. So far, the DNA-binding domain (DBD) has been the focus of structural investigations, in part because most cancer-causing mutations in p53 are found in this domain. At the moment, several structures of the p53 wild-type and mutated DNA-binding domain in isolation as well as in complex with DNA from human and mouse have been determined (88–98). In addition, the structure of the DNA-binding domain of human p63 [Protein Data Bank (PDB) identifier (ID) 2RMN] and C. elegans CEP-1 protein (99) were solved. Although p53 and p63 show a high-sequence identity within the DNA-binding domain with all amino acids that contact DNA being conserved, the DBD of CEP-1 shows a low-sequence identity of only 20% (99). Despite this low-sequence identity, the overall fold of all three proteins is very similar (Fig. S2). The main difference is the existence of small α-helices in regions that have loops in p53 and p63. These loops are involved in contacting the DNA and coordinating an important zinc ion. However, despite some reorientations of several important side chains that are crucial for contacting DNA in human p53, DNA-binding experiments have revealed an almost identical sequence preference even between CEP-1 and human p53, showing a significant degree of structural conservation of the DBD and its sequence preference throughout evolution (99).
Although the DBD does not reflect the diversity in the biological functions of the p53 family members, other structural elements show significant differences. The longest isoforms of both p63 and p73 contain an additional small globular domain that was identified as a SAM domain (31), which is absent in p53. SAM domains are small protein–protein interaction modules with a tendency to form oligomers (100). Although the function of the p63 SAM domain is still debated, mutations in this domain cause the Hay–Wells syndrome that is characterized by skin abnormalities such as aleopecia, scalp infections, dystrophic nails, hypodontia, ankyloblepharon, and cleft lip and/or cleft palate (54, 55, 101). Structure determination of the C terminus of the C. elegans protein CEP-1 revealed the existence of a SAM domain in this protein as well (102). This similarity between CEP-1 and p63/p73 further supports the notion already suggested by the functional analysis that CEP-1 is more closely related to p63 than p53 and that p63 is the evolutionary oldest mammalian family member (2, 102).
Structural investigation of the C terminus of CEP-1 revealed additional surprises. A hallmark of the entire p53 protein family is its ability to form tetramers through a specialized oligomerization domain (OD), which seems to be important for its biological function (103–106). The OD is a dimer of dimers (107, 108) (Fig. 1). First, dimers are built through the formation of an antiparallel β-sheet from two monomers, in which each monomer contributes one strand. This small β-sheet is stabilized by two α-helices, one from each monomer, that also arrange in an antiparallel orientation. The final tetramer is created by interaction of the helices of two dimers, forming a four-helix bundle. This arrangement observed in the crystal and NMR structures of the p53 OD (107, 108) was believed to be the prototype for all other members of the p53-protein family. The structure determination of the C terminus of CEP-1 revealed a dimeric OD that consists of one β-strand and one α-helix per monomer and therefore, constitutes exactly one-half of the OD of p53 (102). The SAM domains of each monomer are closely attached to this OD and crucial for stabilization of the dimer.
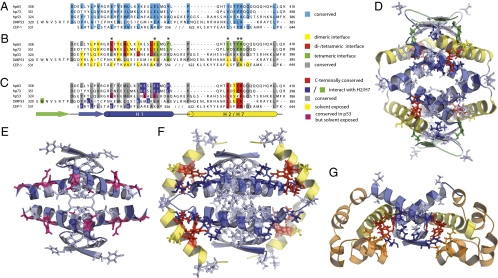
Fig. 1.
