Abstract
The process of nutrient transfer through an ecosystem is an important determinant of production, food-chain length, and species diversity. The general view is that the rate and efficiency of nutrient transfer up the food chain is constrained by herbivore-specific capacity to secure N-rich compounds for survival and production. Using feeding trials with artificial food, we show, however, that physiological stress-response of grasshopper herbivores to spider predation risk alters the nature of the nutrient constraint. Grasshoppers facing predation risk had higher metabolic rates than control grasshoppers. Elevated metabolism accordingly increased requirements for dietary digestible carbohydrate-C to fuel-heightened energy demands. Moreover, digestible carbohydrate-C comprises a small fraction of total plant tissue-C content, so nutrient transfer between plants and herbivores accordingly becomes more constrained by digestible plant C than by total plant C:N. This shift in herbivore diet to meet the altered nutrient requirement increased herbivore body C:N content, the C:N content of the plant community from which grasshoppers select their diet, and grasshopper fecal C:N content. Chronic predation risk thus alters the quality of animal and plant tissue that eventually enters the detrital pool to become decomposed. Our results demonstrate that herbivore physiology causes C:N requirements and nutrient intake to become flexible, thereby providing a mechanism to explain context dependence in the nature of trophic control over nutrient transfer in ecosystems.
Keywords: ecological stoichiometry, metabolism, nutrient balance, physiological stress, predator–prey interaction
Trophic dynamics—the transfer of energy and materials among consumers in ecosystems—govern primary and secondary production, food-chain length, trophic biomass, and species diversity (1–4). Current theory holds that the primary constraint on terrestrial trophic dynamics arises from the mismatch between herbivore nutritional demands and the nutritional quality of plant resources (5–7). Herbivores often have high demands for N-rich proteins for growth and reproduction and must regulate body nutrient contents within low C:N levels (5, 8, 9), but their plant resources contain high levels of indigestible carbohydrates and limiting levels of N-rich proteins (i.e., high C:N ratio) (10, 11). In this view, the rate and efficiency of nutrient transfer up the food chain is constrained by herbivore-specific capacity to secure N-rich compounds.
However, herbivores occupy intermediate levels within food chains and, thus, must often trade-off plant nutrient consumption against predation risk (12, 13). Consequently, predation risk may represent an additional constraint on trophic transfer. Predation risk induces stress, and stress is known to alter organismal physiological nutrient demand and balance (14). Accordingly, we develop the case here that consideration of physiological effects of predation risk on herbivore nutrient demand may represent an integrative way to explore how predators may control trophic dynamics. The general stress paradigm holds that prey exposed to elevated risk of predation increase their mass-specific metabolism (15–18) and allocate resources from growth and reproduction to functions that increase survivorship (19). In nutrient-limited systems, increased energetic demands for maintenance may increase the overall demand for digestible carbohydrate-C, and thereby lower the quantity of energy that can be allocated to production (20–23). Consequently, stress-induced constraints on herbivore production should lower the demand for N-rich proteins (24). Herbivores also have low capacity to store excess nutrients (24), and hence should seek plants with high digestible carbohydrate content to minimize the costs of ingesting and excreting excess N. Such stress-induced shift in nutrient demand may be especially important in terrestrial systems in which digestible carbohydrate represents a small fraction of total plant carbohydrate-C, and may be limiting even under risk-free conditions (25). Moreover, stress responses include break down of body proteins to produce glucose (i.e., gluconeogenesis) (14), which requires excretion of N-rich waste compounds (ammonia or primary amines) (26). Such rebalancing of nutrient intake and allocation in response to predation risk should influence nutrient transfer by altering the C and N content of herbivore body tissue, herbivore excreta and egesta, and plant resources.
We evaluated this prediction using a widely distributed generalist grasshopper herbivore (Melanoplus femurrubrum) that is known to face a trade-off between the need to select nutrients from a mixture of grassland grass and herb species while avoiding risk of predation by a hunting spider (Pisuarina mira) (27). Experimentation with this model system in northeastern Connecticut has shown that grasshoppers routinely alter their diets as predation risk from P. mira increases and leads to substantial changes in N-cycling (28). Our purpose here is to reveal the mechanistic basis for such an outcome, and thereby meet the broader need to understand the general role of predators on herbivore nutrient intake and consequent trophic dynamics (12, 29, 30).
We conducted metabolic measurements and laboratory feeding experiments with artificial diets to resolve why grasshoppers change their nutrient intake in response to predation risk. To explore possible consequences to ecosystem functions, we reared M. femurrubrum grasshoppers in the field in the absence and presence of predation risk, and subsequently measured their respiration rates, body C:N content, and waste material. Finally we estimated the consequences of predation risk on elemental availability and cycling within the ecosystem.
