Abstract
NAD(P)H oxidases (Noxs) produce O2− and play an important role in cardiovascular pathophysiology. The Nox4 isoform is expressed primarily in the mitochondria in cardiac myocytes. To elucidate the function of endogenous Nox4 in the heart, we generated cardiac-specific Nox4−/− (c-Nox4−/−) mice. Nox4 expression was inhibited in c-Nox4−/− mice in a heart-specific manner, and there was no compensatory up-regulation in other Nox enzymes. These mice exhibited reduced levels of O2− in the heart, indicating that Nox4 is a significant source of O2− in cardiac myocytes. The baseline cardiac phenotype was normal in young c-Nox4−/− mice. In response to pressure overload (PO), however, increases in Nox4 expression and O2− production in mitochondria were abolished in c-Nox4−/− mice, and c-Nox4−/− mice exhibited significantly attenuated cardiac hypertrophy, interstitial fibrosis and apoptosis, and better cardiac function compared with WT mice. Mitochondrial swelling, cytochrome c release, and decreases in both mitochondrial DNA and aconitase activity in response to PO were attenuated in c-Nox4−/− mice. On the other hand, overexpression of Nox4 in mouse hearts exacerbated cardiac dysfunction, fibrosis, and apoptosis in response to PO. These results suggest that Nox4 in cardiac myocytes is a major source of mitochondrial oxidative stress, thereby mediating mitochondrial and cardiac dysfunction during PO.
Keywords: cardiac hypertrophy, NAD(P)H oxidase, superoxide, mitochondria
Oxidative stress plays an important role in regulating a wide variety of cellular functions, including gene expression, cell growth, and death (1). Reactive oxygen species (ROS) posttranslationally modulate signaling molecules and transcription factors (2, 3). A number of molecules in the heart are subjected to oxidative posttranslational modification (OPTM), and their functions, such as enzymatic activity and subcellular localization, are regulated by OPTM (4). For example, conserved cysteine resides in class II histone deacetylases (HDACs) are oxidized in response to hypertrophic stimuli, thereby leading to the nuclear export of HDACs and cardiac hypertrophy (5). Furthermore, mitochondrial proteins containing the “iron-sulfur cluster” involved in oxidative phosphorylation are extremely sensitive to OPTM (4).
Oxidative stress in the heart is increased in response to hypertrophy and heart failure. It has been suggested that increases in oxidative stress in the failing heart are primarily due to the functional uncoupling of the respiratory chain caused by inactivation of complex I (6). However, increased ROS in the failing myocardium may also be due to impaired antioxidant capacity, such as reduced activity of Cu/Zn superoxide dismutase and catalase (7), or stimulation of enzymatic sources, including xanthine oxidase, cyclooxygenase, nitric oxide synthase, and nonphagocytic NAD(P)H oxidases (Noxs). Noxs are major enzymes responsible for production of superoxide (O2−) by transferring electrons across the membrane from NAD(P)H to molecular oxygen (8, 9). In a guinea pig model of progressive left ventricular (LV) hypertrophy, expression of p22phox, p67phox, p47phox, and Nox2, and Nox activity are up-regulated during the progression of cardiac hypertrophy to failure (10). Activation of Nox was also observed in human heart failure, as evidenced by enhanced p47phox staining in the sarcolemmal membrane (11). Previous studies with systemic Nox2 knockout mice have suggested that Nox2 is required for induction of cardiac hypertrophy by angiotensin II but not by pressure overload (PO) (reviewed in ref. 12). This observation has suggested the involvement of another Nox isoform in oxidative stress and cardiac dysfunction during PO. The Nox4 isoform is expressed in a wide variety of organs, including the heart (13). Using a newly developed specific anti-Nox4 monoclonal antibody and transgenic mice with cardiac-specific overexpression of Nox4, we have recently shown (i) that Nox4 is expressed primarily in mitochondria in cardiac myocytes, (ii) that expression of Nox4 is up-regulated in response to PO, and (iii) that increased expression of Nox4 in transgenic mice enhances oxidative stress and cardiac dysfunction (13). Importantly, however, the involvement of endogenous Nox4 in oxidative stress and cardiac dysfunction has not been demonstrated yet because of the absence of specific loss-of-function animal models for Nox4.