Comparison of oligomerization domains (ODs) in the p53 family. (A) Structural sequence alignment of oligomerization domains in the p53 family. Conserved residues are colored in blue. (B) Conserved residues that are involved in the dimeric and/or the tetrameric interface are marked with different colors. Residues that are part of the different oligomerization interfaces of p63, p73, and either CEP-1 or DMP53 but are not involved in intersubunit interactions of the p53 OD structure are indicated with an asterisk. (C) The last α-helix (H2 in p63 and p73 and DMP53; H7 in CEP-1) possesses a conserved motif (shown in red and yellow) that is also partially conserved in the C-terminal regulatory domain of p53. Residues that interact with this C-terminal conserved motif are shown in blue and green. Conserved but solvent-exposed residues in the OD of p53 are depicted in pink. They could potentially be involved in interactions with a proposed second helix in the C-terminal regulatory domain of p53 (Fig. 2). (D) Structure of the tetramerization domain of DMP53 [Protein Data Bank (PDB) identifier (ID) 2RP4]. (E) Structure of the p53 OD (PDB ID 1C26). (F) Structure of the tetramerization domain of human p73 (p73 TD, PDB ID 2KBY). (G) Structure of the CEP-1 OD (PDB ID 2RP5) composed of a dimeric OD and a SAM domain (colored in orange). The structures are colored according to the scheme used in C.
CEP-1 is not the only invertebrate p53-like protein that shows low-sequence homology outside of the DBD. Insects have sequences in which the OD cannot be identified from sequence comparison (102). However, their sequences are shorter and more p53-like. Structure determination of the OD of DMP53 (D. melanogaster p53) revealed that the C terminus contains an OD that forms tetramers, albeit again with a different topology. In DMP53, all secondary-structure elements are doubled: each monomer contains two β-strands and two α-helices; however, the overall structure as a dimer of dimers is conserved (102). Deletion of either the additional β-strand or the additional α-helix results in the disintegration of the entire domain. Like the CEP-1 OD, the DMP53 OD also needs additional stabilization elements that are absent in p53.
These structural investigations of the ODs of invertebrate members of the p53-protein family suggested that the p53 OD might not be the prototype for the entire family but the evolutionary endpoint. These considerations implied that the ODs of p63 and p73, which are evolutionary older than p53, might also have additional stabilization elements that are not present in the structure of the OD of p53. Structure determination of the OD of p73, both by NMR spectroscopy (109) as well as x-ray crystallography (110), revealed the existence of an additional helix at the C terminus of the OD. This additional helix reaches across the tetramerization interface and contacts the other dimer, thus stabilizing the tetrameric structure of the entire tetramerization domain (TD). NMR investigations of the p73 TD without the additional helix revealed that the truncated domain forms an equilibrium of several dimeric and tetrameric species, confirming the importance of the additional helix for maintaining the tetrameric state. Further NMR investigations of the p63 TD confirmed that this domain contains an additional helix as well (109). As mentioned above, p53-like proteins exist in many invertebrate species (40–42); some of them even contain several genes that can be divided into a more p63/p73-like group and a more p53-like group based on the presence or absence of a SAM domain. Furthermore, sequence analysis suggests the existence of a second helix in the oligomerization domain of many invertebrate proteins as well, showing that the second helix in the oligomerization domain of p53 family members is an evolutionarily conserved structural element. Table S2 summarizes the results of sequence analysis for the most important groups of species.
The discovery of an identical TD architecture of p63 and p73 suggested that both proteins might be able to form mixed tetramers. Investigation by NMR spectroscopy (109) and MS (110) indeed confirmed the existence of such mixed oligomers. Detailed analysis, surprisingly, showed that a heterotetramer consisting of two homodimers is the thermodynamically most stable oligomer (even more stable than both homotetramers) with all other possible heterooligomers also being formed (109). Interestingly, no interaction between the TD of either p63 or p73 and p53 could be observed, again suggesting a closer evolutionary relationship between p63 and p73.
The heterotetramerization observed in in vitro experiments between the TDs of p63 and p73 might also explain the inhibitory effect of ΔNp63α on TAp73β observed in JHU (Johns Hopkins University)-029 cells, a cell line derived from head and neck squamous-cell carcinoma (HNSCC) (111). In general, inhibitory effects of ΔN isoforms to TA isoforms have been observed in cell-culture experiments (6), and overexpression of ΔN isoforms has been found in several cancer types, leading to the suggestion that ΔNp63 and ΔNp73 might act as oncogenes. In most cases, however, a competition for DNA-binding sites by the ΔN isoforms is assumed to be the mechanism of the dominant negative behavior. In some cases, including the interaction between ΔNp63α and TAp73β in JHU 029 cells, however, direct binding has been observed. In addition to binding mediated through the oligomerization domain, mutations in the DNA-binding domain of p53 were shown to result in interactions with p63 and p73, which lead to transcriptional inactivation of both proteins (112). However, because these mutation-driven interactions do not provide further insight into the evolutionary relationship within the p53 protein family, we do not discuss them here in detail.