Results
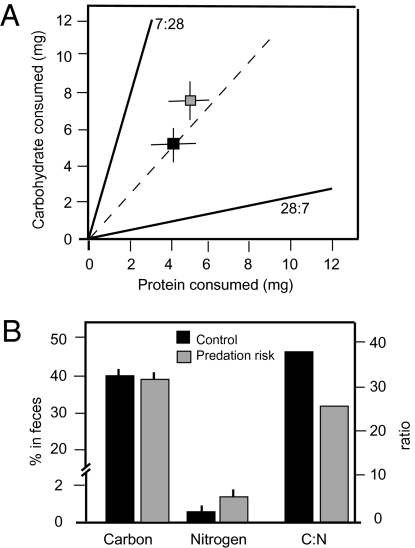
We conducted feeding experiments to discern the nutritional consequences of predation risk. In a 5-wk feeding trial with artificial diets, grasshoppers were randomly allocated to a control (no predator presence) or risk treatment (presence of P. mira spiders), and both groups were offered a choice between two diets. The first diet had high (28%) digestible, protein-low (7%) digestible carbohydrate. We also provided the exact opposite low (7%) digestible protein-high digestible (28%) carbohydrate diet to ensure that grasshoppers had the flexibility to ingest (i.e., were unconstrained relative to potential needs) protein and carbohydrate in any of a wide potential of ratios from 4:1 to 1:4. Metabolic rates (mean ± 1 SE mL CO2 min−1) of grasshoppers facing predation risk (17.5 ± 1.27) were 32% higher (ANCOVA with body mass covariate: F1,15 = 5.06, P = 0.04) than metabolic rates of control grasshoppers (13.2 ± 1.42). Grasshoppers in the risk treatment had slightly elevated digestible-protein intake relative to control conditions (Fig. 1A), but the difference was not significant (P = 0.236). However, grasshoppers in the risk treatment consumed 40% more (ANOVA: F1,32 = 7.22, P = 0.014) digestible carbohydrate than control grasshoppers (Fig. 1A). Grasshoppers in control and risk conditions had similar fecal C contents. However, grasshoppers in the risk conditions released 40% more N than in control conditions (Fig. 1B). This result in turn led to grasshoppers in the risk treatment having 65% lower C:N in fecal material (higher quality fecal matter) than grasshoppers in control conditions (Fig. 1B).
Fig. 1.
Grasshopper nutrient intake and excretion during feeding trials with artificial diets. The experiment was conducted to discern the dietary response of grasshoppers to predation risk. (A) Grasshoppers were presented diets with either 7% protein and 28% digestible carbohydrate or 28% protein and 7% digestible carbohydrate. The solid lines represent intake rails (the balance of nutrients contained in diets) that define the boundaries of the nutritional space for all potential nutrient intake (55). The dashed line represents the nutrient intake target revealed by grasshoppers feeding on the two different diets. In the absence of predation risk, grasshoppers preferred a diet with 1:1 ratio of protein and carbohydrate intake rather than a diet matching their body elemental ratio. Grasshoppers facing risk consumed slightly higher amounts of dietary N than control grasshoppers, and consumed much greater dietary C. (B) Grasshoppers in the feeding trials excreted similar levels of C, reflecting higher respiration of C in risk conditions than in control conditions. Consequently, excess N intake in risk conditions is released in the feces. This result leads to differences in fecal C:N content between risk and control conditions. Values are mean ± SE.
Metabolic rates (mean ±1 SE mL CO2 min−1) of grasshoppers reared in the field for 30 d under chronic predation risk (15.9 ± 0.86) were 40% higher (ANCOVA F1,31 = 12.86, P = 0.001) than grasshoppers reared in no-risk control conditions (11.3 ± 0.91). The body N content of grasshoppers reared under chronic risk of predation (11.4 ± 0.08) was significantly lower (F1,62 = 8.87, P = 0.004) than grasshoppers reared in control (11.7 ± 0.07) conditions. There were no differences (F1,62 = 0.07, P = 0.787) in the C contents of grasshoppers reared under chronic risk of predation (46.9 ± 0.23) and grasshoppers reared in control conditions (46.9 ± 0.22). Consequently, the body C:N ratios of grasshoppers reared under chronic risk of predation (4.1 ± 0.02) were higher (F1,62 = 11.66, P < 0.001) than of grasshoppers reared in control conditions (4.0 ± 0.02). There were no differences (P > 0.25) in grasshopper body mass or length between predation risk and control conditions.
