Abstract
Calmodulin (CaM)-sensitive adenylyl cyclase (AC) in sensory neurons (SNs) in Aplysia has been proposed as a molecular coincidence detector during conditioning. We identified four putative ACs in Aplysia CNS. CaM binds to a sequence in the C1b region of AC-AplA that resembles the CaM-binding sequence in the C1b region of AC1 in mammals. Recombinant AC-AplA was stimulated by Ca2+/CaM. AC-AplC is most similar to the Ca2+-inhibited AC5 and AC6 in mammals. Recombinant AC-AplC was directly inhibited by Ca2+, independent of CaM. AC-AplA and AC-AplC are expressed in SNs, whereas AC-AplB and AC-AplD are not. Knockdown of AC-AplA demonstrated that serotonin stimulation of cAMP-dependent plasticity in SNs is predominantly mediated by this CaM-sensitive AC. We propose that the coexpression of a Ca2+-inhibited AC in SNs, together with a Ca2+/CaM-stimulated AC, would enhance the associative requirement for coincident Ca2+ influx and serotonin for effective stimulation of cAMP levels and initiation of plasticity mediated by AC-AplA.
Keywords: coincidence detector, associative learning, synaptic plasticity, classical conditioning, calcium
Three decades ago, early experimental evidence from two simple invertebrates, Aplysia and Drosophila (1, 2), demonstrated that cAMP plays an important role in learning. Based on studies of classical conditioning in Aplysia, calmodulin (CaM)-sensitive adenylyl cyclase (AC) was proposed to play an associative role in learning (3–5). CaM-sensitive AC was similarly implicated in conditioning in Drosophila (6, 7). Subsequently, CaM-sensitive AC was also found to be important in learning in mammals; mouse mutants that lack CaM-sensitive ACs 1 and 8 displayed deficits in memory (8, 9), and overexpression of AC1 in mice improved memory (10).
The CaM-sensitive AC in Aplysia was hypothesized to serve as an associative coincidence detector that integrates two signals during classical conditioning: (i) Ca2+ influx during sensory neuron (SN) activity triggered by the conditioned stimulus and (ii) serotonin (5-hydroxytryptamine; 5-HT), released by modulatory interneurons during the unconditioned stimulus. This coincidence detector concept was based on cellular electrophysiology and biochemical assays (5, 11–14). Evidence suggesting that CaM-sensitive AC may function as a coincidence detector has also been obtained in Drosophila (15) and in studies of the mammalian AC1 (16). Nevertheless, the proposed role of CaM-sensitive AC as a coincidence detector during learning has not been directly tested in Aplysia or any other system.
Although CaM-sensitive AC was implicated in synaptic plasticity during learning in Aplysia more than 25 years ago, this enzyme from Aplysia had not been characterized at the molecular level. Recent studies have raised questions about the role of the cAMP cascade in associative facilitation at Aplysia SN-to-motor neuron (MN) synapses (17, 18). We have investigated which AC isoforms are expressed in Aplysia CNS and in SNs in particular, and determined whether a CaM-sensitive AC is coupled to 5-HT receptors in the SNs. In these studies, we focused on the biochemical properties and regulation of the two AC isoforms that are expressed in Aplysia SNs. One of these isoforms, AC-AplA, is stimulated by Ca2+/CaM, whereas the second, AC-AplC, is directly inhibited by Ca2+. Knockdown experiments revealed that the Ca2+/CaM-sensitive isoform is responsible for the great majority of 5-HT-induced cAMP-mediated plasticity in SN somata. This demonstrates that the CaM-sensitive AC is indeed dually regulated in those neurons where it has been hypothesized to function as an associative integrator.
Results
In an initial effort to identify AC isoforms expressed in Aplysia CNS, we used degenerate primers corresponding to sequences within the two cytoplasmic regions that are highly conserved among transmembrane ACs in metazoans, the C1a and C2a regions (19, 20). We isolated cDNA clones from Aplysia CNS that corresponded to three distinct sequences based on restriction map analysis and sequencing. Full-length sequences of these putative AC transcripts, AC-AplA, AC-AplB, and AC-AplC, were generated by 5′ and 3′ rapid amplification of cDNA ends (RACE) (GenBank accession nos. AY843025, AY843026, and AY843027). The membrane-associated ACs of multicellular animals have two transmembrane domains, each with approximately six membrane-spanning α-helices, separated by a cytoplasmic C1 domain. A second cytoplasmic domain, C2, is located at the C terminus and a third, relatively short cytoplasmic sequence is at the N terminus (21). Analysis of these putative Aplysia AC isoforms indicated a similar structure, with two hydrophobic domains containing five to seven membrane-spanning α-helices per domain, similar to predictions for mammalian ACs (Fig. S1). Because in transmembrane ACs the C1a and C2a cytoplasmic regions interact to create the nucleotide binding site required for catalytic activity, we predict there is an even number of membrane crossings, namely six. A fourth putative AC, AC-AplD, was identified through a search of the Aplysia EST database (22) and a full-length sequence was obtained by RACE (GenBank accession no. HM030824). Analysis of this transcript indicated a similar topology (Fig. S1D). The predicted C2 domain of AC-AplA is nearly three times the length of the other C2 domains (Table S1). All metazoan transmembrane ACs contain highly conserved sequences in the C1a and C2a regions that together form the ATP-binding pocket in the catalytic core (Fig. S2) (23–25). The four putative Aplysia ACs share this conserved pattern of residues (Fig. S2). All four C1a regions contain the characteristic GDCYYC sequence (19, 20), which is conserved in all transmembrane ACs in metazoans that we have examined, including Trichoplax (Fig. S2C). Moreover, in the purine-binding pocket of the C2a region, all four predicted sequences contain the lysine and aspartate residues that typify ACs, in contrast to guanylyl cyclases (24, 26).