The structure determinations of CEP-1, DMP53, and p73/p63 have shown that the OD structure of p53 is the exception rather than the rule for this protein family and represents the current evolutionary endpoint of a domain that is capable of forming stable tetramers, even without additional stabilizing elements. Careful analysis of all three structures with additional stabilizing elements reveals that in CEP-1, the last helix of the SAM domain (helix 7) plays a similar stabilizing role as helix 2 in Dmp53 and p63/p73 (Fig. 1). Despite some differences in the interaction modes, the interface of helix 2/helix 7 is remarkably conserved throughout the whole p53 family, encompassing two conserved hydrophobic residues and one positively charged amino acid (Fig. S3A). In each case, the two hydrophobic amino acids make important contacts with other hydrophobic amino acids in helix 1, whereas the positively charged amino acid forms a salt bridge with a negatively charged amino acid in helix 1.
C-Terminal Regulatory Domain of p53 and the p53 Activation Code
Interestingly, this conserved set of amino acids in helix 2/7 that is involved in essential stabilizing contacts is also partially conserved in the C-terminal regulatory domain of p53, with L369 and K373 in p53 being equivalent to V386 and R390 in p73. The current model of the activation of p53 is based on the assumption that p53 is kept at low concentrations in normal cells through continuous degradation, for example, by MDM2 (murine double minute 2) and MDMX (17). On DNA damage or other oncogenic signals, p53 becomes stabilized. Originally, it was believed that, in nonstressed cells, p53 exists in a latent conformation that is not capable to bind DNA (26, 113–115). A decisive role in this latent conformation was ascribed to the C-terminal domain (356–393), because deletion of these amino acids or binding of an antibody to this domain increased the DNA-binding affinity (116). NMR-based structural investigations, however, did not reveal any conformational influence of the C-terminal domain or binding to other p53 domains, thus suggesting that the function of the C-terminal domain is not that of an allosteric regulator (28). Further studies have suggested that the C-terminal domain interacts with DNA with no sequence specificity and competes with the DNA-binding domain (117, 118) or specifically interacts with damaged DNA (27, 119–121). In other studies, it was shown that a negative inhibitory effect of the C-terminal domain could only be observed in small, naked DNA oligomers, whereas no such effect could be seen in larger DNA molecules either with or without bound histones (122). These data, combined with experiments that showed binding of p53 to non–B-type DNA structures (123–125), suggested that the inability of wild-type p53 to bind to short oligonucleotides is caused by the inability of these short DNA molecules to adopt a structure that can be recognized by p53. Modification or deletion of the C-terminal domain might, therefore, be required to allow p53 to adopt a conformation that enables binding to DNA structures that are normally not recognized by wild-type p53.
