Abstract
Soluble oligomeric aggregates of the amyloid-β peptide (Aβ) have been implicated in the pathogenesis of Alzheimer’s disease (AD). Although the conformation adopted by Aβ within these aggregates is not known, a β-hairpin conformation is known to be accessible to monomeric Aβ. Here we show that this β-hairpin is a building block of toxic Aβ oligomers by engineering a double-cysteine mutant (called Aβcc) in which the β-hairpin is stabilized by an intramolecular disulfide bond. Aβ40cc and Aβ42cc both spontaneously form stable oligomeric species with distinct molecular weights and secondary-structure content, but both are unable to convert into amyloid fibrils. Biochemical and biophysical experiments and assays with conformation-specific antibodies used to detect Aβ aggregates in vivo indicate that the wild-type oligomer structure is preserved and stabilized in Aβcc oligomers. Stable oligomers are expected to become highly toxic and, accordingly, we find that β-sheet-containing Aβ42cc oligomers or protofibrillar species formed by these oligomers are 50 times more potent inducers of neuronal apoptosis than amyloid fibrils or samples of monomeric wild-type Aβ42, in which toxic aggregates are only transiently formed. The possibility of obtaining completely stable and physiologically relevant neurotoxic Aβ oligomer preparations will facilitate studies of their structure and role in the pathogenesis of AD. For example, here we show how kinetic partitioning into different aggregation pathways can explain why Aβ42 is more toxic than the shorter Aβ40, and why certain inherited mutations are linked to protofibril formation and early-onset AD.
Keywords: amyloid-β peptide, protein aggregation, protein structure, protofibril, β-hairpin conformation
Alzheimer’s disease (AD) is linked to the formation of neurotoxic oligomeric aggregates of the amyloid-β peptide (Aβ) in the brain and several such aggregates have been described (1, 2). These aggregates have, for instance, been identified in vivo in brain tissue of humans as 70 kDa or larger aggregates containing Aβ dimers (3), or from transgenic AD mice as smaller aggregates called Aβ*56 (4). Toxic Aβ oligomers and protofibrils have also been made in vitro, such as oligomeric ADDLs prepared by dilution from organic solvents (5, 6), smaller globulomers formed in SDS-containing solvents (7), oligomers formed in water at low pH (8, 9), and larger nonfibrillar aggregates known as protofibrils (10, 11). However, it is not clear if and how these different aggregates are related to the pathogenesis of AD. Indeed, although there is a large body of data on the conformation and “cross-β” packing of Aβ in amyloid fibrils (12, 13), which are the end-products of aggregation, little is known about the basic building blocks of the oligomers and protofibrils that precede them. Here we use protein engineering to address these issues and to provide a method to stabilize toxic Aβ oligomers for structural and functional studies.
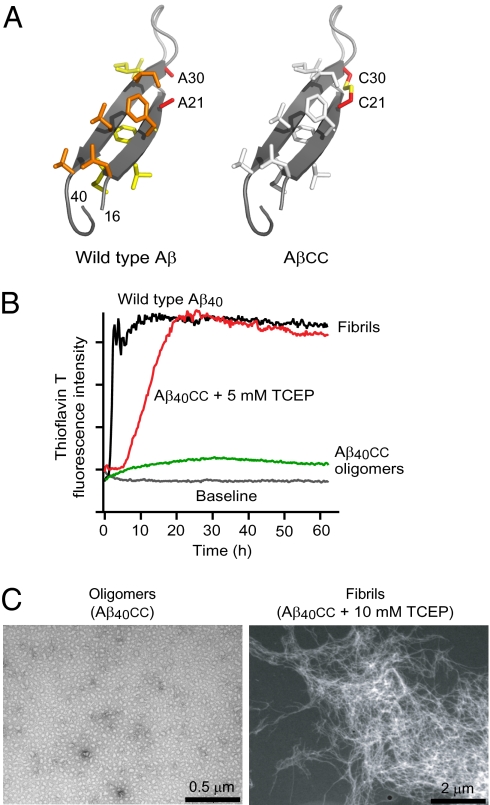
Monomeric Aβ does not adopt a unique conformation in water solution. Nevertheless, NMR experiments (14) and molecular modeling (15) suggest that the central and C-terminal hydrophobic regions of Abeta have a propensity to form extended beta-strand conformations with a connecting turn between them. Such a “hairpin” conformation (Fig. 1A) is in fact also induced when Aβ forms a complex with a phage-display selected Affibody-binding protein (16, 17). The hairpin is topologically similar to the conformation of Aβ in fibrils. However, there is a distinct difference in that the hydrogen bonds are intramolecular, resulting in antiparallel β-strands, whereas they are intermolecular in fibrils, resulting instead in parallel β-sheets.
Fig. 1.
Protein engineering. (A) (Left) The β-hairpin conformation of Aβ40 observed in complex with an Affibody binding protein (17). Nonpolar side chains at the two hydrophobic faces are shown as sticks and colored yellow and orange, respectively. The Ala21 and Ala30 methyls are located in close proximity on opposite β-strands. (Right) Model of the AβA21C/A30C double mutant (Aβcc) in which the β-hairpin conformation is locked by a disulfide bond. (B) ThT fluorescence assays of Aβ40cc aggregation in the absence or presence of TCEP reducing agent compared with wild-type Aβ40 aggregation. (C) TEM micrographs of β-sheet oligomers of Aβ40cc (Left) and of fibrils formed in presence of TCEP (Right).