The fecal N content of grasshoppers reared under chronic risk of predation (3.8 ± 0.23) did not differ from grasshoppers reared in control (3.7 ± 0.22) conditions. However, C contents of grasshoppers reared under chronic risk of predation (44.1 ± 0.22) was higher than of grasshoppers reared in control conditions (41.1 ± 0.24) (F1,62 = 9.21, P < 0.001). This process resulted in 5% higher (F1,62 = 3.95, P = 0.05) fecal C:N ratios of grasshoppers reared under chronic risk of predation (11.6 ± 0.20) than of grasshoppers reared in control conditions (11.1 ± 0.16).
In the study system, grasshopper diet-shift in response to predation risk can alter plant species composition via selective foraging for certain plant species and by mitigating plant-plant competition (27). We calculated the consequences of shifting species composition on total plant C:N content. We measured the C and N contents of individual herb species that comprise the grassland plant community. Those values, in conjunction with measured differences in plant species composition between risk and risk-free treatments (Table S1) resulted in an estimated 10% increase (F1,12 = 31.7, P < 0.001) in C:N content of herbs in risk treatments compared with control conditions.
Discussion
Theory about consumer-nutrient limitation and trophic transfer in terrestrial ecosystems suggests that C occurs in excess, whereas N and P are the most limiting to herbivore nutrition, population dynamics, and ecosystem impacts (3, 5, 6). This conclusion is drawn from observations of large mismatches in gross elemental C relative to N and P content between herbivore body tissue and plant resources, and the questionable assumption (31) that there is a homeostatic C:N ratio that does not depend on food-web structure. Our results suggest that this perspective gives an incomplete picture of herbivore-nutrient limitation because not all available C is in a nutritionally useable form (9, 30) and that both nutrient requirements and body C:N can be variable between environmental conditions (31). Indeed, in our particular case, grasshoppers freely chose intake levels that exacerbated C ingestion and lead to heightened N release in feces. The proportional digestible carbohydrate-C intake in risk treatments appeared to be driven by the proportional increase in metabolic rate caused by predation risk. This finding offers support to the idea (9, 25) that, in addition to being protein-N-limited, terrestrial grazing herbivores may, under certain conditions, also be energy- (i.e., digestible carbohydrate) limited.
The observed changes in grasshopper nutritional balance are consistent with predictions that are based on the physiological-stress paradigm, with one exception. We predicted that grasshopper reared under chronic risk of predation should release more N in their feces. Indeed, measurements in the artificial diet experiment reveal 40% increase in feces N. However, grasshoppers reared under chronic risk of spider predation in the field released feces with relatively higher C contents (and hence, higher C:N) compared with grasshoppers reared in risk-free environments. Grasshopper feces include egesta and excretions. Thus, the observed differences between the laboratory and the field may result from higher amounts of indigestible structural carbohydrates in natural forages of grasshoppers, especially plants that were selected under risky conditions. Understanding the link between stress, resource quality, and nutrient intake and release represents an important frontier in analyses of herbivore nutrition (26, 30) that may further add to understanding context-dependency in ecosystem-nutrient dynamics.
The mechanism causing the diet-shift identified here has implication for advancing foraging theory because it differs from mechanisms of diet-shift assumed by current theory predicting predation risk effects in food chains (e.g., 32–34). This theory is based on the argument that prey decrease the risk of predation by altering their foraging time budgets (e.g., reduce movement activity, increase vigilance), seeking temporal and spatial foraging refuges, and consuming resources that require less handling time. In all of these cases, predation risk is assumed to impose constraints on prey by directly forcing them to make alternative resource choices—a nutrient cost of predation risk (35). This theory implies that risky conditions force prey into a situation leading to less favorable nutrient intake, and hence nutrient imbalance. This theory further assumes that factors that mediate the risk of predation (e.g., habitat structure or time), and not variation in the nutritional value of alternative resources, is what should govern prey diet-shifts. We show, however, that the shift in resource consumption resulting from prey stress-response to predation risk is not the consequence of a directly imposed predator constraint, but rather an active choice by prey to achieve a new nutrient balance that matches the new nutritional requirements that accompany the adaptive stress-responses.
Our work expands upon previous understanding (36, 37) that the nutrient content of body tissue is not only constrained by dietary nutrient composition, but may vary with constraints imposed by internal physiological state in response to predation risk. Moreover, the magnitude of within-species variation in body elemental content is comparable to the magnitude across species variation observed in terrestrial insect species (31). Together, these insights suggest that body C:N within a species may be quite malleable, as opposed to being a fixed species property (5, 8), thereby requiring reconsideration of how trophic transfer of material and energy between plants and herbivore is constrained.