In light of the structures of the ODs of other members of the p53 protein family and particularly, the structures of the TDs of p73/p63 and the partial conservation of crucial residues in p53, it is intriguing to speculate that activation of p53 entails the formation of a helix in the C-terminal regulatory domain that stabilizes its tetrameric state. This model is further supported by the conservation of two glutamates in helix 1 that are, in p73, part of the interaction interface with helix 2 (Fig. S3B). Both glutamates are solvent-exposed in the structures of the p53 OD, indicating that they would be able to interact with a potential helix 2. Structural analysis of the C-terminal regulatory domain, however, has not produced any evidence for the formation of a helix so far (28); nevertheless, in complex with binding partners, different secondary-structure elements have been observed (Table S3). Indeed, secondary-structure prediction of the C-terminal regulatory sequence of human p53 predicts only a weak α-helical propensity with a significant dip at the position of G374, including the two highly conserved lysines (K372 and K373) (Fig. 2). However, the C-terminal regulatory domain is known to be the target of multiple posttranslational modifications such as phosphorylation, acetylation, methylation, ubiquitination, neddylation, and sumoylation (Table S4). Mimicking posttranslational modifications in secondary-structure predictions by replacing serines and threonines that are known to become phosphorylated with glutamates and selected lysines that are known to become acetylated with glutamines significantly enhances the α-helical propensity and predicts the existence of a helix from R363 to F385. Interestingly, the predicted helical propensity for this potential helix 2 is almost as high as the predicted helical propensity for the canonical helix 1 of the OD (Fig. 2). However, it is unlikely that all possible posttranslational modifications will occur simultaneously, and some of these modifications are also mutually exclusive (for example, ubiquitination and acetylation on the same lysine). If posttranslational modifications result in the formation of the second helix that stabilizes the tetrameric state, this helix formation is likely triggered by a special modification pattern. Given the high degree and the different variety of modifications in the C-terminal regulatory domain, the p53-activation code is likely to be complex, possibly even resulting in different structures connected with different functions. A direct influence of the second helix on the function has already been documented for p73: the second helix was found to be preferentially involved in regulating genes involved in cell-cycle progression (126), which implies that the oligomeric structure might play an important role in the selection of the target genes. Different oligomeric structures might further be stabilized by interaction with proteins that are known to bind to the OD, such as 14-3-3, S100 family members, p34cdc2 53BP1, or TMS1 (127–132). In the case that helix 2 exists in p53, evolution in mammals would have turned a structural element present in p63 and p73 into a switchable element with regulatory functions in p53. Therefore, the evolutionary memory of p53 might be mirrored in structural elements in the C terminus of p53. Investigating the p53-activation code will be an important step in fully understanding the activation process of this most important tumor suppressor.
Fig. 2.
Proposed second helix in the C-terminal regulatory domain of human p53. (A) Sequence of the oligomerization and C-terminal regulatory domain of human p53. Several posttranslational modifications (Table S4) are known that depend on the cellular context. Black bars indicate attractive (i, i + 4) interactions in case the appropriate serine is phosphorylated. Red bars indicate repulsive (i, i + 3) or (i, i + 4) interactions that are resolved if a participating lysine is acetylated. (B) Secondary structure prediction of the C-terminal regulatory domain of human p53 using GOR IV (133). Glutamine was used as an acetyl-mimetic for lysine, and glutamate was used as a phospho-mimetic for serine.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001069107/-/DCSupplemental.
References
- 1.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 2.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 3.Levine AJ, Oren M. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 5.Kaghad M, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 7.Schmale H, Bamberger C. A novel protein with strong homology to the tumor suppressor p53. Oncogene. 1997;15:1363–1367. doi: 10.1038/sj.onc.1201500. [DOI] [PubMed] [Google Scholar]