We have previously proposed that intermediate oligomeric species consist of Aβ subunits in the hairpin conformation and that a conformational change results in the formation of Aβ subunits held together by intermolecular hydrogen bonds that are seeds for polymerization into amyloid fibrils (17). There is also experimental evidence for the hairpin in oligomers of the globulomer kind (18), as well as for antiparallel β-sheet secondary structure in Aβ oligomers formed in TBS buffer or in cell culture medium (19). We therefore set out to test if stabilizing Aβ in the hairpin conformation observed in the Affibody complex would promote the formation of oligomeric aggregates but not fibrils, and whether such stabilized oligomers would possess antigenic and neurotoxic characteristics similar to those of wild-type Aβ oligomers found in AD.
Results
Protein Engineering.
The structure of Aβ40 in complex with the ZAβ3 Affibody (PDB accession No. 2OTK) was examined for sites suitable for disulfide engineering that would constrain it in its hairpin conformation. Ala21 and Ala30 are ideally suited for this purpose, as their β-carbons are located on opposite β-strands at a distance of 4.2 Å from each other (Fig. 1A). Molecular modeling showed that a Cys21/Cys30 disulfide can be accommodated with favorable conformational energy and without perturbing the hairpin structure in which 12 backbone hydrogen bonds are predicted to form in Aβ40cc.
We produced Aβcc in Escherichia coli bacteria by coexpression with the ZAβ3 Affibody (20). This Affibody binds Aβ in the hairpin conformation, which allows for the formation and purification of monomeric Aβcc with an intramolecular disulfide bond. Recombinant Aβ produced in this way contains an N-terminal methionine residue, but this does not affect its biophysical properties (20).
Aβcc with an Intact Disulfide Bond Cannot Form Amyloid Fibrils.
Our hairpin hypothesis postulates that Aβcc should not form amyloid fibrils as long as the intramolecular disulfide is intact, and experiments show that this is indeed the case. Thioflavin T (ThT) fluorescence assays of fibril formation demonstrate that oxidized Aβ40cc remains soluble in a weakly ThT-binding state, whereas the addition of tris-2-carboxyethyl-phosphine (TCEP) reducing agent to break the disulfide bond results in accelerating ThT binding to levels equal to those observed with wild-type Aβ40 fibrils (Fig. 1B). Transmission electron microscopy (TEM) confirms that oxidized Aβ40cc forms oligomeric aggregates, whereas reduced Aβ40cc forms fibrils (Fig. 1C). In addition, the longer Aβ42cc cannot form amyloid fibrils in its oxidized state (Fig. S1) but, as described below, instead forms β-sheet containing oligomers and protofibrils.
Formation of Oligomers with Different Secondary-Structure Content.
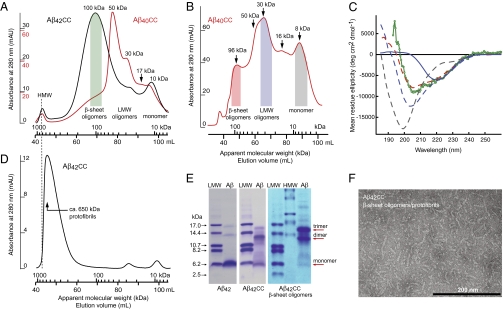
Blocking of fibril formation allows for the enrichment of oligomeric aggregates that are otherwise only transiently formed by wild-type Aβ (1, 2). Purified Aβ40cc and Aβ42cc were denatured into monomeric form in 7 M guanidinium chloride and subjected to size exclusion chromatography (SEC) with native (nondenaturing) phosphate buffer at pH 7.2 as running buffer. Different oligomers then form spontaneously during SEC. The SEC chromatograms of Aβ40cc and Aβ42cc reveal the separation of distinct oligomeric species with apparent molecular weights of ∼16, ∼30, ∼50, and ∼100 kDa, in addition to monomer (at 8–10 kDa) and high molecular weight (HMW) aggregates (larger than ∼1,000 kDa) eluting at the void volume (Fig. 2 A and B). Aβ42cc and Aβ40cc form oligomers with similar molecular weights and higher concentration favors the formation of larger oligomers of both species, as expected (Fig. S2). Sometimes, ∼185-kDa oligomers are also observed; these are not well separated in SEC, but can be distinguished in the SEC profiles in Fig. 2B and Fig. S1A.
Fig. 2.