AC-AplA and AC-AplC Resemble AC Isoforms That Are Regulated by Ca2+.
To identify the CaM-sensitive AC in Aplysia, which had been proposed to play an associative role in classical conditioning, we compared the four Aplysia sequences with the C1a and C2a regions of all nine mammalian and four of the Drosophila transmembrane ACs. In mammals, AC1 and AC8 are stimulated by Ca2+/CaM (27–29). ACs 5 and 6 are highly similar isoforms that are directly inhibited by Ca2+ (30). ACs 2, 4, and 7 are closely related, Ca2+-independent ACs. AC3 is inhibited by CaM kinase II (31). AC9 is regulated by CaM kinase II, PKC, and calcineurin (32, 33). The C1a region of AC-AplA is particularly similar (72%) to rutabaga, the Ca2+/CaM-sensitive AC in Drosophila (34) (Fig. S3), which suggested that AC-AplA may be the Ca2+/CaM-sensitive AC in Aplysia CNS (Fig. S4). AC-AplA is also similar to ACs 1, 5, and 6, as is rutabaga AC. However, AC-AplC is the Aplysia isoform most similar to AC5 and AC6 (Fig. S4). Based on this analysis, we predicted that AC-AplA might be stimulated by Ca2+/CaM and that AC-AplC might be Ca2+-inhibited. The C1a region of AC-AplB is not highly similar to any single group of ACs, whereas the C2a domain of AC-AplB is most similar to members of the AC2/4/7 group (Figs. S3 and S4). AC-AplD has a relatively low similarity to all of the mammalian ACs; however, it is most similar to AC9 and to Drosophila AC 35C, the Drosophila homolog of AC9 (19) (Fig. S4). None of the identified Aplysia AC isoforms resembled AC3.
AC-AplA Contains a Ca2+/CaM-Binding Domain.
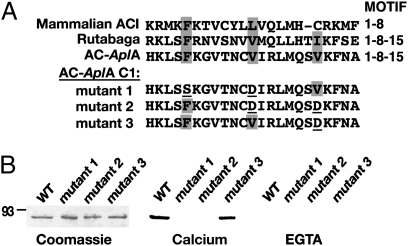
To determine which cytoplasmic domains interact with CaM, we expressed the N-terminal and C1 and C2 domains of each of the four putative ACs. In CaM overlay assays, we observed that CaM bound to the C1 domain of AC-AplA, in a Ca2+-dependent manner, but not to the other cytoplasmic domains (Fig. S5). The C1b region of AC-AplA contains a sequence with similarity to the CaM-binding region of mammalian AC1 (28) (Fig. 1). CaM-binding motifs are relatively diverse, and include the 1-8-14 pattern of hydrophobic residues (35, 36). AC1 contains a 1-8 motif, where the 1 and 8 residues are both required for CaM binding (28). Both AC-AplA and rutabaga AC contain a 1-8-15 pattern of hydrophobic residues within the C1b region (37). A 25-aa peptide from AC-AplA containing this 1-8-15 sequence bound CaM. To determine which hydrophobic amino acids mediated CaM binding, we mutated the motif in the AC-AplA C1 domain at positions 1 and 8 (F498S/V505D), positions 8 and 15 (V505D/V512D), or positions 15 alone (V512D). CaM binding was eliminated in both the F498S/V505D and V505D/V512D mutants (Fig. 1B). In contrast, CaM binding was not substantially affected in the V512D mutant, indicating that residue 15 does not contribute to the affinity for CaM. Thus, the CaM-binding motif of AC-AplA has a 1-8 pattern of hydrophobic residues, as does mammalian AC1 (28).
Fig. 1.