- 8.Senoo M, et al. A second p53-related protein, p73L, with high homology to p73. Biochem Biophys Res Commun. 1998;248:603–607. doi: 10.1006/bbrc.1998.9013. [DOI] [PubMed] [Google Scholar]
- 9.Trink B, et al. A new human p53 homologue. Nat Med. 1998;4:747–748. doi: 10.1038/nm0798-747. [DOI] [PubMed] [Google Scholar]
- 10.Flores ER, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 11.Gong JG, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 12.Rossi M, Sayan AE, Terrinoni A, Melino G, Knight RA. Mechanism of induction of apoptosis by p73 and its relevance to neuroblastoma biology. Ann N Y Acad Sci. 2004;1028:143–149. doi: 10.1196/annals.1322.015. [DOI] [PubMed] [Google Scholar]
- 13.Kovalev S, Marchenko N, Swendeman S, LaQuaglia M, Moll UM. Expression level, allelic origin, and mutation analysis of the p73 gene in neuroblastoma tumors and cell lines. Cell Growth Differ. 1998;9:897–903. [PubMed] [Google Scholar]
- 14.Ichimiya S, et al. Genetic analysis of p73 localized at chromosome 1p36.3 in primary neuroblastomas. Med Pediatr Oncol. 2001;36:42–44. doi: 10.1002/1096-911X(20010101)36:1<42::AID-MPO1011>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Ichimiya S, et al. p73 at chromosome 1p36.3 is lost in advanced stage neuroblastoma but its mutation is infrequent. Oncogene. 1999;18:1061–1066. doi: 10.1038/sj.onc.1202390. [DOI] [PubMed] [Google Scholar]
- 16.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: Hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 17.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: Defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 20.Vilgelm A, El-Rifai W, Zaika A. Therapeutic prospects for p73 and p63: Rising from the shadow of p53. Drug Resist Updat. 2008;11:152–163. doi: 10.1016/j.drup.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 23.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: Drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 24.McKeon F, Melino G. Fog of war: The emerging p53 family. Cell Cycle. 2007;6:229–232. doi: 10.4161/cc.6.3.3876. [DOI] [PubMed] [Google Scholar]
- 25.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 26.Hupp TR, Lane DP. Allosteric activation of latent p53 tetramers. Curr Biol. 1994;4:865–875. doi: 10.1016/s0960-9822(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman J, Prives C. Activation of p53 sequence-specific DNA binding by short single strands of DNA requires the p53 C-terminus. Cell. 1995;81:1021–1029. doi: 10.1016/s0092-8674(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 28.Ayed A, et al. Latent and active p53 are identical in conformation. Nat Struct Biol. 2001;8:756–760. doi: 10.1038/nsb0901-756. [DOI] [PubMed] [Google Scholar]
- 29.De Laurenzi V, et al. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med. 1998;188:1763–1768. doi: 10.1084/jem.188.9.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Laurenzi VD, et al. Additional complexity in p73: Induction by mitogens in lymphoid cells and identification of two new splicing variants epsilon and zeta. Cell Death Differ. 1999;6:389–390. doi: 10.1038/sj.cdd.4400521. [DOI] [PubMed] [Google Scholar]
- 31.Chi SW, Ayed A, Arrowsmith CH. Solution structure of a conserved C-terminal domain of p73 with structural homology to the SAM domain. EMBO J. 1999;18:4438–4445. doi: 10.1093/emboj/18.16.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada N, Ozaki T, Ichimiya S, Todo S, Nakagawara A. Identification of a transactivation activity in the COOH-terminal region of p73 which is impaired in the naturally occurring mutants found in human neuroblastomas. Cancer Res. 1999;59:2810–2814. [PubMed] [Google Scholar]
- 33.Ozaki T, et al. Deletion of the COOH-terminal region of p73alpha enhances both its transactivation function and DNA-binding activity but inhibits induction of apoptosis in mammalian cells. Cancer Res. 1999;59:5902–5907. [PubMed] [Google Scholar]
- 34.Serber Z, et al. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol. 2002;22:8601–8611. doi: 10.1128/MCB.22.24.8601-8611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straub WE, et al. The C-terminus of p63 contains multiple regulatory elements with different functions. Cell Death Dis. 2010;1:e5. doi: 10.1038/cddis.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujita K, et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–1142. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, et al. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009;23:278–290. doi: 10.1101/gad.1761609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourdon JC, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courtois S, de Fromentel CC, Hainaut P. p53 protein variants: Structural and functional similarities with p63 and p73 isoforms. Oncogene. 2004;23:631–638. doi: 10.1038/sj.onc.1206929. [DOI] [PubMed] [Google Scholar]