Biophysical and biochemical characterization of Aβcc oligomers and protofibrils. (A) Formation and separation of Aβ42cc (black) and Aβ40cc (red) oligomers during SEC on a Superdex 200 PG 16/600 column. Monomer peptide samples were loaded in denaturing buffer and eluted with native phosphate buffer at pH 7.2. Apparent molecular weights and classification of eluted oligomers have been indicated. HMW aggregates elute with the void volume. Sample amounts: 2.7 mg Aβ40cc and 1.7 mg Aβ42cc; see also Fig. S2. (B) SEC as in A showing separation of Aβ40cc oligomers on a Superdex 75 PG 16/60 column on which smaller aggregates become better separated. Sample amount: ∼ 3 mg. (C) CD (mean residue ellipticity) of SEC fractions pooled as indicated by shaded areas in A and B. Dashed lines: 8-kDa monomer (gray, 12 μM), 30-kDa LMW oligomers (blue, 16 μM), and 96-kDa β-sheet oligomers (red, 8 μM) of Aβ40cc. Green: ∼100-kDa β-sheet oligomers of Aβ42cc (13 μM). Solid blue line: the 30-kDa LMW oligomer fraction of Aβ40cc after concentration and heat treatment showing formation of β-sheet oligomers. (D) SEC of concentrated Aβ42cc β-sheet oligomers (1 mL, 145 μM), which form protofibrils with an average apparent molecular weight of ∼ 650 kDa. The dotted line shows that these are smaller than HMW aggregates in A. (E) SDS/PAGE of wild-type Aβ42 (Left), purified but unfractionated Aβ42cc (Center), and Aβ42cc β-sheet oligomers formed during SEC as in A (Right). The right lanes in all panels contain Aβ samples, and other lanes contain high and low molecular weight standards (HMW and LMW). Weights corresponding to monomer and SDS-resistant dimers and trimers have been indicated. The loading buffer contained 2.5 mM TCEP (heat-stable reducing agent) to completely break all disulfide bonds. (F) TEM micrograph of a concentrated sample of Aβ42cc β-sheet oligomers (190-μM monomer concentration) showing assembly of β-sheet oligomers into protofibrils.
Circular dichroism (CD) spectroscopy (Fig. 2C) reveals that Aβ40cc and Aβ42cc oligomers with an apparent molecular weight of ∼100 kDa contain ∼40% β-sheet secondary structure (β-sheet oligomers). Low molecular-weight (LMW) oligomers display a “random coil” CD spectrum similar to that of a disordered peptide and therefore lack regular secondary structure. LMW oligomers may, however, convert into either β-sheet oligomers or HMW aggregates and β-sheet oligomers may assemble further into protofibrils (Fig. 2D), as described below. The presence of antiparallel β-sheet in the larger oligomers was confirmed by Fourier transform infrared (FTIR) spectroscopy (Fig. S3).
Differences Between Aβ40cc and Aβ42cc Oligomerization.
There is a clear difference between the aggregation patterns of Aβ40cc and Aβ42cc, as the latter more readily forms β-sheet oligomers (Fig. 2A and Fig. S2). However, Aβ40cc LMW oligomers form β-sheet oligomers when they are concentrated to ∼0.6 mM and subjected to a 10-min heat treatment at 60 °C (Fig. 2C and Fig. S4). Hence, a kinetic barrier for formation of β-sheet oligomers is more easily overcome by Aβ42cc than by Aβ40cc. This finding explains the different size distributions observed in SEC of Aβ42cc and Aβ40cc (Fig. 2A) and it may also explain differences in toxicity, as described in the Discussion.
β-Sheet Oligomers Contain SDS-Stable Dimers and Trimers.
Aggregated Aβ isolated from the brains of AD patients contains neurotoxic dimeric and trimeric species that are either formed in SDS-containing solutions or are resistant to denaturation in SDS/PAGE electrophoresis experiments. It has been suggested that such species may constitute building blocks of toxic Aβ aggregates (3, 18). If this suggestion is true and if the aggregates formed by Aβcc are structurally identical to wild-type aggregates, then one would expect Aβcc to form SDS-stable dimers. We find that dimeric and trimeric bands of purified but unfractionated Aβ42cc are more prominent in SDS/PAGE than those of wild-type Aβ42. But more importantly, ∼100 kDa β-sheet oligomer fractions of Aβ42cc are only observed as dimer and trimer units in SDS/PAGE with Coomassie staining (Fig. 2E). The absence of a monomer band implies that dimers and trimers form in the oligomers and remain stable in SDS-containing buffers.
β-Sheet Oligomers Can Form Protofibrils.
Pooling and concentrating SEC fractions containing Aβ42cc or Aβ40cc β-sheet oligomers results in the formation of higher-order aggregates (Fig. 2D), which are distinct from the HMW aggregates observed to elute in the void of the SEC profiles in Fig. 2A and B (also shown by antibody assays below). TEM images of such samples (Fig. 2F) show spherical oligomers with an average diameter of ∼6 nm, as well as longer and curved aggregates with the same diameter. The morphology of the larger aggregates is such that they appear to be assemblies of the 6-nm oligomers. Their size and appearance are also very similar, if not indistinguishable, to those of protofibrils formed by wild-type Aβ; one may, for instance, compare Fig. 2F to figure 1C in the work of Goldsbury et al. (figure 1C in ref. 21). A globular protein, which in SEC elutes at a volume corresponding to that of the β-sheet oligomers (∼100 kDa) will have a diameter of ∼6 nm, in agreement with the size of the small spherical oligomers. A molecular weight of ∼100 kDa per 6-nm oligomer subunit in Aβcc protofibrils is also close to the observed mass per length of wild-type Aβ protofibrils: 114 ± 12 kDa per 6 nm (21). Hence, it appears that the smaller particles in Fig. 2F are β-sheet oligomers and that these associate to form protofibrils. Aβ40cc β-sheet oligomers also form protofibrils upon concentration. Dilution of protofibril samples followed by SEC suggest that they are stable (Fig. 2D). Such diluted samples, which we will call β-sheet oligomers/protofibrils, were used for antibody and neurotoxicity experiments described in the following section.