CaM-binding sequence within C1b region of AC-AplA. (A) (Upper) Alignment of the CaM-binding domain of human AC1 and the corresponding sequences in AC-AplA and rutabaga AC; the 1-8 or 1-8-15 pattern of hydrophobic residues is indicated by shading. (Lower) Mutations of these three hydrophobic residues in AC-AplA are shown with substitutions at positions 1 and 8 (mutant 1), 8 and 15 (mutant 2), or 15 alone (mutant 3). (B) CaM overlay assays to determine which hydrophobic residues in the 1-8-15 motif in the C1b region of AC-AplA (amino acids 498–512) are critical for CaM binding. Coomassie-stained gel (Left). CaM overlay assays in the presence of 1 mM Ca2+ (Center) or 10 mM EGTA (Right). Note the double mutants F498S/V505D and V505D/V512D both eliminated CaM binding, whereas the single mutation V512D did not affect CaM binding, suggesting that residues 1 and 8 are necessary for CaM binding. Note also that CaM binding was Ca2+-dependent.
AC-AplA Is a Ca2+-/CaM-Regulated AC, Whereas AC-AplC Is Inhibited by Ca2+.
To explore Ca2+ regulation of the hypothesized CaM-sensitive and Ca2+-inhibited ACs AC-AplA and AC-AplC, we expressed these two AC isoforms in High Five insect cells. Expression was confirmed with isoform-specific antibodies (Fig. S6). Membranes from High Five cells expressing AC-AplA or AC-AplC displayed a 6.0 ± 1.5-fold (n = 4) and 7.3 ± 2.5-fold (n = 3) increase, respectively, in basal AC activity as compared with membranes from control cells infected with baculovirus encoding β-gal (Fig. 2 A1 and B1). AC-AplA and AC-AplC were assayed with Ca2+/Mg2+/EGTA buffers with free Ca2+ ranging from 50 nM to ≥40 μM. In the presence of CaM, we observed 3.1 ± 0.4-fold stimulation as Ca2+ increased to 1.7 μM (n = 4) (Fig. 2A). No stimulation was seen in the absence of CaM (Fig. 2A3). AC-AplC was inhibited when Ca2+ was increased above 100 nM; inhibition reached a plateau by 10 μM Ca2+ (39.2 ± 4.5% of basal activity, n = 3) (Fig. 2B). This inhibition of AC-AplC was CaM-independent (Fig. 2B3).
Fig. 2.
Ca2+ regulation of AC-AplA and AC-AplC. (A) AC-AplA is stimulated by Ca2+/CaM. (A1) AC activity in membranes from High Five insect cells infected with baculovirus encoding either AC-AplA or β-gal, assayed in EGTA/Ca2+/Mg2+ buffers with free Ca2+ concentrations ranging from 5 nM to 350 μM, in the presence of 1 μM CaM. Absolute AC activity from a representative experiment (mean ± SD of five replicate assays). Note both AC-AplA and native insect AC (β-gal) display stimulation by Ca2+, although the absolute maximal activity of the cells expressing recombinant Aplysia AC is substantially greater. (A2) Calculated activity of AC-AplA as a function of [Ca2+]. To calculate the Ca2+ stimulation of AC-AplA, for each Ca2+ concentration, the absolute activity in membranes from cells infected with β-gal virus was subtracted from the activity in membranes from cells infected with AC-AplA virus. Data represent means ± SEM of AC activity from four separate experiments using membranes from independent populations of High Five cells; each membrane preparation was assayed in five replicates. AC activity at each Ca2+ concentration was normalized to the basal activity at 5 nM Ca2+. (A3) The stimulation of AC-AplA by Ca2+ is CaM-dependent. Data are means ± SEM of data from three experiments on separate populations of cells (F3,6 = 23.7, P = 0.001). (B) AC-AplC is inhibited by Ca2+, independently of CaM. (B1) AC activity in membranes from High Five cells infected with baculovirus encoding either AC-AplC or β-gal, assayed at a range of free Ca2+ concentrations from 5 nM to 44 μM, in the presence of 1 μM CaM. Absolute AC activity from a representative experiment (mean ± SD of five replicate assays). (B2) Activity of AC-AplC, calculated as in A2. Note the activity of AC-AplC decreased as the concentration of free Ca2+ increased. (B3) The inhibition of AC-AplC by Ca2+ is independent of CaM. Data are means ± SEM of data from three experiments on separate populations of cells (F3,6 = 42.8, P < 0.001). In A1, A2, B1, and B2, some error bars are smaller than symbols. *P < 0.05 for pairwise, posthoc comparisons with 50 nM Ca2+ + CaM.
Ca2+-Regulated AC Isoforms Are Enriched in Aplysia Sensory Neurons.
To assess which of the AC isoforms are expressed in Aplysia mechanosensory neurons, we carried out quantitative real-time PCR (qRT-PCR) assays for all four AC isoforms. Assays were linear down to <10 copies (Fig. S7). Assays on cDNA from SN clusters gave a wide range of values for AC-AplA and AC-AplC, depending on the population of animals. AC-AplA message was present at >1000 copies per SN. AC-AplC mRNA varied from 10 to >1000 copies per SN. In contrast, AC-AplB and AC-AplD were present at <1 copy per SN, consistent with contamination from other cells, such as glia. Thus, it appears that both AC-AplA and AC-AplC are expressed in SNs, whereas the other two AC isoforms are not.