- 40.Lane DP, et al. Mdm2 and p53 are highly conserved from placozoans to man. Cell Cycle. 2010;9:540–547. doi: 10.4161/cc.9.3.10516. [DOI] [PubMed] [Google Scholar]
- 41.Lane DP, et al. The Mdm2 and p53 genes are conserved in the Arachnids. Cell Cycle. 2010;9:748–754. doi: 10.4161/cc.9.4.10616. [DOI] [PubMed] [Google Scholar]
- 42.Pankow S, Bamberger C. The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE. 2007;2:e782. doi: 10.1371/journal.pone.0000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: Role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- 44.Ollmann M, et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101:91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 45.Brodsky MH, et al. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 46.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 47.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer. 2009;9:831–841. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 48.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 49.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 50.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbluth JM, Pietenpol JA. The jury is in: p73 is a tumor suppressor after all. Genes Dev. 2008;22:2591–2595. doi: 10.1101/gad.1727408. [DOI] [PubMed] [Google Scholar]
- 52.Moll UM, Slade N. p63 and p73: Roles in development and tumor formation. Mol Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 53.Celli J, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- 54.McGrath JA, et al. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet. 2001;10:221–229. doi: 10.1093/hmg/10.3.221. [DOI] [PubMed] [Google Scholar]
- 55.Rinne T, Bolat E, Meijer R, Scheffer H, van Bokhoven H. Spectrum of p63 mutations in a selected patient cohort affected with ankyloblepharon-ectodermal defects-cleft lip/palate syndrome (AEC) Am J Med Genet A. 2009;149A:1948–1951. doi: 10.1002/ajmg.a.32793. [DOI] [PubMed] [Google Scholar]
- 56.Barrow LL, et al. Analysis of the p63 gene in classical EEC syndrome, related syndromes, and non-syndromic orofacial clefts. J Med Genet. 2002;39:559–566. doi: 10.1136/jmg.39.8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 58.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knudson AG., Jr Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatachalam S, et al. Retention of wild-type p53 in tumors from p53 heterozygous mice: Reduction of p53 dosage can promote cancer formation. EMBO J. 1998;17:4657–4667. doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schumacher B, Hofmann K, Boulton S, Gartner A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr Biol. 2001;11:1722–1727. doi: 10.1016/s0960-9822(01)00534-6. [DOI] [PubMed] [Google Scholar]
- 62.Schumacher B, et al. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 2005;12:153–161. doi: 10.1038/sj.cdd.4401539. [DOI] [PubMed] [Google Scholar]
- 63.Tomasini R, et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomasini R, et al. TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc Natl Acad Sci USA. 2009;106:797–802. doi: 10.1073/pnas.0812096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 66.Flores ER, et al. Tumor predisposition in mice mutant for p63 and p73: Evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–373. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 67.Nemajerova A, Palacios G, Nowak NJ, Matsui S, Petrenko O. Targeted deletion of p73 in mice reveals its role in T cell development and lymphomagenesis. PLoS ONE. 2009;4:e7784. doi: 10.1371/journal.pone.0007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Losada J, Wu D, DelRosario R, Balmain A, Mao JH. p63 and p73 do not contribute to p53-mediated lymphoma suppressor activity in vivo. Oncogene. 2005;24:5521–5524. doi: 10.1038/sj.onc.1208799. [DOI] [PubMed] [Google Scholar]
- 69.Senoo M, Manis JP, Alt FW, McKeon F. p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell. 2004;6:85–89. doi: 10.1016/j.ccr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Keyes WM, et al. p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc Natl Acad Sci USA. 2006;103:8435–8440. doi: 10.1073/pnas.0602477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo X, et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat Cell Biol. 2009;11:1451–1457. doi: 10.1038/ncb1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keyes WM, et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong H, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawamura T, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marión RM, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Utikal J, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meletis K, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 80.Lee KH, et al. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci USA. 2010;107:69–74. doi: 10.1073/pnas.0909734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin T, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 82.Cicalese A, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 83.Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 84.Colaluca IN, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 85.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 86.Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Curr Biol. 1995;5:931–936. doi: 10.1016/s0960-9822(95)00183-7. [DOI] [PubMed] [Google Scholar]