Recognition of Aβcc by a Physiologically Relevant Conformation-Specific Antibody.
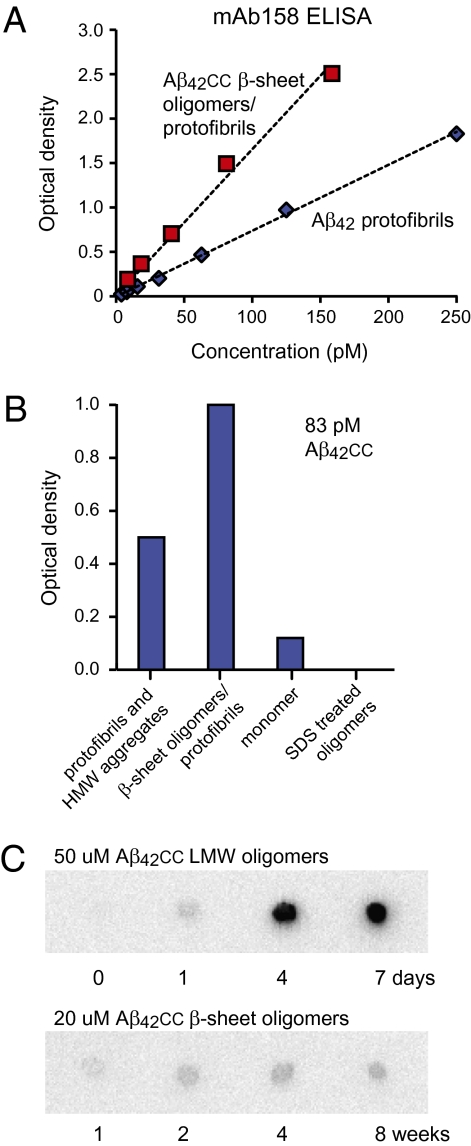
We used two different conformation-specific antibodies that bind wild-type Aβ aggregates to examine if their binding epitopes are preserved in Aβcc and to shed light on how the many Aβcc aggregates are related. The mAb158 monoclonal antibody (22) was selected based on its affinity for protofibrils of Aβ42 carrying the Arctic mutation (11). The mAb158 antibody also recognizes protofibrils of wild-type Aβ42, a protofibrillar form that is present in the medium of APP-expressing cells, and Aβ aggregates in brains of transgenic mice (22). ELISA experiments show that β-sheet oligomers/protofibrils of Aβ42cc bind mAb158 with the same affinity, or better, than wild-type protofibrils (Fig. 3A). This assay is conformation-specific because monomeric Aβ42cc or SDS-treated β-sheet oligomers are not recognized by mAb158 (Fig. 3B). β-Sheet oligomers of Aβ40cc that have been converted from LMW oligomers by concentration and heat treatment are also recognized by mAb158 (Fig. S4). Hence, β-sheet Aβcc oligomers expose a mAb158 conformational epitope that is also present in wild-type Aβ protofibrils in vitro and in vivo.
Fig. 3.
Recognition of Aβcc oligomers by antibodies that are conformation specific for wild-type Aβ aggregates. (A) mAb158 antibody sandwich ELISA detection (22) of Aβ42cc β-sheet oligomers/protofibrils compared with detection of wild-type Aβ42 protofibrils. (B) mAb158 ELISA analysis of different Aβ42cc species at 83 pM total peptide concentrations. (C) Aging of Aβ42cc LMW and β-sheet oligomers at 37 °C followed by A11 antibody dot blot analysis showing that A11 binding aggregates form from LMW oligomers but not from β-sheet oligomers. Concentrations were equalized before blotting.
Aβcc Can Access Two Distinct Aggregation Pathways.
In addition to oligomers and protofibrils, Aβcc also forms HMW aggregates (Fig. 2A and Fig. S2). These aggregates are less homogeneous and contain both large amorphous aggregates and long protofibrils (Fig. S5), the latter of which may account for mAb158 binding observed for this fraction (Fig. 3B). Interestingly, the HMW SEC fractions also bind the A11 antibody (Fig. S6), which also is conformation-specific (23). However, β-sheet oligomers/protofibrils of Aβcc that bind mAb158 do not bind A11, and these are therefore structurally distinct from the HMW aggregates. To identify which oligomeric species represent the precursors of the A11 binding HMW aggregates of Aβcc, we aged both LMW and β-sheet oligomer fractions of Aβ42cc at 37 °C. (LMW oligomers were kept in diluted form to avoid the transformation into β-sheet oligomers). Within a few days, LMW oligomer samples of Aβ42cc assemble into large, stable A11-positive aggregates with a diameter of 19 to 25 nm, whereas β-sheet oligomers do not form A11-binding species even after 8 wk of aging (Fig. 3C and Fig. S6). These experiments show that the conformational specificity of A11 is different from that of mAb158: mAb158 binds an epitope present in aggregates containing Aβ42cc or Aβ40cc β-sheet oligomers, whereas A11 binds aggregates that are formed by LMW Aβ40cc or Aβ42cc oligomers or monomeric samples. There must therefore be two aggregation pathways for Aβcc with different end products: the β-sheet pathway, involving aggregation of β-sheet oligomers into protofibrils (which are detected in the mAb158 ELISA), and the coil pathway, involving aggregation of monomer or disordered LMW oligomers into HMW A11-binding aggregates (which are also detectable in vivo) (23). (The naming of the aggregation pathways reflects the secondary-structure content of the originating oligomers.)