The feeding response of grasshoppers in response to predation risk has broader implications for re-examining the nature of trophic dynamics in ecosystems (13). This is because trophic dynamics may not be strictly regulated by factors impacting herbivores at the population scale, such as the distribution and abundance of plant resources or predation pressure, as is currently treated by much ecological theory (12, 13). Rather, understanding trophic dynamics, and hence ecosystem-scale properties, may require perspectives that consider the emergent consequences of individual-scale herbivore behavioral and physiological plasticity in response to predation risk (12, 21, 38).
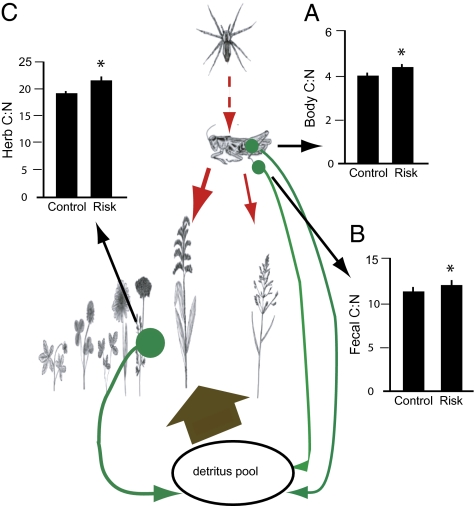
To this end, the physiological mechanism we document here may alter the nutritional quality of material input to the soil detritus pool via three pathways (Fig. 2). Chronic predation risk leads to lower N content in herbivore body tissue, and thus a 6% higher C:N ratio than control conditions, available for decomposition. Chronic predation also leads to a concomitant of 7% higher fecal C content, and thus a 5% higher feces C:N ratio than in control conditions (Fig. 2B). Finally, chronic predation risk leads to a reduction in the quality (10% higher C:N content than in control conditions) of herb plant tissue that eventually becomes litter. This reduction may in turn alter the functioning of below-ground communities and consequently change decomposition rates (39, 40). At this stage, we cannot make general predictions about the direction or magnitude of predator-induced changes in detrital quality on soil-nutrient pools because the impacts of quality and quantity of organic materials on below-ground processes and properties is an area of debate in soil ecology (41, 42). Nonetheless, the magnitude of observed differences in quality of plant and animal tissues between control and risk conditions can translate into a lowering of N-mineralization rates by 60% (43). Chronic predation risk may also have effects that flow up the food chain by reducing the nutrient quality of herbivore prey available to predators, consistent with theoretical predictions (44).
Fig. 2.
Pathways through which C:N change impacts the ecosystem. Changes in herbivore physiological demand for C and N in response to predation risk can lead to shifts in C:N content of organic material along four pathways that eventually lead to the organic-matter pool to be decomposed. (A) C:N composition of herbivore body tissue. (B) C:N composition of herbivore feces. (C) Changes in C:N content of the herb community as a result of mediation of Solidago-competitive dominance by herbivory in the face of predation risk. Solid red arrows indicate direct trophic interactions; dashed red arrows indicate a nontrophic fear effect. Arrow thickness indicates food preference under risk conditions. Green arrows indicate the source and fate of tissue C and N in the ecosystem. Values are mean ± SE. *, significant difference (P < 0.05).
The findings reported here derive predominately from laboratory experiments using a model system. Nevertheless, the identified physiological mechanism can explain the basis for the consistently observed diet-shift exhibited by M. femurrubrum grasshoppers in response to predation risk by P. mira spiders under field conditions. This diet-shift in turn explains patterns in plant-species composition and soil-nutrient properties within the grassland ecosystem in which these species naturally reside (cf. 27, 28, 45). Thus, we show that a research program that combines largely reductionist laboratory and field studies can provide complementary insights that enable the scaling from individual-level behavior and physiology to whole-ecosystem functioning. More generally, predation risk is a ubiquitous feature of trophic dynamics in terrestrial landscapes (46–48), as is heightened stress caused by fear of predation (46) and flexible diet choice in response to such a stressor (26, 29), suggesting that the mechanism we observed here may be scalable to other types of systems.