The expression of AC-AplA and AC-AplC was confirmed by immunoblot on desheathed pleural ganglia, which contain the largest population of these mechanoreceptor SNs (Fig. 3). In both pleural ganglion and CNS membranes, the antibody against the C2b domain of AC-AplA stained a major band at ≈115 kDa; in many immunoblots, an additional weaker band was visible at 200 kDa (Fig. 3A), similar to the predicted size of 208 kDa (Fig. S6). To distinguish whether the lower molecular weight band represents a truncated form of AC-AplA or an unrelated protein, we compared immunoblots of CNS with antibodies against two different domains of AC-AplA (Fig. 3B). Both antibodies stained a band at 115 kDa, suggesting that this smaller 115-kDa protein, which includes the C2b region, represents the primary form of AC-AplA that is expressed in CNS. This band is comparable in size to the other Aplysia and mammalian AC isoforms (Table S1 and Fig. S6). The smaller AC-AplA band was not due to proteolysis during preparation (SI Methods). We speculate that the 115-kDa band represents a functionally active AC with a short C2 domain produced by proteolytic processing. In contrast, rutabaga AC, which also has a long C2 domain (Table S1), is >200 kDa and does not appear to be expressed in a truncated form (34). The antibody against the C2b domain of AC-AplC stained a band at ≈125 kDa, similar to the predicted size (Fig. 3A).
Fig. 3.
Immunoblot of AC-AplA and AC-AplC in pleural ganglion and CNS membranes. (A) Antibodies against sequences in the C2b domains of AC-AplA and AC-AplC recognize bands in membranes from pleural ganglia, which are enriched for SNs. Preincubation with antigen peptide blocked staining. The high molecular weight bands recognized by the anti-AC-AplA antibody are indicated by arrowheads. (B) To confirm the identity of the predominant protein recognized by anti-AC-AplA antibody, which is substantially smaller than the predicted molecular weight, we probed CNS preparations with antibodies against two distinct domains of AC-AplA. Note that the C1b and C2b antibodies recognized identical bands. (This same lower molecular weight band has been observed in immunoblots on pleural ganglion sensory neuron clusters.)
AC-AplA Is Expressed in SN Somata, Processes, and Growth Cones, Whereas AC-AplC Is Primarily Expressed in SN Somata.
Using immunocytochemistry, we determined the expression pattern of AC-AplA and AC-AplC in SNs (Fig. S8). SNs were cultured either alone or together with the L7 MN. AC-AplA immunoreactivity was observed in SN somata and processes. Both the postsynaptic MNs and regions where pre- and postsynaptic processes overlapped showed similarly intense staining. Interestingly, intense AC-AplA staining was observed in growth cones. AC-AplC immunoreactivity was also observed in SN and MN somata and processes; however, staining in processes and growth cones was noticeably weaker than in somata (Fig. S8).
AC-AplA Is Coupled to 5-HT Receptors in Aplysia SNs.
We asked which of the AC isoforms are coupled to 5-HT receptors in the SNs. Pairing activity and Ca2+ influx with 5-HT enhance two forms of 5-HT-initiated, cAMP-dependent modulation in SNs: spike broadening and increased excitability (3, 14, 38). We therefore predicted that in SNs, 5-HT receptors are coupled to the Ca2+/CaM-sensitive AC, AC-AplA. To test the role of specific AC isoforms in 5-HT-initiated modulation in SNs, we knocked down expression of AC-AplA or AC-AplC by injecting morpholino antisense (39). To assess the consequence of AC knockdown, we measured an effect of 5-HT known to be mediated entirely by cAMP: broadening of the SN action potential in 100 mM tetraethylammonium (TEA) and 20 μM nifedipine (at these concentrations, TEA blocks K+ channels and nifedipine blocks Ca2+ channels that are modulated by PKC; SI Methods) (40, 41). Control SNs were injected either with Alexa 594 dextran alone, which was used to identify all injected neurons, or with control morpholino. Because AC-AplB is not expressed in SNs, morpholino for AC-AplB served as an additional control. In SNs injected with AC-AplA morpholino, spike broadening was reduced significantly by 72–80%, depending on the control (F4,28 = 5.3, P = 0.003; Fig. 4). Injection of morpholino for AC-AplC resulted in a nonsignificant reduction in spike broadening. In SNs in which expression of AC-AplA was knocked down, there was some remaining spike broadening (114%). It seemed possible that this residual 5-HT response might be mediated by AC-AplC. To test this hypothesis, we injected SNs with a combination of morpholinos for AC-AplA and AC-AplC. This combination produced no greater reduction in spike broadening as compared with AC-AplA morpholino alone. To confirm the contribution of AC-AplA to 5-HT-initiated modulation in SNs, we used dsRNA as an alternate means to knock down expression. Injection of dsRNA for AC-AplA significantly reduced spike broadening by 75–77% relative to the Alexa control or dsRNA for AC-AplB (F2,18 = 9.714, P = 0.001) (Fig. 4). We did not attempt to further explore the possible modest contribution of AC-AplC to the spike-broadening response; given the large contribution of AC-AplA, which accounted for ≈75% of the broadening response, and the variability in spike broadening across experiments, it would be difficult to detect a possible small contribution of AC-AplC. In summary, AC-AplA accounts for the great majority of 5-HT-initiated spike broadening in the SNs.