Neurotoxicity of Aβcc Oligomers.
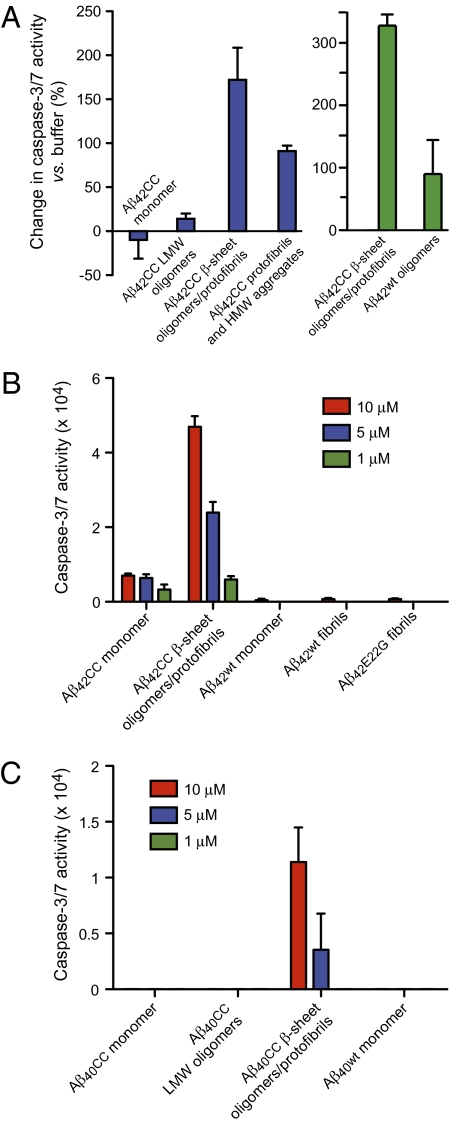
The neurotoxicity of different Aβcc aggregates was assayed by measuring their ability to induce apoptosis, indicated by the level of caspase-3/7 activity in a human neuroblastoma cell line, SH-SY5Y. Aβ42cc aggregates pooled from SEC all induce apoptosis 24 h after addition to the cells, but β-sheet oligomer fractions are considerably more toxic than LMW or HMW fractions of Aβ42cc. In fact, the toxicity is comparable or higher than that of wild-type Aβ42 oligomers, as one would expect for a stabilized toxic aggregate (Fig. 4A). Nerve cell apoptosis is dose-dependent and measurable after 24 h of treatment at 1-μM peptide concentrations and the β-sheet Aβ42cc oligomers are 50 times more potent than wild-type Aβ42 monomer or amyloid fibril samples (Fig. 4B). In contrast, the equivalent monomer and LMW oligomer fractions of Aβ40cc do not induce apoptosis. However, when Aβ40cc LMW oligomers are transformed to β-sheet oligomers/protofibrils, as described above, these start to induce caspase activity, but at lower levels compared with Aβ42cc (Fig. 4C). The neurotoxicity experiments therefore suggest that the actively toxic species are β-sheet oligomers of Aβ42cc and Aβ40cc, and larger protofibrillar aggregates that form when these are concentrated, as they have to be before the apoptosis assays. The results are also consistent with the lower propensity of Aβ40cc monomers to form β-sheet oligomers and these are, once formed, intrinsically less toxic than those of Aβ42cc. The fact that LMW oligomers and HMW aggregates of Aβ42cc also induce apoptosis (as shown in Fig. 4A) does not contradict that β-sheet oligomers/protofibrils are the primary toxic species, because LMW and β-sheet oligomers are not completely separated in SEC (Fig. 2 A and B) and large protofibrils are present in HMW fractions (Fig. S5). Overall, the neurotoxicity of different Aβ42cc and Aβ40cc aggregates (Fig. 4) matches specific recognition in the mAb158 ELISA (Fig. 3 A and B, and Fig. S4), indicating that toxic aggregates formed by Aβcc are to be found along the β-sheet aggregation pathway.
Fig. 4.