It is widely understood in community ecology that trophic interactions are controlled by the interplay between bottom-up (nutrient supplies) and top-down (predation) processes. Still, ecosystem ecology has been less inclined to embrace this conceptualization, preferring instead to emphasize a dominance of bottom-up nutrient control of ecosystem processes, such as nutrient dynamics (but see refs. 49–51). Our study shows that by controlling the relative flow of nutrients up the food chain to top predators and down the food chain to the organic-matter pool, herbivore physiological responses to environmental context can help to integrate nutrient dynamics with herbivore behavior in such a way that makes top-down and bottom-up trophic control of ecosystem function synergistic, thereby providing the means to integrate community and ecosystem perspectives about the control of nutrient dynamics. This finding leads to the testable hypothesis that trophic dynamics are neither bottom-up- nor top-down-regulated, but instead may be an emergent property of herbivore regulation of nutrient balance that emanates from the middle of the food chain (12, 38).
Materials and Methods
Artificial Diet Experiment.
We created two synthetic diets (52) that had opposite digestible carbohydrate and protein ratios by mass (7:28 vs. 28:7). We captured 22 third-instar grasshopper nymphs in the field and immediately transferred them to the laboratory. We weighed the grasshoppers and placed them individually in plastic terraria, in which we placed two dishes of synthetic diet (52) and a 29.6-mL plastic water container. One-half of the terraria were randomly designated a risk treatment that contained a transparent plastic cylinder (5 cm depth × 13 cm height) with a screen lid containing a water container and an individual Pisaurina spider fed with grasshopper nymphs. We placed the terraria in random locations on shelving exposed to natural light in the laboratory. We placed opaque partitions between the terraria. We varied protein (p) and digestible carbohydrate (c) to produce the following diet proportions: p7:c28; p28:c7 (all values are expressed on a percentage dry-weight basis). We weighed each food dish and food to the nearest 0.1 mg. We placed 10 additional food dishes in empty plastic terraria to account for humidity changes. Every 5 d we removed the food dishes, weighed them, and returned them. We collected feces from all terraria, freeze-dried them for 48 h, ground them to homogenous powder, and measured their C and N content using a CHN autoanalyzer (with ascorbic acid as a primary standard). We also measured the grasshopper metabolic rate (see details below). We ended the experiment after 5 wk when most grasshopper larvae were fifth instar.
Grasshopper Metabolic-Rate Measurements.
We measured grasshopper standard metabolic rate as the rate of carbon dioxide emission (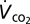 ) in an incurrent flow-through system. Air was analyzed by an infra-red CO2 analyzer (Qubit S151; 1 ppm resolution) after water vapor was removed. We used an airflow rate of 200 mL/min. Average ambient temperature within the respirometry chamber was 23.4 ± 0.3 °C. Following food deprivation of 16 h (water was available), we weighed grasshoppers (± 0.1 mg), placed in a transparent 50 mL (9.2 cm length × 2.0 cm depth) metabolic chamber and allowed them to recover from handling for at least 10 min before measurements commenced. Most animals ceased exploratory movements within 5 min after insertion into the chamber and subsequently remained immobile. We measured the mean minimal steady-state
) in an incurrent flow-through system. Air was analyzed by an infra-red CO2 analyzer (Qubit S151; 1 ppm resolution) after water vapor was removed. We used an airflow rate of 200 mL/min. Average ambient temperature within the respirometry chamber was 23.4 ± 0.3 °C. Following food deprivation of 16 h (water was available), we weighed grasshoppers (± 0.1 mg), placed in a transparent 50 mL (9.2 cm length × 2.0 cm depth) metabolic chamber and allowed them to recover from handling for at least 10 min before measurements commenced. Most animals ceased exploratory movements within 5 min after insertion into the chamber and subsequently remained immobile. We measured the mean minimal steady-state 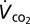 for 10 min. The analyzer provides fractional CO2 concentration (parts-per-million), yet standard metabolic rate should be reported as a rate, so we transformed the recordings as
for 10 min. The analyzer provides fractional CO2 concentration (parts-per-million), yet standard metabolic rate should be reported as a rate, so we transformed the recordings as 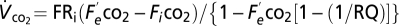 , where
, where 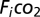 = incurrent fractional concentration of CO2;
= incurrent fractional concentration of CO2; 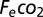 = excurrent fractional concentration of CO2; FR = flow rate (mL min−1); RQ = respiratory quotient, assumed equal to 0.85 in herbivorous animals (53). For metabolic measurements of field-reared grasshoppers, we captured 34 fourth-fifth instar grasshopper nymphs from the field mesocosms (see below) and immediately transferred them to individual 7 × 2 × 15-cm clear plastic chambers in the laboratory.