Fig. 4.
cAMP-dependent modulation of SN action potential by 5-HT is mediated by AC-AplA. SNs in the pleural ganglion ventro-caudal cluster were injected either with antisense morpholino or dsRNA for AC-AplA or AC-AplB or antisense morpholino for AC-AplC. Alexa 594 dextran was included with morpholinos or dsRNA to identify injected neurons. Injection of control morpholino or Alexa 594 dextran served as a control. Because AC-AplB is not expressed in SNs, morpholino or dsRNA for AC-AplB was used as a third control. After injection, clusters were maintained in culture for 4 d before electrophysiology. Spike-broadening responses to 5-HT were measured in the presence of 100 mM TEA and 20 μM nifedipine; under these conditions, 5-HT-induced spike broadening is mediated entirely by cAMP-dependent phosphorylation (40, 41) (SI Methods). SNs injected with either morpholino or dsRNA for AC-AplA showed significantly less spike broadening than control neurons. (A) Traces recorded before and after 3–4 min of exposure to 10 μM 5-HT; each pair of traces is recorded from a different SN. (B) Group data for 5-HT-induced spike broadening for SNs treated with either dsRNA or morpholino. SNs injected with morpholino for AC-AplA showed significantly less broadening of the action potential than control SNs injected with either Alexa dextran (P < 0.001), control morpholino (P = 0.008), or morpholino for AC-AplB (P = 0.002; all P values for individual pairwise, posthoc comparisons were made with the Sidak adjustment; SI Methods) (** indicates P < 0.01) (F4,28 = 5.31 P = 0.003). SNs injected with dsRNA for AC-AplA showed significantly less broadening of the action potential than control SNs injected with Alexa dextran (P = 0.003) or dsRNA for AC-AplB (P = 0.006) (F4,28 = 9.71, P = 0.001). There was no significant (NS) reduction in the spike-broadening response of SNs injected with morpholino for AC-AplC (P = 0.416, 1.0, and 0.982 for Alexa control, control morpholino, and AC-AplB morpholino, respectively, for pairwise, posthoc comparisons). (C) Effect of double knockdown of AC-AplC and AC-AplA. To further assess whether some of the action potential broadening induced by 5-HT might be mediated by AC-AplC, we tested whether double knockdown using morpholinos for both AC-AplC and AC-AplA produced greater reduction in the spike-broadening response, as compared with AC-AplA morpholino alone. SNs injected with both morpholinos showed no greater reduction in spike broadening compared with SNs injected with AC-AplA morpholino alone. The data for AC-AplA morpholino are from B (** indicates P < 0.01).
Discussion
Four Putative ACs Are Expressed in Aplysia CNS.
We identified four distinct putative AC isoforms in CNS. The secondary protein structures predicted from all four transcripts resemble the structure of transmembrane ACs in metazoans (Fig. S1). Moreover, all four predicted proteins share highly conserved sequences typically found in metazoan ACs (Fig. S2). The presence of these key features of metazoan ACs suggests that all four genes code for functionally active AC enzymes. We focused on the functional properties of the two isoforms that are expressed in Aplysia SNs: AC-AplA and AC-AplC. Heterologous expression of either of these transcripts increased cAMP-synthesizing activity (Fig. 2), demonstrating that each codes for an enzymatically active AC.
AC-AplA Is Stimulated by Ca2+/CaM, Whereas AC-AplC Is Inhibited by Ca2+.
Our primary goal was to identify and characterize the Ca2+/CaM-sensitive AC in Aplysia SNs. The dually regulated Ca2+/CaM-sensitive AC has been hypothesized to function during classical conditioning as a molecular site of associative convergence for Ca2+ influx accompanying SN activity and the modulatory neurotransmitter 5-HT. Of the 12 cytoplasmic domains of the cloned Aplysia AC isoforms, only the C1 domain of AC-AplA bound CaM. Recombinant AC-AplA was stimulated by Ca2+/CaM more than 3-fold. Recombinant AC-AplC was directly inhibited by submicromolar Ca2+, much like AC5 and AC6 (42). Because inhibition was detected as Ca2+ increased just above 100 nM, AC-AplC should be modulated by small increases in Ca2+ with very modest spike activity. Although the C1a region of AC-AplB does not closely resemble any single group of ACs, its C2a domain is relatively similar to ACs 2, 4, and 7 (Figs. S3 and S4). AC2 is stimulated by PKC (21, 43). Lorenzetti et al. (44) have found in B51 neurons that PKC increases cAMP levels, and that these cells express AC-AplB. They suggest that during operant conditioning, a PKC-modulated AC in B51 serves as a coincidence detector for dopamine and for Ca2+ influx triggered by bursts of activity. Thus, AC-AplB, much like AC-AplA, may serve as an associative integrator during conditioning. AC-AplD is more similar to AC9 than other mammalian isoforms (Fig. S3). All four Aplysia AC isoforms have clear orthologs in Lottia gigantean, a prosobranch gastropod mollusk (24). Lottia, like most invertebrates, has an ortholog of mammalian AC3. As yet, no AC3-type AC has been found in Aplysia.