Mitochondria are the major source of ROS during aging and heart failure (6, 14, 15). Increased production of ROS in the failing heart leads to mitochondrial permeability transition (mPT) (16), which causes matrix swelling, outer membrane rupture, release of apoptotic signaling molecules, such as cytochrome c, from the intermembrane space, and irreversible injury to the mitochondria (17). Up-regulation of Nox4 may have a direct influence on increases in mitochondrial oxidative stress and consequent mitochondrial dysfunction and cell death during heart failure. Importantly, NAD(P)H oxidase has been assumed to be unlikely as a source of ROS in the mitochondria (18–21).
Thus, important goals in this work were (i) to demonstrate the involvement of Nox4 in oxidative stress at baseline and in response to PO, (ii) to elucidate the role of Nox4 in mediating pathological hypertrophy in response to PO, and (iii) to show the importance of endogenous Nox4 in mediating OPTM and regulating the function of mitochondrial proteins during heart failure, using genetically altered mouse models.
Results
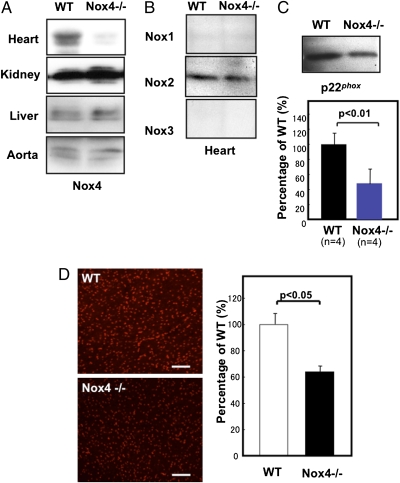
We generated mice with cardiac-specific deletion of Nox4 (c-Nox4−/−) (Fig. S1). Although protein expression of Nox4 was detected in the heart, kidneys, liver, and aorta in wild type (WT) mice, it was attenuated (−78%) selectively in the heart in c-Nox4−/− mice (Fig. 1A). Expression of Nox2 was not significantly affected in c-Nox4−/− compared with WT mice, and neither Nox1 nor Nox3 was detected in WT or c-Nox4−/− mouse hearts (Fig. 1B), indicating that no compensatory up-regulation of other Nox enzymes occurs. Expression of p22phox protein, but not mRNA, was reduced to about 50% in c-Nox4−/− mice, consistent with the notion that Nox stabilizes p22phox (Fig. 1C and Fig. S2 A and B) (18).
Fig. 1.
Expression of Nox4, Nox1-3, and p22phox, and the level of O2− in c-Nox4−/− mouse hearts. (A) Expression of Nox4 protein in the heart and other organs in c-Nox4−/− mice. (B) Expression of other members of the Nox family in the heart. (C) Expression of p22phox in the heart. (Lower) shows quantification of p22phox expression. (D) The level of O2− production in the heart at baseline was evaluated with dihydroethidium (DHE) staining. (Scale bars, 50 μm.) In A–D, the results shown are representative of three to five experiments.
Dihydroethidium staining of LV myocardial sections, a relatively specific indicator of O2−, was significantly weaker in c-Nox4−/− mouse hearts than in WT ones (Fig. 1D). The O2− producing activity of the whole heart was also evaluated by lucigenin chemiluminescent assay. The O2− dismutase (SOD)-inhibitable chemiluminescent activity was significantly lower in c-Nox4−/− hearts than in WT ones (Fig. S2C). These results suggest that endogenous Nox4 plays a critical role in mediating O2− production in the heart at baseline.
We have previously shown that Nox4 is partially localized in mitochondria in cardiac myocytes (13). To further evaluate the functional significance of Nox4 in mitochondria, we obtained microsomal, cytosolic, and mitochondrial fractions from heart homogenates of WT and c-Nox4−/− mice, confirmed the purity of each fraction as described previously (13), and then conducted lucigenin chemiluminescent assays. The detected SOD-inhibitable component of chemiluminescence ranged from greatest to least in the order of mitochondria, microsomes, and cytosol in WT mice, consistent with the previous report (13), but was significantly attenuated in the microsomal and mitochondrial fractions prepared from c-Nox4−/− mice (Fig. S2D). These results suggest that Nox4 plays an important role in mediating O2− production primarily in mitochondria and modestly in the microsomes.