Neurotoxicity of Aβcc to SH-SY5Y human neuroblastoma cells. Aβcc samples, except β-sheet oligomers/protofibrils in C, were prepared and isolated by SEC, as in Fig 2A, concentrated to ∼75 to 250 μM in phosphate buffer at pH 7.2, and added to cell cultures at 1-, 5-, or 10-μM concentrations. Caspase-3/7 activity reporting on apoptosis was measured after 24 h of treatment. (A) Aβ42cc induced apoptosis following treatment with 10 μM of different species. Blue: comparison of different Aβ42cc species. Green: Aβ42cc oligomers compared with wild-type Aβ42 oligomers in another experiment. (B) Dose-dependence of apoptosis induced by Aβ42cc species compared with that of wild-type Aβ42 monomer and fibrils and Aβ42E22G. (C) Apoptosis induced by different Aβ40cc species and wild-type Aβ40. The sample marked “Aβ40cc LMW oligomers” is a 75-kDa SEC oligomer fraction. The Aβ40cc β-sheet oligomers/protofibrils were formed by pooling and concentrating monomer and LMW oligomer SEC fractions and heating the concentrated sample as described in the text and in Fig. S4.
Discussion
Do Aβcc Oligomers Mimic Wild-Type Aβ Oligomers?
Aβcc contains alanine to cysteine replacements, at positions 21 and 30, designed to stabilize a β-hairpin conformation by forming an intramolecular disulfide cross-link. The hairpin is predicted to comprise residues 17 to 23 and 30 to 36 as antiparallel β-strands connected by a turn involving residues 25 to 29. There is considerable evidence that such a conformation is accessible in monomeric Aβ: (i) it forms in complex with binding proteins (16, 17), (ii) its secondary structure elements persistently appear in computer simulations of different Aβ fragments (15, 24, 25), and (iii) NMR data suggest that turn formation of residues 24 to 28 nucleates monomer folding (26). We previously postulated that metastable Aβ oligomers contain hairpin subunits and that conversion into a related cross-β conformation transforms oligomers into fibril seeds and primes these for the runaway aggregation that is typical of amyloid fibril formation.
Here we test if soluble Aβ oligomers indeed consist of hairpins as constituent monomer building blocks and, if this is the case, if hairpin stabilization then provides a method for stabilization of physiologically relevant oligomers. We find that a Cys21-Cys30 disulfide prevents the formation of amyloid fibrils, resulting instead in the formation of stable oligomeric aggregates and protofibrils. These aggregates are indistinguishable from wild-type Aβ aggregates in TEM and they contain the dimeric and trimeric SDS-resistant units that are regarded as fingerprints of neurotoxic Aβ in vivo. Conformation-specific antibodies that recognize wild-type Aβ in the brains of AD patients (23) and transgenic mice (22) also recognize Aβcc oligomers. Finally, β-sheet oligomers and protofibrillar species formed by Aβ42cc or Aβ40cc are powerful inducers of apoptosis in neuronal cells in culture. Aβcc oligomers are therefore biologically functional, and we propose that they constitute an appropriate model for structural and functional studies of oligomers relevant to the pathogenesis of AD. Aβcc may potentially also be used for therapy development based on immunization or for small-molecule drug discovery.
Architecture of Neurotoxic Aβ Oligomers and Protofibrils.
The conformation of Aβcc in toxic β-sheet oligomers is most likely a β-hairpin, as shown in Fig. 1A, but β-hairpin conformations in which the turn is shifted from residues 25 to 29 toward, for instance, 24 to 28 or 23 to 27 may in fact also be compatible with a Cys21/Cys30 disulfide. The proposition that toxic Aβ oligomers are built of hairpin monomer subunits arranged to form larger units of antiparallel β-sheet secondary structure is in agreement with other observations. Infrared spectroscopy shows that antiparallel (and not parallel) β-sheet is present in oligomeric aggregates (19, 27). A β-hairpin similar to what is described here is also observed in synthetic Aβ globulomers (18). Aβ species with a mass equivalent to that of a dimer has been directly linked to disease (3). It is not certain that these are chemically identical to dimers observed in vitro, but the present data linking neurotoxicity, β-sheet oligomers, and SDS-resistant dimers and trimers are consistent with such a view.
Our experiments are not conclusive with regard to the precise molecular weight of a minimal β-sheet oligomer from which protofibrils assemble. SEC elution volumes suggest ∼100 kDa, but SEC columns are calibrated with globular protein standards and any disorder will result in a larger Stokes radius and an overestimate of the molecular weight. For example, the disordered nature of monomeric Aβ makes it appear larger in SEC than a globular protein of the same molecular weight. It is therefore not clear if oligomers of ∼16, ∼30, and ∼50 kDa correspond to dimers, tetramers, and hexamers of Aβcc, respectively, or if some other stoichiometry applies. However, when also considering that disordered regions may remain in the ∼100-kDa β-sheet oligomers, these should contain at least 12 monomer subunits. Hence, a dodecamer stoichiometry of toxic Aβ oligomers that has been favored in other research (4, 28–30) is consistent with our data.
Electron microscopy images indicate that β-sheet oligomers of ∼6 nm in diameter assemble into protofibrils of variable lengths, thereby implying that the protofibrils are composed of β-sheet oligomers. Aβcc protofibrils are morphologically very similar to wild-type protofibrils. These protofibrils are also recognized in the mAb158 monoclonal antibody ELISA (22), which is conformation-specific for protofibrils of wild-type Aβ42.
With regard to the structural differences between β-sheet and LMW oligomers, it is possible that the latter either do not form β-hairpins with intramolecular hydrogen bonds (despite the disulfide) or that hydrogen-bonded β-hairpins do not assemble into larger β-sheet structures in LMW oligomers.