- 87.Sah VP, et al. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995;10:175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- 88.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 89.Khoo KH, Joerger AC, Freund SM, Fersht AR. Stabilising the DNA-binding domain of p53 by rational design of its hydrophobic core. Protein Eng Des Sel. 2009;22:421–430. doi: 10.1093/protein/gzp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suad O, et al. Structural basis of restoring sequence-specific DNA binding and transactivation to mutant p53 by suppressor mutations. J Mol Biol. 2009;385:249–265. doi: 10.1016/j.jmb.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 91.Malecka KA, Ho WC, Marmorstein R. Crystal structure of a p53 core tetramer bound to DNA. Oncogene. 2009;28:325–333. doi: 10.1038/onc.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boeckler FM, et al. Targeted rescue of a destabilized mutant of p53 by an in silico screened drug. Proc Natl Acad Sci USA. 2008;105:10360–10365. doi: 10.1073/pnas.0805326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon E, Kim DY, Suh SW, Kim KK. Crystal structure of the mouse p53 core domain in zinc-free state. Proteins. 2008;70:280–283. doi: 10.1002/prot.21608. [DOI] [PubMed] [Google Scholar]
- 94.Joerger AC, Ang HC, Fersht AR. Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc Natl Acad Sci USA. 2006;103:15056–15061. doi: 10.1073/pnas.0607286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ho WC, Fitzgerald MX, Marmorstein R. Structure of the p53 core domain dimer bound to DNA. J Biol Chem. 2006;281:20494–20502. doi: 10.1074/jbc.M603634200. [DOI] [PubMed] [Google Scholar]
- 96.Canadillas JM, et al. Solution structure of p53 core domain: Structural basis for its instability. Proc Natl Acad Sci USA. 2006;103:2109–2114. doi: 10.1073/pnas.0510941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao K, Chai X, Johnston K, Clements A, Marmorstein R. Crystal structure of the mouse p53 core DNA-binding domain at 2.7 A resolution. J Biol Chem. 2001;276:12120–12127. doi: 10.1074/jbc.M011644200. [DOI] [PubMed] [Google Scholar]
- 98.Kitayner M, et al. Diversity in DNA recognition by p53 revealed by crystal structures with Hoogsteen base pairs. Nat Struct Mol Biol. 2010;17:423–429. doi: 10.1038/nsmb.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huyen Y, et al. Structural differences in the DNA binding domains of human p53 and its C. elegans ortholog Cep-1. Structure. 2004;12:1237–1243. doi: 10.1016/j.str.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Kim CA, Bowie JU. SAM domains: Uniform structure, diversity of function. Trends Biochem Sci. 2003;28:625–628. doi: 10.1016/j.tibs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Rinne T, Brunner HG, van Bokhoven H. p63-associated disorders. Cell Cycle. 2007;6:262–268. doi: 10.4161/cc.6.3.3796. [DOI] [PubMed] [Google Scholar]
- 102.Ou HD, Löhr F, Vogel V, Mäntele W, Dötsch V. Structural evolution of C-terminal domains in the p53 family. EMBO J. 2007;26:3463–3473. doi: 10.1038/sj.emboj.7601764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chène P. The role of tetramerization in p53 function. Oncogene. 2001;20:2611–2617. doi: 10.1038/sj.onc.1204373. [DOI] [PubMed] [Google Scholar]
- 104.Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of a minimal transforming domain of p53: Negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stürzbecher HW, et al. A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992;7:1513–1523. [PubMed] [Google Scholar]
- 106.Chan WM, Siu WY, Lau A, Poon RY. How many mutant p53 molecules are needed to inactivate a tetramer? Mol Cell Biol. 2004;24:3536–3551. doi: 10.1128/MCB.24.8.3536-3551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeffrey PD, Gorina S, Pavletich NP. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science. 1995;267:1498–1502. doi: 10.1126/science.7878469. [DOI] [PubMed] [Google Scholar]
- 108.Lee W, et al. Solution structure of the tetrameric minimum transforming domain of p53. Nat Struct Biol. 1994;1:877–890. doi: 10.1038/nsb1294-877. [DOI] [PubMed] [Google Scholar]