Despite significantly attenuated O2− production, the cardiac phenotype was normal in c-Nox4−/− mice at baseline. Specifically, organ weights, echocardiographically evaluated cardiac dimensions and left ventricular function, and histological findings in the heart in c-Nox4−/− mice were not significantly different from those in WT mice (Table S1).
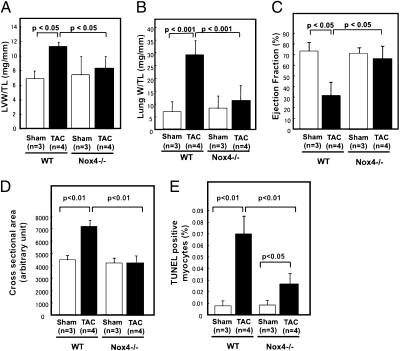
We have previously shown that protein expression of Nox4 in the heart is up-regulated in response to PO (13). We further investigated the role of endogenous Nox4 in the heart in mediating cardiac hypertrophy and LV dysfunction in response to PO. We applied transverse aortic constriction (TAC) to WT and c-Nox4−/− mice for 4 wk. The mortality rate was significantly higher (P < 0.05) in WT (31%) than in c-Nox4−/− mice (14%). The pressure gradient across the TAC did not differ significantly between WT and c-Nox4−/− mice at 4 wk (Fig. S3A). As expected, in WT mice PO significantly increased LV weight/tibial length (LVW/TL), which was accompanied by signs of heart failure, namely, an increase in lung weight/tibial length (LungW/TL) and decrease in echocardiographically evaluated LV ejection fraction (EF) and fractional shortening. However, changes in these parameters in response to PO were significantly attenuated in c-Nox4−/− mice (vs. Δ in WT mice, −74, −83, −90, and −69%, respectively) (Fig. 2 A–C and Fig. S3 B and C), suggesting that endogenous Nox4 plays an essential role in mediating cardiac hypertrophy and LV dysfunction in response to PO. TAC tended to increase LVW/TL and LungW/TL in Nox2−/− mice (Fig. S4), suggesting that the function of Nox during PO is isoform-dependent. TAC-induced increases in LV myocyte cross-sectional area were abolished (vs. Δ in WT mice, −98%) in c-Nox4−/− mice (Fig. 2D and Fig. S5A). TAC-induced increases in LV interstitial fibrosis and TUNEL positive cardiac myocytes were significantly attenuated (−51 and −68%, respectively) in c-Nox4−/− mice (Fig. 2E and Fig. S5B). These results suggest that endogenous Nox4 plays an essential role in mediating cardiac hypertrophy, fibrosis, and myocardial cell death in response to PO.
Fig. 2.
The role of endogenous Nox4 in mediating various cardiac phenotypes in response to PO. c-Nox4−/− mice and WT littermates were subjected to either TAC or sham operation. (A) LVW/TL and (B) LungW/TL in response to 4 wk TAC were determined. (C) Ejection fraction, an index of LV systolic function, was obtained echocardiographically. (D) LV myocyte cross-sectional area was evaluated histologically. (E) Apoptosis was evaluated by TUNEL staining. In A–E, bar graphs indicate mean ± SEM obtained from three to five experiments.