Differences Between Aβ40 and Aβ42 Aggregation and Toxicity.
We find that Aβ42cc more readily forms β-sheet oligomers than Aβ40cc. This occurs as a result of the presence of a kinetic barrier to oligomer formation that can only be overcome by Aβ40cc with the aid of heating and concentration. Wild-type Aβ42 is considered to be more toxic than Aβ40. It is possible that this difference in toxicity reflects different barriers to β-sheet oligomer formation that we observe here with Aβcc.
Several studies also indicate differences in the aggregation pathways of wild-type Aβ42 and Aβ40 (29) and multiple pathways for amyloid formation (1). We propose an aggregation scheme (Fig. 5) in which monomeric Aβ can form disordered LMW oligomers or larger β-sheet oligomers, and that LMW oligomers can convert into β-sheet oligomers. β-Sheet oligomers can associate into protofibrils recognized in the mAb158 ELISA. LMW oligomers, on the other hand, can also aggregate into A11-binding species, which cannot be formed by β-sheet oligomers or protofibrils. β-Sheet oligomer formation by Aβ40cc is slower than by Aβ42cc, and this results in different populations of the two peptides (at a given concentration) aggregating along the LMW oligomer → A11-binding HMW-aggregate pathway and the mAb158-binding β-sheet oligomer → protofibril pathway.
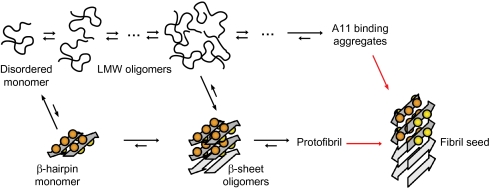
Fig. 5.
Aβ aggregation via two pathways. One pathway involves LMW oligomers without regular secondary structure and eventually large nonfibrillar aggregates binding the A11 antibody (coil pathway; Upper) and the other involves assembly into β-sheet oligomers, or coil oligomers that are converted into β-sheet oligomers, which are building blocks of mAb158 binding protofibrils (β-sheet pathway; Lower). Neurotoxic Aβ aggregates are formed along the β-sheet pathway. The scheme is consistent with the present studies of Aβcc and overall features can be reconciled with a large body of work on wild-type and naturally occurring Aβ mutants as discussed in the text. Red arrows reflect the interconversion of Aβ subunits from β-hairpin conformation in soluble aggregates to cross-β structure in fibril seeds and mature amyloid fibrils.
It is not clear from which aggregate fibril seeds are formed in Aβcc after the addition of reducing agent. However, protofibrils have been suggested to constitute the penultimate intermediate in amyloid fibril formation (1, 10), and fibril formation can also occur in solutions containing A11-binding aggregates (31). In the reaction scheme in Fig. 5, we therefore allow for seed formation by both these aggregates. Still, the aggregation equilibria also allow for other possibilities, such as seeding directly from small β-sheet oligomers as suggested previously (17), or by secondary nucleation events (32).
Possible Link Between β-Sheet Oligomer Formation and Early-Onset AD.
Arguments relating toxicity to aggregation pathway may be extended to include inherited mutations in Aβ that are linked to higher toxicity and early-onset AD. For example, Aβ with the Arctic E22G mutation forms protofibrils more rapidly than wild-type Aβ (11), which in our aggregation scheme is equivalent to a higher rate of β-sheet oligomer formation (aggregation along the β-sheet pathway). In accordance with such a mechanism, the AβE22G peptide more easily forms SDS-resistant higher-order aggregates (20), which we also associate to β-sheet oligomers. An experimental linkage between kinetic partitioning of mutant Aβ peptides into different aggregation pathways and their toxicity would, if it can be further substantiated, constitute an attractive mechanism to rationalize the relationship between inherited mutations and early-onset AD.
Conclusions
We have engineered an Aβ peptide variant (Aβcc) in which a β-hairpin is stabilized by a disulfide bond. Aβcc forms oligomeric aggregates in which conformational and biological properties of wild-type Aβ oligomers are preserved, and amyloid formation is prevented. Aβ42cc and Aβ40cc both aggregate via two distinct pathways that can be distinguished using conformation-specific antibodies. The coil pathway involves formation of less structured LMW oligomers with apparent molecular weights of 50 kDa or less, which can aggregate further into nonfibrillar HMW species. The β-sheet aggregation pathway involves initial formation of structured β-sheet oligomers with an apparent molecular weight of 100 kDa or more, which also contain SDS-stable dimeric and trimeric units. β-Sheet oligomers can associate into protofibrils that are morphologically identical to wild-type protofibrils and detected in a mAb158 antibody ELISA, which is specific for wild-type protofibrils. Aggregates on the β-sheet aggregation pathway are neurotoxic and induce neuronal apoptosis. A kinetic barrier for formation of β-sheet oligomers makes Aβ40cc more prone to aggregate along the coil pathway, but once formed, β-sheet oligomers of Aβ40cc induce neuronal apoptosis as well. Kinetic partitioning into two aggregation pathways in which one contains the neurotoxic aggregates may explain why Aβ40 is less toxic than Aβ42 and why certain Aβ mutations lead to early onset AD.