= excurrent fractional concentration of CO2; FR = flow rate (mL min−1); RQ = respiratory quotient, assumed equal to 0.85 in herbivorous animals (53). For metabolic measurements of field-reared grasshoppers, we captured 34 fourth-fifth instar grasshopper nymphs from the field mesocosms (see below) and immediately transferred them to individual 7 × 2 × 15-cm clear plastic chambers in the laboratory.
Field-Rearing Experimental Design.
We placed 30 pairs of cylindrical 0.25 m2 × 1 m mesocosms over natural vegetation in a meadow at the Yale-Myers Research Forest in northeastern Connecticut. We stocked six second-instar M. femurrubrum grasshopper nymphs to each mesocosm. One day later, we added one adult P. mira spider to a randomly assigned mesocosm in each pair. We rendered spiders ineffective at subduing prey to induce only risk effects by gluing their chelicerae with nontoxic, quick-drying cement. This process does not change spider activity and grasshoppers do not seem to distinguish between manipulated and unmanipulated spiders (54). After 30 d, we collected and immediately placed grasshoppers to individual containers in the laboratory, according to pair and cage of origin, for metabolic rate measurement and measurements of body-percent C and N content by mass.
Grasshopper Body C:N Content.
We evaluated field-rearing conditions on C:N content of 67 fourth-fifth instar grasshopper nymphs collected from the field mesocosms. We reduced variation because of recent food consumption by removing grasshopper gut contents under dissecting microscope. We freeze-dried the empty gut and body for 48 h, ground them to homogenous powder, and measured their percent C and N contents by mass.
Grasshopper Fecal C:N Content.
We collected feces from individual grasshoppers that were collected from the two rearing conditions and housed individually in plastic chambers for metabolic measurements (see above). All feces that were released by an individual during the 16-h fasting period before metabolic measurements was collected and freeze-dried for 48 h. Each individual's feces was ground to a homogenous powder and measured for percent C and N content by mass.
Calculation of Herb Plant C:N Potentially Entering the Soil Organic-Matter Pool.
We collected random leaves from several herb species that comprise the meadow community (Table S2) and analyzed their C:N contents. We used the abundance of each species in control and risk conditions based on data compiled from previous experiments (27) and their associated C:N contents to calculate the total C and N contained in the herb community. The field experiments testing for ecosystem effects of predation risk used nondestructive plant-sampling techniques to avoid biasing C:N via vegetation clipping. We therefore sampled herb species outside of experimental areas and subjected plant samples (five independent leaves for each species) to C:N analysis. C and N values for the plants are presented in Table S2. M. femurrubrum grasshoppers at the study trade-off the need to select nutrients from a mixture of grassland grass (specifically Poa pratensis) and herb species (specifically goldenrod Solidago rugosa) when avoiding hunting spider (P. mira) predators. Experimentation has shown that these grasshoppers routinely increase their dietary proportion, especially of the competitively dominant plant S. rugosa, as predation risk from P. mira increases (27). We therefore considered S. rugosa and other herb species as two functional groups as regards herbivore foraging. We then calculated the C and N contribution of the species within each functional group (weighed by their proportion of total abundance in the herb community) to the organic matter available for decomposition under risk-free and risk conditions. The values for replicates within treatments and average values are presented in Table S1.
Supplementary Material
Acknowledgments
We thank A. Beckerman, S. Behmer, M. Bradford, P. Raymond, and G. Trussell for their helpful advice and discussion, and A. Dagaeff, F. Douglas, K. Hughes, J. Price, and A. Whitney, who helped with field and laboratory work. This work was supported US National Science Foundation Grant 0515014 (to O.J.S.) and a Donnelley Environmental Postdoctoral fellowship, Yale Institute for Biospheric Studies (to D.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009300107/-/DCSupplemental.