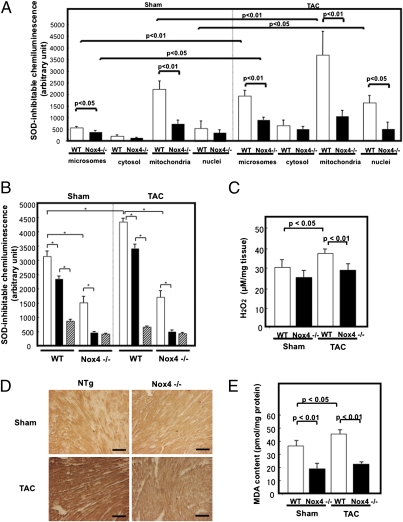
To evaluate the role of Nox4 in mediating oxidative stress during heart failure, mice were subjected to TAC for 4 wk. Subcellular fractionation and immunoblot analyses showed that Nox4 expression in cardiac mitochondria, microsomes, and nucleus is significantly increased by TAC for 4 wk, and that up-regulation of Nox4 after TAC is attenuated in c-Nox4−/− mice (Fig. S6). Lucigenin chemiluminescent assays indicated that the SOD-inhibitable component of the O2− production in mitochondrial microsomal and nuclear fractions in WT mouse hearts is significantly elevated after TAC. However, increases in O2− production in these fractions in response to TAC were attenuated significantly in c-Nox4−/− mice (Fig. 3A). Because electron leakage from the respiratory chain could be another source of O2− production in isolated mitochondria, O2− production was further analyzed in the presence of NADH and rotenone, an inhibitor of mitochondrial respiratory chain complex I, and diphenylene iodonium (DPI), an inhibitor of Nox and other flavin containing enzymes. Rotenone treatment partially reduced the mitochondrial SOD-inhibitable O2− production, and the addition of DPI further reduced it in WT mice with either sham operation or TAC, suggesting that O2− originates from both complex I and flavin containing oxidases. In c-Nox4−/− mice, rotenone significantly reduced the mitochondrial SOD-inhibitable O2− production, but the addition of DPI failed to reduce it further (Fig. 3B). TAC-induced increases in O2− production in the presence of succinate and antimycin A, which represents O2− produced by complex III, were lower than those in O2− derived from NADH and similar in WT and c-Nox 4−/− mice (Fig. S7A). Interestingly, in WT mice, TAC primarily increased the DPI-sensitive component of O2− production rather than the rotenone-sensitive one. Furthermore, cardiac-specific deletion of Nox4 completely abolished the TAC-induced increase in the DPI-sensitive component of O2− production, thereby preventing an overall increase in O2− production from mitochondria. Taken together, these results suggest that Nox4, rather than electron leakage from the respiratory chain, plays a major role in mediating increases in mitochondrial O2− production in response to PO. The O2− produced by Nox4 is rapidly converted to H2O2, which may also be generated directly by Nox4. Amplex Red assays showed that the production of H2O2 in cardiac muscle is significantly increased by PO in control but not in c-Nox4−/− hearts (Fig. 3C), suggesting that endogenous Nox4 critically regulates the level of H2O2 in the heart under PO conditions. Further investigation is required to determine whether or not Nox4 directly produces H2O2.
Fig. 3.
The level of oxidative stress in response to PO. c-Nox4−/− mice and WT littermates were subjected to either TAC for 4 wk or sham operation. (A) Microsomal, cytosolic, mitochondrial, and nuclear fractions were prepared from WT and c-Nox4−/− mouse hearts. NADH-dependent and SOD-inhibitable O2− release was measured by the lucigenin chemiluminescent method. (B) Effects of rotenone and DPI on O2− production by the mitochondrial fraction (*P < 0.05). (C) The level of H2O2 in LV blocks from WT and c-Nox4−/− mouse hearts was evaluated with the Amplex Red method. (D) LV myocardial sections obtained from WT and c-Nox4−/− mice were subjected to immunostaining with anti–8-OHdG antibody. (Scale bars, 50 μm.) (E) MDA content was measured in heart homogenates from WT and c-Nox4−/− mice. In A, B, C, and E, bar graphs indicate mean ± SEM obtained from three to five experiments.
The TAC-induced increases in 8-OHdG staining, an indicator of oxidative DNA damage, in LV myocardium were significantly attenuated in c-Nox4−/− mice (Fig. 3D). Both MDA and MDA + 4-HAE contents, indicators of lipid peroxidation, in the LV myocardium at baseline and after TAC were also attenuated in c-Nox4−/− mice (Fig. 3E and Fig. S7B). These results suggest that endogenous Nox4 plays an essential role in mediating oxidative stress in the heart under PO.