Both AC-AplA and AC-AplC Are Expressed in SNs, and AC-AplA Is Enriched in Presynaptic Processes and in Growth Cones.
Based on qRT-PCR, both AC-AplA and AC-AplC are expressed in SNs, but not AC-AplB or AC-AplD. The expression of AC-AplA and AC-AplC in SNs was confirmed by immunoblot and immunocytochemistry. Immunocytochemistry on cultured neurons revealed that AC-AplA and AC-AplC are expressed both presynaptically in SNs and postsynaptically in L7 motor neurons. In SNs, immunoreactive AC-AplA was equally observed in presynaptic processes and in somata, whereas AC-AplC was preferentially localized to somata. This suggests that AC-AplA may play important roles in short-term synaptic modulation, as well as in regulation of transcription cascades that are important in long-term synaptic plasticity. Interestingly, activation of PKA at presynaptic loci has been implicated in synaptic tagging in the establishment of long-term synaptic facilitation (45). AC-AplA is also expressed in growth cones. This CaM-sensitive AC may serve to mediate interactions between Ca2+ signals and the cAMP pathways, both of which have been implicated in growth cone guidance (46, 47).
In SNs, 5-HT Stimulation of cAMP Levels Is Predominantly Mediated by AC-AplA.
CaM-sensitive AC has been hypothesized to function as a molecular convergence site for Ca2+ influx and 5-HT in SNs during associative synaptic plasticity (11). These two stimuli coincide temporally during classical conditioning when SN activity precedes a tail shock, which releases 5-HT. We explored whether the CaM-sensitive AC, AC-AplA, mediates the rise in cAMP levels stimulated by 5-HT (48–50). SNs treated with morpholino or dsRNA for AC-AplA showed a 72–80% reduction in the cAMP-dependent spike-broadening response to 5-HT. In contrast, morpholino for AC-AplC did not significantly affect the spike-broadening response (Fig. 4).
Thus, in the SN somata, 5-HT stimulation of the cAMP pathway is largely or exclusively mediated via the CaM-sensitive AC, AC-AplA. Because AC-AplC immunoreactivity was weaker in SN processes than in somata, we suggest that at presynaptic sites, AC-coupled 5-HT receptors also activate primarily AC-AplA. As a consequence, most or all of the stimulation of cAMP synthesis by 5-HT is subject to modulation by Ca2+/CaM. We conclude that in the SNs, the CaM-sensitive AC is able to provide a locus for associative integration of Ca2+ influx and 5-HT, as had been predicted earlier based on cellular studies (13, 14).
A Possible Role for AC-AplC in Associative Integration.
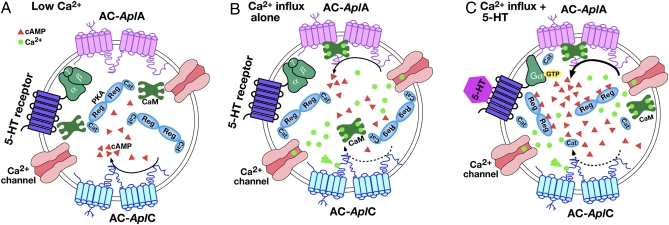
Whereas the role of a CaM-regulated AC may be important in associative integration of SN activity and modulatory inputs, the functional role of a Ca2+-inhibited AC in the SNs is less obvious. The coexpression of the Ca2+-inhibited AC-AplC and the CaM-stimulated AC-AplA in the presynaptic sensory neurons suggests a possible role for Ca2+ inhibition in enhancing the function of AC-AplA as a coincidence detector. Earlier biochemical and cellular studies on Aplysia neurons observed that activity and Ca2+ alone are unable to effectively activate the cAMP cascade (38, 51). Rather remarkably, cAMP-dependent kinase makes no contribution to posttetanic potentiation produced by trains of 40 action potentials at these SN-MN synapses (52), suggesting that even intense activity, which increases presynaptic Ca2+ levels substantially, does not effectively increase cAMP levels. Coexpression of a Ca2+-inhibited AC with the CaM-stimulated AC could make Ca2+ a relatively ineffective stimulator of cAMP levels because inhibition of AC-AplC should at least partially offset the stimulation of AC-AplA. In contrast, when a Ca2+ increase is paired with arrival of 5-HT, CaM should potentiate the stimulation of AC-AplA by Gs (Fig. 5). Thus, the coexpression of AC-AplC and AC-AplA should work to minimize the nonassociative effect of activity and Ca2+ influx on cAMP levels, while permitting the associative activation of AC-AplA by Ca2+ and transmitter. The mammalian homolog to AC-AplC, the Ca2+-inhibited AC5, is highly expressed in regions of mammalian CNS, such as striatum (42); however the possible functional role of Ca2+ inhibition of AC5 is not understood. We propose that in mammalian brain, as in Aplysia CNS, Ca2+-inhibited AC may function to enhance the associative requirement for coincident Ca2+ influx and transmitter for effective elevation of cAMP levels mediated by CaM-sensitive AC.