References
- 1.Lindeman RL. The trophic-dynamic aspect of ecology. Ecology. 1942;23:399–418. [Google Scholar]
- 2.Post DM. The long and short of food-chain length. Trends Ecol Evol. 2002;17:269–277. [Google Scholar]
- 3.Gruner DS, et al. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol Lett. 2008;11:740–755. doi: 10.1111/j.1461-0248.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat. 1959;93:145–159. [Google Scholar]
- 5.Elser JJ, et al. Nutritional constraints in terrestrial and freshwater food webs. Nature. 2000;408:578–580. doi: 10.1038/35046058. [DOI] [PubMed] [Google Scholar]
- 6.Hillebrand H, et al. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol Lett. 2009;12:516–527. doi: 10.1111/j.1461-0248.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 7.Sterner RW, Elser JJ. Ecological Stoichiometry. Princeton: Princeton University Press; 2002. [Google Scholar]
- 8.Fagan WF, et al. Nitrogen in insects: Implications for trophic complexity and species diversification. Am Nat. 2002;160:784–802. doi: 10.1086/343879. [DOI] [PubMed] [Google Scholar]
- 9.Raubenheimer D, Simpson SJ, Mayntz D. Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct Ecol. 2009;23:4–16. [Google Scholar]
- 10.Karasov WH, Martinez del Rio C. Physiological Ecology. NJ: Princeton Press University Princeton; 2007. [Google Scholar]
- 11.Robbins CT. Wildlife Feeding and Nutrition. San Diego: Academic Press; 1983. [Google Scholar]
- 12.Schmitz OJ. Herbivory from individuals to ecosystems. Annu Rev Ecol Syst. 2008;39:133–152. [Google Scholar]
- 13.Schmitz OJ. Resolving Ecosystem Complexity. Princeton: Princeton University Press; 2010. [Google Scholar]
- 14.Wingfield JC, Ramenofsky M. Hormones and the behavioral ecology of stress. In: Baum PHM, editor. Stress Physiology in Animals. Sheffield, UK: Sheffield Academic Press; 1999. pp. 1–51. [Google Scholar]
- 15.Woodley CM, Peterson MS. Measuring responses to simulated predation threat using behavioral and physiological metrics: The role of aquatic vegetation. Oecologia. 2003;136:155–160. doi: 10.1007/s00442-003-1236-1. [DOI] [PubMed] [Google Scholar]
- 16.Sunardi, Asaeda T, Manatunge J. Physiological responses of topmouth gudgeon, Pseudorasbora parva, to predator cues and variation of current velocity. Aquat Ecol. 2007;41:111–118. [Google Scholar]
- 17.Slos S, Stoks R. Predation risk induces stress proteins and reduces antioxidant defense. Funct Ecol. 2008;22:637–642. [Google Scholar]
- 18.Beckerman AP, Wieski K, Baird DJ. Behavioural versus physiological mediation of life history under predation risk. Oecologia. 2007;152:335–343. doi: 10.1007/s00442-006-0642-6. [DOI] [PubMed] [Google Scholar]
- 19.Wingfield JC, et al. Ecological bases of hormone-behavior interactions: The “emergency life history stage.”. Am Zool. 1998;38:191–206. [Google Scholar]
- 20.DuRant SE, Hopkins WA, Talent LG. Energy acquisition and allocation in an ectothermic predator exposed to a common environmental stressor. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:442–448. doi: 10.1016/j.cbpc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Trussell GC, Ewanchuk PJ, Matassa CM. The fear of being eaten reduces energy transfer in a simple food chain. Ecology. 2006;87:2979–2984. doi: 10.1890/0012-9658(2006)87[2979:tfober]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.McPeek MA. The growth/predation risk trade-off: So what is the mechanism? Am Nat. 2004;163:E88–E111. doi: 10.1086/382755. [DOI] [PubMed] [Google Scholar]
- 23.McPeek MA, Grace M, Richardson JML. Physiological and behavioral responses to predators shape the growth/predation risk trade-off in damselflies. Ecology. 2001;82:1535–1545. [Google Scholar]
- 24.Sterner RW. Modelling interactions of food quality and quantity in homeostatic consumers. Freshw Biol. 1997;38:473–481. [Google Scholar]
- 25.Anderson TR, Boersma M, Raubenheimer D. Stoichiometry: Linking elements to biochemicals. Ecology. 2004;85:1193–1202. [Google Scholar]
- 26.Christianson D, Creel S. A nutritionally mediated risk effect of wolves on elk. Ecology. 2010;91:1184–1191. doi: 10.1890/09-0221.1. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz OJ. Top predator control of plant biodiversity and productivity in an old-field ecosystem. Ecol Lett. 2003;6:156–163. [Google Scholar]