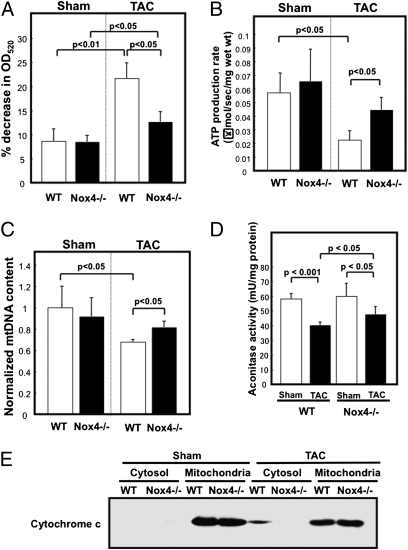
Because Nox4 critically regulates the level of oxidative stress in mitochondria, we hypothesized that Nox4 promotes mitochondrial dysfunction during PO. To test this hypothesis, we evaluated mitochondrial function in WT and c-Nox4−/− mice with or without PO. First, isolated mitochondria were challenged with calcium overload and the rate of mitochondrial swelling was evaluated by light scattering, where decreases in the absorbance reflect passive swelling of the mitochondrial matrix. TAC-induced decreases in absorbance were significantly inhibited in c-Nox4−/− mice compared with WT mice (Fig. 4A), suggesting that TAC-induced mitochondrial swelling, most likely caused by mPTP opening, is attenuated in c-Nox4−/− mice. Second, the rate of ATP production was significantly decreased in WT mice in response to TAC but was well maintained in c-Nox4−/− mice (Fig. 4B). Third, the amount of mitochondrial DNA in the heart after TAC was significantly greater in c-Nox4−/− than in WT mice (Fig. 4C). Fourth, TAC-induced suppression of aconitase activity, a well established marker of mitochondrial oxidative damage, was significantly attenuated in c-Nox4−/− mice (Fig. 4D). Finally, the TAC-induced release of cytochrome c into the cytosolic fraction seen in WT mice was significantly attenuated in c-Nox4−/− mice (Fig. 4E). These results suggest that endogenous Nox4 plays an essential role in mediating mitochondrial dysfunction in the heart during pathological hypertrophy.
Fig. 4.
Mitochondrial function is preserved in c-Nox4−/− mice during PO. The cardiac mitochondrial fraction was prepared from c-Nox4−/− mice and WT littermates subjected to TAC or sham operation. (A–D) Mitochondrial function was evaluated by the mitochondrial swelling assay (A), ATP production assay (B), quantitative real-time PCR for mitochondrial DNA (C), and aconitase assay (D). (E) Release of cytochrome c into the cytosol was evaluated with immunoblot analyses. In A–D, bar graphs indicate mean ± SEM obtained from three to four experiments.
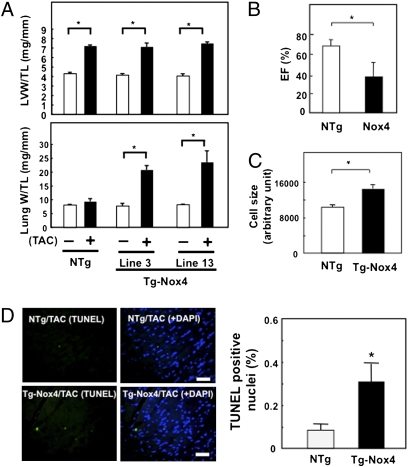
To examine whether enhanced expression of Nox4 during PO stimulates cardiac dysfunction, transgenic mice with cardiac-specific overexpression of Nox4 (Tg-Nox4) (13) were subjected to TAC for 2 wk. The cardiac phenotype of Tg-Nox4 subjected to sham operation was normal at 3–4 mo. Although TAC did not enhance cardiac hypertrophy, as determined by LVW/TL, TAC significantly increased LungW/TL in two lines of Tg-Nox4 mice compared with nontransgenic (NTg) mice (Fig. 5A). Echocardiographic measurements showed that up-regulation of Nox4 induces LV dilation and reduces LV systolic function in response to TAC (Fig. 5B and Fig. S8A). Taken together, these results indicate that up-regulation of Nox4 stimulates TAC-induced cardiac dysfunction without significant changes in cardiac hypertrophy at the organ level. Histological analyses indicated that overexpressed Nox4 increased LV myocyte cross-sectional area, as determined by anti-wheat germ agglutinin (WGA) staining (Fig. 5C and Fig. S8B), fibrosis, as determined by PASR staining (Fig. S8C), and apoptosis, as determined by TUNEL staining (Fig. 5D), in response to PO. Thus, increased expression of Nox4 enhances pathological changes in the heart, such as fibrosis and apoptosis, and enlargement of remaining cardiac myocytes without increases in overall heart weight. Although the number of cycling α-actinin positive cells as evaluated by Ki67 staining was significantly increased after TAC in nontransgenic hearts, it did not change in c-Nox4−/− hearts (Fig. S8D). Because Nox4 overexpression was driven by the α-myosin heavy chain (αMHC) promoter, Nox4 should be overexpressed in both cardiac myocytes and cardiac progenitor cells (22). Thus, the enhancement of TAC-induced LV dysfunction and the apparent lack of cardiac hypertrophy in Tg-Nox4 may be in part mediated through suppression of myocyte renewal as well.