- 109.Coutandin D, et al. Conformational stability and activity of p73 require a second helix in the tetramerization domain. Cell Death Differ. 2009;16:1582–1589. doi: 10.1038/cdd.2009.139. [DOI] [PubMed] [Google Scholar]
- 110.Joerger AC, et al. Structural evolution of p53, p63, and p73: Implication for heterotetramer formation. Proc Natl Acad Sci USA. 2009;106:17705–17710. doi: 10.1073/pnas.0905867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–2225. doi: 10.1038/sj.onc.1210311. [DOI] [PubMed] [Google Scholar]
- 113.Hupp TR, Meek DW, Midgley CA, Lane DP. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 114.Hupp TR, Sparks A, Lane DP. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell. 1995;83:237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 115.Halazonetis TD, Kandil AN. Conformational shifts propagate from the oligomerization domain of p53 to its tetrameric DNA binding domain and restore DNA binding to select p53 mutants. EMBO J. 1993;12:5057–5064. doi: 10.1002/j.1460-2075.1993.tb06199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, et al. p53 domains: Identification and characterization of two autonomous DNA-binding regions. Genes Dev. 1993;7:2575–2586. doi: 10.1101/gad.7.12b.2575. [DOI] [PubMed] [Google Scholar]
- 118.Anderson ME, Woelker B, Reed M, Wang P, Tegtmeyer P. Reciprocal interference between the sequence-specific core and nonspecific C-terminal DNA binding domains of p53: Implications for regulation. Mol Cell Biol. 1997;17:6255–6264. doi: 10.1128/mcb.17.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee S, Elenbaas B, Levine A, Griffith J. p53 and its 14 kDa C-terminal domain recognize primary DNA damage in the form of insertion/deletion mismatches. Cell. 1995;81:1013–1020. doi: 10.1016/s0092-8674(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 120.Selivanova G, et al. The single-stranded DNA end binding site of p53 coincides with the C-terminal regulatory region. Nucleic Acids Res. 1996;24:3560–3567. doi: 10.1093/nar/24.18.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reed M, et al. The C-terminal domain of p53 recognizes DNA damaged by ionizing radiation. Proc Natl Acad Sci USA. 1995;92:9455–9459. doi: 10.1073/pnas.92.21.9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Espinosa JM, Emerson BM. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell. 2001;8:57–69. doi: 10.1016/s1097-2765(01)00283-0. [DOI] [PubMed] [Google Scholar]
- 123.Kim E, Albrechtsen N, Deppert W. DNA-conformation is an important determinant of sequence-specific DNA binding by tumor suppressor p53. Oncogene. 1997;15:857–869. doi: 10.1038/sj.onc.1201412. [DOI] [PubMed] [Google Scholar]
- 124.Kim E, et al. Influence of promoter DNA topology on sequence-specific DNA binding and transactivation by tumor suppressor p53. Oncogene. 1999;18:7310–7318. doi: 10.1038/sj.onc.1203139. [DOI] [PubMed] [Google Scholar]
- 125.Jett SD, Cherny DI, Subramaniam V, Jovin TM. Scanning force microscopy of the complexes of p53 core domain with supercoiled DNA. J Mol Biol. 2000;299:585–592. doi: 10.1006/jmbi.2000.3759. [DOI] [PubMed] [Google Scholar]
- 126.Nyman U, et al. Protein kinase C-dependent phosphorylation regulates the cell cycle-inhibitory function of the p73 carboxy terminus transactivation domain. Mol Cell Biol. 2009;29:1814–1825. doi: 10.1128/MCB.00585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajagopalan S, Jaulent AM, Wells M, Veprintsev DB, Fersht AR. 14-3-3 activation of DNA binding of p53 by enhancing its association into tetramers. Nucleic Acids Res. 2008;36:5983–5991. doi: 10.1093/nar/gkn598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fernandez-Fernandez MR, Veprintsev DB, Fersht AR. Proteins of the S100 family regulate the oligomerization of p53 tumor suppressor. Proc Natl Acad Sci USA. 2005;102:4735–4740. doi: 10.1073/pnas.0501459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wagner P, Fuchs A, Götz C, Nastainczyk W, Montenarh M. Fine mapping and regulation of the association of p53 with p34cdc2. Oncogene. 1998;16:105–111. doi: 10.1038/sj.onc.1201510. [DOI] [PubMed] [Google Scholar]
- 130.Wagner P, Fuchs A, Prowald A, Montenarh M, Nastainczyk W. Precise mapping of the tms1 binding site on p53. FEBS Lett. 1995;377:155–158. doi: 10.1016/0014-5793(95)01329-6. [DOI] [PubMed] [Google Scholar]
- 131.Huang J, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 132.Kachirskaia I, et al. Role for 53BP1 Tudor domain recognition of p53 dimethylated at lysine 382 in DNA damage signaling. J Biol Chem. 2008;283:34660–34666. doi: 10.1074/jbc.M806020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garnier J, Gibrat JF, Robson B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 1996;266:540–553. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.