- 28.Schmitz OJ. Predators have large effects on ecosystem properties by changing plant diversity, not plant biomass. Ecology. 2006;87:1432–1437. doi: 10.1890/0012-9658(2006)87[1432:phleoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc Biol Sci. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behmer ST. Insect herbivore nutrient regulation. Annu Rev Entomol. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- 31.Bertram SM, Bowen M, Kyle M, Schade JD. Extensive natural intraspecific variation in stoichiometric (C:N:p) composition in two terrestrial insect species. J Insect Sci. 2008;8:1–7. doi: 10.1673/031.008.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrams PA. Foraging time optimization and interactions in food webs. Am Nat. 1984;124:80–96. [Google Scholar]
- 33.Krivan V, Schmitz OJ. Trait and density mediated indirect interactions in simple food webs. Oikos. 2004;107:239–250. [Google Scholar]
- 34.Bolker B, Holyoak M, Krivan V, Rowe L, Schmitz O. Connecting theoretical and empirical studies of trait-mediated interactions. Ecology. 2003;84:1101–1114. [Google Scholar]
- 35.Hawlena D, Pérez-Mellado V. Change your diet or die: Predator-induced shifts in insectivorous lizard feeding ecology. Oecologia. 2009;161:411–419. doi: 10.1007/s00442-009-1375-0. [DOI] [PubMed] [Google Scholar]
- 36.Perkins MC, Woods HA, Harrison JF, Elser JJ. Dietary phosphorus affects the growth of larval Manduca sexta. Arch Insect Biochem Physiol. 2004;55:153–168. doi: 10.1002/arch.10133. [DOI] [PubMed] [Google Scholar]
- 37.Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol Lett. 2003;6:96–101. [Google Scholar]
- 38.Schmitz OJ, et al. From individuals to ecosystem function: Toward an integration of evolutionary and ecosystem ecology. Ecology. 2008;89:2436–2445. doi: 10.1890/07-1030.1. [DOI] [PubMed] [Google Scholar]
- 39.Aerts R. Nitrogen partitioning between resorption and decomposition pathways: A trade-off between nitrogen use efficiency and litter decomposibility? Oikos. 1997;80:603–606. [Google Scholar]
- 40.Taylor BR, Parkinson D, Parsons WFJ. Nitrogen and lignin content as predictors of litter decay rates: A microcosm test. Ecology. 1989;70:97–104. [Google Scholar]
- 41.Bradford MA, Fierer N, Reynolds JF. Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct Ecol. 2008;22:964–974. [Google Scholar]
- 42.Hattenschwiler S, Tiunov AV, Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Syst. 2005;36:191–218. [Google Scholar]
- 43.Schmitz OJ. Effects of predator functional diversity on grassland ecosystem function. Ecology. 2009;90:2339–2345. doi: 10.1890/08-1919.1. [DOI] [PubMed] [Google Scholar]
- 44.Abrams PA. Predators that benefit prey and prey that harm predators: Unusual effects of interacting foraging adaptations. Am Nat. 1992;140:573–600. [Google Scholar]
- 45.Schmitz OJ, Kalies EL, Booth MG. Alternative dynamic regimes and trophic control of plant succession. Ecosystems (N Y) 2006;9:659–672. [Google Scholar]
- 46.Creel S, Christianson D. Relationships between direct predation and risk effects. Trends Ecol Evol. 2008;23:194–201. doi: 10.1016/j.tree.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz OJ, Krivan V, Ovadia O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol Lett. 2004;7:153–163. [Google Scholar]
- 48.Heithaus MR, Wirsing AJ, Burkholder D, Thomson J, Dill LM. Towards a predictive framework for predator risk effects: The interaction of landscape features and prey escape tactics. J Anim Ecol. 2009;78:556–562. doi: 10.1111/j.1365-2656.2008.01512.x. [DOI] [PubMed] [Google Scholar]
- 49.Loreau M. Consumers as maximizers of matter and energy flow in ecosystems. Am Nat. 1995;145:22–42. [Google Scholar]
- 50.DeAnglis DL. Dynamics of Nutrient Cycling and Food Webs. London: Chapman and Hall; 1992. [Google Scholar]
- 51.Carpenter SR, Cottingham KL, Schindler DE. Biotic feedbacks in lake phosphorus cycles. Trends Ecol Evol. 1992;7:332–336. doi: 10.1016/0169-5347(92)90125-U. [DOI] [PubMed] [Google Scholar]
- 52.Behmer ST, Joern A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc Natl Acad Sci USA. 2008;105:1977–1982. doi: 10.1073/pnas.0711870105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogowitz GL, Chappell MA. Energy metabolism of eucalyptus-boring beetles at rest and during locomotion: Gender makes a difference. J Exp Biol. 2000;203:1131–1139. doi: 10.1242/jeb.203.7.1131. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz OJ, Beckerman AP, Obrien KM. Behaviorally mediated trophic cascades: Effects of predation risk on food web interactions. Ecology. 1997;78:1388–1399. [Google Scholar]
- 55.Raubenheimer D, Simpson SJ. The geometry of compensatory feeding in the locust. Anim Behav. 1993;45:953–964. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.