Fig. 5.
The effects of aortic banding in Tg-Nox4 mouse hearts. Tg-Nox4 mice and NTg littermates were subjected to either TAC or sham operation for 2 wk. (A) LVW/TL and LungW/TL. (B) LV ejection fraction (%), an index of LV systolic function, was evaluated echocardiographically. (C) LV myocyte cross-sectional area was evaluated by WGA staining. (Scale bars, 50 μm.) (D) Apoptosis was quantitated with TUNEL staining. (Scale bars, 50 μm.) In A–D, bar graphs indicate mean ± SEM obtained from four to eight experiments (*P < 0.05).
Discussion
Our results suggest that Nox4 localized in mitochondria in cardiac myocytes is a major source of O2− production in the heart. In particular, up-regulation of Nox4 is primarily responsible for the increased mitochondrial O2− production in response to PO in the mouse heart. Furthermore, Nox4 plays a critical role in mediating mitochondrial dysfunction, apoptosis in cardiac myocytes, and eventual LV dysfunction in response to PO. To our knowledge, the role of endogenous Nox4 in mediating oxidative stress and pathological hypertrophy has not been clearly demonstrated thus far.
Our loss-of-function mouse model uniquely allowed us to demonstrate the role of endogenous Nox4 in mediating O2− production in mitochondria. Although mitochondrial O2− production consists of both rotenone- and DPI-sensitive components, because the DPI-sensitive component is abolished in c-Nox4−/− mice, Nox4 must represent the DPI-sensitive component of O2− production. Expression of Nox4 in mitochondria is up-regulated in response to PO, thereby contributing to increases in O2− production in mitochondria and overall enhancement of oxidative stress in cardiac myocytes. Importantly, PO primarily increased the DPI-sensitive component of O2− production in mitochondria in WT mice, whereas the increase in O2− production under PO was abolished in c-Nox4−/− without significant changes in the rotenone-sensitive O2− production. Although electron leakage from complex I of the electron transport chain is responsible for an increase in oxidative stress under mechanical loading and during heart failure (6), our results clearly suggest that mitochondrial Nox4 plays a major role in mediating increased production of O2− from mitochondria in failing hearts.
O2− produced in mitochondria is rapidly converted into H2O2, which is readily diffusible in the intracellular space. However, increased production of O2− and/or H2O2 from mitochondrial Nox4 effectively oxidizes mitochondrial proteins, including aconitase (23). Here we demonstrate that both oxidative modification and consequent functional impairment of mitochondrial proteins during PO were significantly attenuated in c-Nox4−/− mouse hearts. Thus, endogenous Nox4 localized in mitochondria plays an important role in mediating mitochondrial dysfunction. Because mitochondrial dysfunction facilitates electron leakage, thereby inducing ROS-induced ROS release (24), down-regulation of Nox4 may reduce oxidative stress not only by reducing its own O2− production but also by inhibiting mitochondrial dysfunction. Although mitochondrial O2− presumably produced by complex I and III during PO was not significantly attenuated in c-Nox4−/− mice under our experimental conditions, it may become more prominent and Nox4-dependent during later stages of heart failure.