Fig. 5.
Coexpression of Ca2+-inhibited AC, together with dually regulated Ca2+/CaM-sensitive AC, enhances associative specificity of stimulation of cAMP pathway. (A) In the absence of Ca2+ influx or 5-HT, AC-AplC shows a low level of basal activity (curved arrow), producing modest levels of cAMP (red triangles). (B) Ca2+ influx during unpaired activity activates CaM, stimulating AC-AplA. At the same time, Ca2+ (green circles) binds to AC-AplC (green arrow), inhibiting the basal activity of this AC isoform (broken curved arrows represent loss of basal cAMP synthesis). The direct Ca2+ inhibition of AC-AplC reduces the net rise in cAMP levels when AC-AplA is stimulated by Ca2+ influx alone. (C) When activity and Ca2+ influx are paired with the arrival of 5-HT (hexagon), activation of AC-AplA is enhanced (thick curved arrow). With pairing there is a greater net increase in cAMP levels, resulting in effective activation of PKA.
Methods
AC Cloning.
Total RNA was extracted from Aplysia CNS using TRizol (Invitrogen), purified on an RNAqueous column (Ambion), treated with DNase H, and reverse-transcribed using random hexamers. A degenerate cloning strategy using highly conserved sequences within the C1a and C2a regions was used to identify Aplysia AC isoforms (see SI Methods for details). Amplified PCR products were cloned into pCR2.1, and distinct clones were identified based on restriction mapping and DNA sequencing. 5′ and 3′ RACE was performed using a SmartRace Kit (Clontech). To verify each AC RACE sequence, PCR was performed on three independent cDNA preparations, using gene-specific primers located within the 5′ and 3′ UTRs. Sequences for AC- AplA, -B, and -C were deposited in GenBank (accession nos. AY843027, AY843025, and AY843026).
Antigen Peptide Selection, Antibody Production, and Purification.
Antibodies were generated in rabbits (Covance) against thyroglobulin-conjugated peptides (Bio-Synthesis) corresponding to nonconserved sequences within the hypervariable C2b regions of each AC or the calmodulin-binding region of AC-AplA. Antibodies were affinity-purified on antigen peptide columns before use for immunoblotting and immunocytochemistry (see SI Methods for details).
Supplementary Material
Acknowledgments
Drs. Steven Munger and Frank Margolis provided assistance with the design of molecular biology experiments and Drs. Tony Gover and Brian Hagen provided help with confocal imaging. This research was supported by National Institutes of Health Grant MH-55880.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY843025, AY843026, AY843027, and HM030824).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004451107/-/DCSupplemental.
References
- 1.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: Possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 2.Byers D. A review of the behavior and biochemistry of dunce, a mutation of learning in Drosophila. Basic Life Sci. 1980;16:467–474. doi: 10.1007/978-1-4684-7968-3_33. [DOI] [PubMed] [Google Scholar]
- 3.Abrams TW. Activity-dependent presynaptic facilitation: An associative mechanism in Aplysia. Cell Mol Neurobiol. 1985;5:123–145. doi: 10.1007/BF00711089. [DOI] [PubMed] [Google Scholar]
- 4.Kandel ER, Schwartz JH. Molecular biology of learning: Modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 5.Ocorr KA, Walters ET, Byrne JH. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci USA. 1985;82:2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudai Y, Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984;47:119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- 7.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 8.Wei F, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–726. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 9.Wong ST, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- 11.Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: Dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yovell Y, Kandel ER, Dudai Y, Abrams TW. A quantitative study of the Ca2+/calmodulin sensitivity of adenylyl cyclase in Aplysia, Drosophila, and rat. J Neurochem. 1992;59:1736–1744. doi: 10.1111/j.1471-4159.1992.tb11005.x. [DOI] [PubMed] [Google Scholar]
- 13.Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983;219:405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: Activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 15.Livingstone MS. Genetic dissection of Drosophila adenylate cyclase. Proc Natl Acad Sci USA. 1985;82:5992–5996. doi: 10.1073/pnas.82.17.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayman GA, et al. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–25405. [PubMed] [Google Scholar]
- 17.Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by long-term potentiation of sensorimotor synapses. Science. 1997;278:467–471. doi: 10.1126/science.278.5337.467. [DOI] [PubMed] [Google Scholar]
- 18.Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- 19.Cann MJ, Chung E, Levin LR. A new family of adenylyl cyclase genes in the male germline of Drosophila melanogaster. Dev Genes Evol. 2000;210:200–206. doi: 10.1007/s004270050304. [DOI] [PubMed] [Google Scholar]
- 20.Premont RT. Identification of adenylyl cyclases by amplification using degenerate primers. Methods Enzymol. 1994;238:116–127. doi: 10.1016/0076-6879(94)38011-2. [DOI] [PubMed] [Google Scholar]