Materials and Methods
Complete discussions of molecular modeling, peptide production and purification, SDS/PAGE, fibril formation assays, CD, infrared spectroscopy, TEM, atomic force microscopy, mAb158 ELISA, A11 dot blot, and neurotoxicity assays are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor Andreas Barth at Stockholm University for providing expertise on FTIR spectroscopy. This work was supported by Grants 2008-3475 (to T.H.) and 2006-2822 (to L.L.) from the Swedish Research Council, grants from the Medical Research Council and Engineering and Physical Sciences Research Council (to C.M.D. and L.M.L.), grants from the Wellcome Trust (to C.M.D), and grants from Hjärnfonden, Alzheimerfonden, and Uppsala University Hospital (to L.L.). T.H. and A.S. are part of the Mucosal Immunobiology and Vaccine Center supported by the Swedish Foundation for Strategic Research.
Footnotes
Conflict of interest statement: A.S. and T.H. are shareholders of MIVAC Development AB, Gothenburg, Sweden.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001740107/-/DCSupplemental.
References
- 1.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. Amyloid beta-protein assembly and Alzheimer disease. J Biol Chem. 2009;284:4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesné S, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 5.De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 6.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gellermann GP, et al. Abeta-globulomers are formed independently of the fibril pathway. Neurobiol Dis. 2008;30:212–220. doi: 10.1016/j.nbd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang THJ, et al. Structural studies of soluble oligomers of the Alzheimer β-amyloid peptide. J Mol Biol. 2000;297(1):73–87. doi: 10.1006/jmbi.2000.3559. [DOI] [PubMed] [Google Scholar]
- 10.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 11.Nilsberth C, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 12.Maji SK, Wang L, Greenwald J, Riek R. Structure-activity relationship of amyloid fibrils. FEBS Lett. 2009;583:2610–2617. doi: 10.1016/j.febslet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Nelson R, et al. Structure of the cross-β spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou L, et al. Solution NMR studies of the A β(1-40) and A β(1-42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. J Am Chem Soc. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 15.Mitternacht S, Staneva I, Härd T, Irbäck A. Comparing the folding free-energy landscape of Aβ42 variants with different aggregation properties. Proteins. 2010;78:2600–2608. doi: 10.1002/prot.22775. [DOI] [PubMed] [Google Scholar]
- 16.Grönwall C, et al. Selection and characterization of Affibody ligands binding to Alzheimer amyloid β peptides. J Biotechnol. 2007;128(1):162–183. doi: 10.1016/j.jbiotec.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer W, Grönwall C, Jonsson A, Ståhl S, Härd T. Stabilization of a β-hairpin in monomeric Alzheimer’s amyloid-β peptide inhibits amyloid formation. Proc Natl Acad Sci USA. 2008;105:5099–5104. doi: 10.1073/pnas.0711731105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, et al. Structural characterization of a soluble amyloid β-peptide oligomer. Biochemistry. 2009;48:1870–1877. doi: 10.1021/bi802046n. [DOI] [PubMed] [Google Scholar]
- 19.Cerf E, et al. Antiparallel β-sheet: A signature structure of the oligomeric amyloid β-peptide. Biochem J. 2009;421:415–423. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- 20.Macao B, et al. Recombinant amyloid beta-peptide production by coexpression with an affibody ligand. BMC Biotechnol. 2008;8:82. doi: 10.1186/1472-6750-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsbury C, Frey P, Olivieri V, Aebi U, Müller SA. Multiple assembly pathways underlie amyloid-β fibril polymorphisms. J Mol Biol. 2005;352:282–298. doi: 10.1016/j.jmb.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Englund H, et al. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]
- 23.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 24.Lam AR, Teplow DB, Stanley HE, Urbanc B. Effects of the Arctic (E22—>G) mutation on amyloid beta-protein folding: Discrete molecular dynamics study. J Am Chem Soc. 2008;130:17413–17422. doi: 10.1021/ja804984h. [DOI] [PubMed] [Google Scholar]
- 25.Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE. The Alzheimer’s peptides Abeta40 and 42 adopt distinct conformations in water: A combined MD / NMR study. J Mol Biol. 2007;368:1448–1457. doi: 10.1016/j.jmb.2007.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habicht G, et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Abeta protofibrils. Proc Natl Acad Sci USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barghorn S, et al. Globular amyloid beta-peptide oligomer—a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viola KL, Velasco PT, Klein WL. Why Alzheimer’s is a disease of memory: The attack on synapses by A beta oligomers (ADDLs) J Nutr Health Aging. 2008;12(1):51S–57S. doi: 10.1007/BF02982587. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y-R, Glabe CG. Distinct early folding and aggregation properties of Alzheimer amyloid-beta peptides Abeta40 and Abeta42: Stable trimer or tetramer formation by Abeta42. J Biol Chem. 2006;281:24414–24422. doi: 10.1074/jbc.M602363200. [DOI] [PubMed] [Google Scholar]
- 32.Knowles TPJ, et al. An analytical solution to the kinetics of breakable filament assembly. Science. 2009;326:1533–1537. doi: 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.