The biological toxicity of O2− is due to its capacity to inactivate the “iron-sulfur cluster” containing enzymes (25), thereby liberating free iron in the cell, which can undergo Fenton chemistry and generate the highly reactive hydroxyl radical. TAC-induced inhibition of aconitase, an enzyme in the TCA cycle, was attenuated in c-Nox4−/− mice. Because Nox4 preferentially utilizes NADH as an electron donor (26), these results not only support our hypothesis that Nox4 is involved in mitochondrial oxidative stress but also raise an exciting possibility that Nox4 directly regulates the NADH/FADH2 generating enzymes in the TCA cycle, thereby initiating regulatory feedback mechanisms controlling its O2− producing activity.
Combined use of a specific monoclonal antibody (13) and c-Nox4−/− mice allowed us to show that expression of Nox4 in mitochondria is up-regulated by PO. Because down-regulation of Nox4 attenuates and up-regulation of Nox4 facilitates LV dysfunction in response to PO, these results suggest that Nox4 is necessary and sufficient for mediating LV dysfunction in response to PO. Cardiac-specific overexpression of Nox4 enhances cardiac myocyte apoptosis and increases cardiac myocyte size without enhancing LVW/TL in response to PO. Nox4 also suppresses PO-induced increases in the number of cycling cardiac myocytes/progenitor cells (Fig. S8D). We have shown previously that overexpression of Nox4 in cardiac myocytes induces apoptosis but not cardiac hypertrophy in vitro (13). Thus, we speculate that up-regulation of Nox4 primarily induces cardiac myocyte death and/or inhibits replenishment with newly generated cardiac myocytes in response to PO, which in turn causes LV dysfunction and compensatory hypertrophy of the remaining myocytes. By inference, we speculate that down-regulation of Nox4 prevents cardiac myocyte death and/or enhances cardiac myocyte renewal, thereby preserving LV function.
Because Nox4 was either up- or down-regulated in a cardiac-specific manner in our models, the extent of cardiac fibrosis is regulated by Nox4 expressed in cardiac myocytes. However, Nox4 expressed in cardiac fibroblasts may also play an important role in mediating cardiac fibrosis by stimulating myofibroblast differentiation (27). In fact, our preliminary results suggest that overexpression of Nox4 induces proliferation of cardiac fibroblasts (Fig. S8E). Thus, cardiac myocytes may not be the only cell type in the heart affected by Nox4 in the development of pathological hypertrophy. Targeting Nox4 may inhibit pathological hypertrophy by acting through multiple cell types in the heart.
Although expression of other Nox isoforms, primarily Nox2, in the heart does not seem to be affected in c-Nox4−/− mice, protein expression of p22phox, a heterodimeric partner of Nox, is reduced to about 50%. Because p22phox is stabilized by the presence of Nox in the membrane (18), the reduced level of p22phox protein but not mRNA in c-Nox4−/− mice is most likely due to the absence of its heterodimeric partner. Consistently, protein expression of p22phox was also reduced in Nox2−/− mice (Fig. S2B). It is formally possible that down-regulation of p22phox affects the activity of other Nox isoforms, including Nox2. However, we believe that this possibility is remote because Nox2 and Nox4 have distinct subcellular localizations. Furthermore, c-Nox4−/− mice exhibited a cardiac phenotype in response to TAC distinct from that of Nox2−/− mice (28, 29) (Fig. S4), indicating the specificity of isoform-specific down-regulation.
In summary, our results suggest that Nox4 in cardiac myocytes is a critical mediator of oxidative stress and cardiac dysfunction during PO. Because of its mitochondrial localization and up-regulation, Nox4 is a major source of O2− and/or H2O2 production in the heart, thereby playing an important role in mediating mitochondrial dysfunction during PO. We propose that Nox4 could be a target of future treatments for heart failure.
Methods
Generation of c-Nox4−/− Mice.