- 21.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moroz LL, et al. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα.GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 24.Sossin WS, Abrams TW. Evolutionary conservation of the signaling proteins upstream of cyclic AMP-dependent kinase and protein kinase C in gastropod mollusks. Brain Behav Evol. 2009;74:191–205. doi: 10.1159/000258666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 26.Baker DA, Kelly JM. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol Microbiol. 2004;52:1229–1242. doi: 10.1111/j.1365-2958.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 27.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- 28.Wu Z, Wong ST, Storms DR. Modification of the calcium and calmodulin sensitivity of the type I adenylyl cyclase by mutagenesis of its calmodulin binding domain. J Biol Chem. 1993;268:23766–23768. [PubMed] [Google Scholar]
- 29.Krupinski J, et al. Adenylyl cyclase amino acid sequence: Possible channel- or transporter-like structure. Science. 1989;244:1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura M, Cooper DM. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci USA. 1992;89:6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, Wayman G, Storm DR. Phosphorylation and inhibition of type III adenylyl cyclase by calmodulin-dependent protein kinase II in vivo. J Biol Chem. 1996;271:24231–24235. doi: 10.1074/jbc.271.39.24231. [DOI] [PubMed] [Google Scholar]
- 32.Antoni FA, et al. Ca2+/calcineurin-inhibited adenylyl cyclase, highly abundant in forebrain regions, is important for learning and memory. J Neurosci. 1998;18:9650–9661. doi: 10.1523/JNEUROSCI.18-23-09650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cumbay MG, Watts VJ. Gαq potentiation of adenylate cyclase type 9 activity through a Ca2+/calmodulin-dependent pathway. Biochem Pharmacol. 2005;69:1247–1256. doi: 10.1016/j.bcp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Levin LR, et al. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 35.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 36.Vetter SW, Leclerc E. Novel aspects of calmodulin target recognition and activation. Eur J Biochem. 2003;270:404–414. doi: 10.1046/j.1432-1033.2003.03414.x. [DOI] [PubMed] [Google Scholar]
- 37.Levin LR, Reed RR. Identification of functional domains of adenylyl cyclase using in vivo chimeras. J Biol Chem. 1995;270:7573–7579. doi: 10.1074/jbc.270.13.7573. [DOI] [PubMed] [Google Scholar]
- 38.Eliot LS, Hawkins RD, Kandel ER, Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1994;14:368–383. doi: 10.1523/JNEUROSCI.14-01-00368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heasman J. Morpholino oligos: Making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 40.Dumitriu B, Cohen JE, Wan Q, Negroiu AM, Abrams TW. Serotonin receptor antagonists discriminate between PKA- and PKC-mediated plasticity in Aplysia sensory neurons. J Neurophysiol. 2006;95:2713–2720. [Google Scholar]
- 41.Cohen JE, Onyike CU, McElroy VL, Lin AH, Abrams TW. Pharmacological characterization of an adenylyl cyclase-coupled 5-HT receptor in Aplysia: Comparison with mammalian 5-HT receptors. J Neurophysiol. 2003;89:1440–1455. doi: 10.1152/jn.01004.2002. [DOI] [PubMed] [Google Scholar]
- 42.Guillou JL, Nakata H, Cooper DM. Inhibition by calcium of mammalian adenylyl cyclases. J Biol Chem. 1999;274:35539–35545. doi: 10.1074/jbc.274.50.35539. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa Y. Regulation of cAMP signaling by phosphorylation. Adv Second Messenger Phosphoprotein Res. 1998;32:99–120. doi: 10.1016/s1040-7952(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzetti FD, Baxter DA, Byrne JH. Molecular mechanisms underlying a cellular analog of operant reward learning. Neuron. 2008;59:815–828. doi: 10.1016/j.neuron.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casadio A, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 46.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 47.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 48.Bernier L, Castellucci VF, Kandel ER, Schwartz JH. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3′:5′-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982;2:1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ocorr KA, Byrne JH. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB) Neurosci Lett. 1985;55:113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- 50.Abrams TW, Bernier L, Hawkins RD, Kandel ER. Possible roles of Ca2+ and cAMP in activity-dependent facilitation, a mechanism for associative learning in Aplysia. Soc Neurosci Abstr. 1984;10:269. (abstr) [Google Scholar]
- 51.Yovell Y, Abrams TW. Temporal asymmetry in activation of Aplysia adenylyl cyclase by calcium and transmitter may explain temporal requirements of conditioning. Proc Natl Acad Sci USA. 1992;89:6526–6530. doi: 10.1073/pnas.89.14.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin I, Hawkins RD. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at Aplysia sensory-motor neuron synapses. J Neurosci. 2003;23:7288–7297. doi: 10.1523/JNEUROSCI.23-19-07288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.