We generated Nox4−/− mice using lox-P and homologous recombination strategies (Fig. S1). These mice were backcrossed into a C57BL/6 background. Cardiac-specific deletion of Nox4 was achieved using αMHC-Cre (30). Tg-Nox4 mice were generated on an FVB background with the αMHC promoter (Courtesy of J. Robbins, Children's Hospital, Cincinnati, OH). Systemic Nox2−/− mice were purchased from Jackson Laboratory. All protocols concerning the use of animals were approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey.
Fractionation of the Mouse Heart.
Isolated mouse hearts were homogenized in 10 volumes of ice-cold Buffer A [200 mmol/L mannitol, 50 mmol/L sucrose, 10 mmol/L KCl, 1 mmol/L EDTA, 10 mmol/L Hepes-KOH (pH 7.4), 0.1% BSA, and a mixture of protease inhibitors]. Homogenates were centrifuged at 600 × g for 5 min at 4 °C. Supernatants were then centrifuged at 3,500 × g for 15 min at 4 °C. The pellets were resuspended in Buffer A and centrifuged at 1,500 × g for 5 min. The supernatants were centrifuged at 5,500 × g for 10 min at 4 °C, and then the pellets were suspended as the mitochondrial fraction in PBS containing protease inhibiters. The resultant supernatant was further centrifuged at 100,000 × g for 60 min, and the pellet and the supernatant were used as microsomal and cytosolic fractions, respectively. A nuclear fraction was prepared from mouse hearts with NE-PER Nuclear Extraction Reagent (Thermo Scientific).
Lucigenin Chemiluminescent Assays.
Mouse whole heart homogenates or cytosolic, mitochondrial, microsomal, and nuclear fractions were suspended in 200 μL of an assay buffer composed of 100 mmol/L potassium phosphate (pH 7.0), 10 μmol/L flavin adenine dinucleotide (FAD), 1 mmol/L NaN3, and 1 mmol/L EGTA. After preincubation with 5 μmol/L lucigenin, NADH or NADPH was added to a final concentration of 500 μmol/L (23). The chemiluminescence was continuously monitored using a luminometer. The reaction was terminated by the addition of SOD (100 μg/mL) (13).
Statistical Analysis.
All values are expressed as mean ± SEM. Statistical analyses between groups were done by unpaired Student's t test or one-way ANOVA followed by a post hoc Fisher's comparison test. A value of P < 0.05 was accepted as significant.
Supplementary Material
Acknowledgments
We thank Daniela Zablocki for critical reading of the manuscript. This work was supported in part by Public Health Service Grants HL59139, HL67724, HL69020, HL91469, and AG27211.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002178107/-/DCSupplemental.
References
- 1.Dimmeler S, Zeiher AM. A “reductionist” view of cardiomyopathy. Cell. 2007;130:401–402. doi: 10.1016/j.cell.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Sadoshima J. Redox regulation of growth and death in cardiac myocytes. Antioxid Redox Signal. 2006;8:1621–1624. doi: 10.1089/ars.2006.8.1621. [DOI] [PubMed] [Google Scholar]
- 3.Filomeni G, Rotilio G, Ciriolo MR. Disulfide relays and phosphorylative cascades: Partners in redox-mediated signaling pathways. Cell Death Differ. 2005;12:1555–1563. doi: 10.1038/sj.cdd.4401754. [DOI] [PubMed] [Google Scholar]
- 4.Fu C, et al. Quantitative analysis of redox-sensitive proteome with DIGE and ICAT. J Proteome Res. 2008;7:3789–3802. doi: 10.1021/pr800233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ago T, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Ide T, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 7.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol. 1994;266:H1280–H1285. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 9.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 10.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 11.Heymes C, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 12.Cave AC, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 13.Ago T, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ide T, et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 15.Kinugawa S, et al. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 16.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 17.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 18.Ambasta RK, et al. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 19.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 21.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottage CT, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ago T, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 24.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42:14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 26.Shiose A, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 27.Cucoranu I, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 28.Byrne JA, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 29.Maytin M, et al. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 30.Agah R, et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.