Abstract
Context
Prediction models to identify healthy individuals at high risk of cardiovascular disease have limited accuracy. A low ankle brachial index is an indicator of atherosclerosis and has the potential to improve prediction.
Objective
To determine if the ankle brachial index provides information on the risk of cardiovascular events and mortality independently of the Framingham Risk Score and can improve risk prediction.
Data Sources
Relevant studies were identified by collaborators. A search of MEDLINE (1950 to February 2008) and EMBASE (1980 to February 2008), was conducted using common text words for the term ‘ABI’ combined with text words and Medical Subject Headings to capture prospective cohort designs. Review of reference lists and conference proceedings, and correspondence with experts was conducted to identify additional published and unpublished studies.
Study Selection
Studies were included if (1) participants were derived from a general population (2) ankle brachial index was measured at baseline and (3) subjects were followed up to detect total and cardiovascular mortality.
Data Extraction
Pre-specified data on subjects in each selected study were extracted into a combined dataset and an individual participant data meta-analysis conducted on subjects who had no previous history of coronary heart disease.
Results
Sixteen population cohort studies fulfilling the inclusion criteria were included. During 480,325 person years of follow up of 24,955 men and 23,339 women, the risk of death by ankle brachial index had a reverse J shaped distribution with a normal (low risk) ankle brachial index of 1.11 to 1.40. The 10-year cardiovascular mortality (95% CI) in men with a low ankle brachial index (≤ 0.90) was 18.7% (13.3% to 24.1%) and with normal ankle brachial index (1.11 to 1.40) was 4.4% (3.2% to 5.7%), hazard ratio (95% CI) 4.2 (3.5 to 5.4). Corresponding mortalities in women were 12.6% (6.2% to 19.0%) and 4.1% (2.2% to 6.1%), hazard ratio 3.5 (2.4 to 5.1). The hazard ratios remained elevated on adjusting for Framingham Risk Score, 2.9 (2.3 to 3.7) for men and 3.0 (2.0 to 4.4) for women. A low ankle brachial index (≤0.90) was associated with approximately twice the 10-year total mortality, cardiovascular mortality and major coronary event rate compared with the overall rate in each Framingham category. Inclusion of the ankle brachial index in cardiovascular risk stratification using the Framingham Risk Score would result in reclassification of the risk category and modification of treatment recommendations in approximately 19% of men and 36% of women.
Conclusion
Measurement of the ankle brachial index may improve the accuracy of cardiovascular risk prediction beyond the Framingham Risk Score. Development and validation of a new risk equation incorporating the ankle brachial index is warranted.
Introduction
Major cardiovascular and cerebrovascular events including myocardial infarction and stroke often occur in individuals without known pre-existing cardiovascular disease. The prevention of such events, including the accurate identification of those at risk,1 remains a serious public health challenge. Scoring equations to predict those at increased risk have been developed using cardiovascular risk factors, including cigarette smoking, blood pressure, total and HDL cholesterol, and diabetes mellitus. The Framingham Risk Score (FRS)2,3 is often considered the reference standard but has limited accuracy, tending to over-estimate risk in low risk populations and under-estimate in high risk populations.4 The incorporation of other risk markers, such as metabolic syndrome5 and plasma C-reactive protein (C-RP)6,7 has had partial success in improving prediction and attention is also being given to indicators of asymptomatic atherosclerosis, such as coronary artery calcium (CAC), carotid intima media thickness (IMT) and the ankle brachial index (ABI).1
The ABI, which is the ratio of systolic pressure at the ankle to that in the arm, is quick and easy to measure and has been used for many years in vascular practice to confirm the diagnosis and assess the severity of peripheral artery disease in the legs. However, the ABI is also an indicator of generalised atherosclerosis because lower levels have been associated with higher rates of concomitant coronary and cerebrovascular disease, and with the presence of cardiovascular risk factors.8 Also, in population cohort studies in the USA9-12 and Europe,13-17 a low ABI has been related to an increased incidence of mortality (total and cardiovascular), myocardial infarction and stroke. These increased relative risks have been shown to be independent of baseline cardiovascular disease and risk factors, suggesting that the ABI might have an independent role in predicting cardiovascular events.
The objective of our study was to determine if the ABI provides information on the risk of cardiovascular events and mortality independently of the Framingham Risk Score and can improve risk prediction. In order to enhance the representativeness of our study and to maximise subject numbers, we formed the Ankle Brachial Index (ABI) Collaboration with the intent of including all major observational studies which had investigated longitudinally the ABI and incidence of cardiovascular events and mortality in general populations. At the same time we wished to identify a normal (low risk) level of the ABI which might be used in future studies and in clinical practice.
Methods
The study design was an individual participant data meta-analysis of population-based cohort studies. The criteria for study inclusion were that (1) the study contained participants of any age and sex derived from a general population, i.e. not a specific disease group, (2) ABI was measured at baseline using a technique standardized in each study, (3) subjects were followed up systematically to detect total and cardiovascular mortality.
At initial meetings of epidemiologists interested in the ABI, studies fulfilling the inclusion criteria were identified. A search was carried out of MEDLINE from 1950 to February 2008 and EMBASE from 1980 to February 2008 and of reference lists and conference proceedings to identify possible additional studies. Further studies and unpublished data were sought by discussion between collaborators, cardiovascular epidemiologists and vascular physicians and by correspondence with the Asia Pacific Cohort Studies Collaboration. Possible studies for inclusion were independently assessed for suitability by two collaborators and any lack of clarity or disagreement was resolved by discussion.
The principal authors/lead investigators of studies were invited to join the ABI Collaboration and, following acceptance, were sent a questionnaire enquiring about the availability of specific study data. On reviewing responses to these questionnaires, a set of data which were commonly available was agreed, and each study transferred their relevant data to the co-ordinating centre.
Requested data included individual demographic characteristics (e.g. gender, age, height and weight), baseline clinical co-factors (e.g. systolic/diastolic blood pressure, cholesterol, diabetes and cigarette smoking), details of baseline ABI measurements and information on non-fatal and fatal events during follow up. For these analyses, the subjects included had no previous history of coronary heart disease as defined in each study, a value for ABI recorded at baseline, and follow-up dates or times to events. Data from collaborators were extracted and analysed using SPSS for Windows Release 14.0.0 (September 2005) and SAS/STAT software, Version 9.1 of the SAS System for Windows (Copyright 2002-2003 SAS Institute Inc.).
A Framingham risk score was derived for each subject using the gender-specific prediction formulae proposed by Wilson et al3 based on conventional cardiovascular risk factors (age, total and HDL cholesterol categories, blood pressure categories, diabetes and smoking status). When data on some of the variables necessary to calculate the FRS were incomplete, missing values, amounting to 3.9% of total values, were imputed using the Expectation-Maximization (EM) procedure for multivariate normal data which is implemented in SPSS.
Overall (all studies combined) hazard ratios for ABI, subdivided into ten categories compared with a reference range of 1.11 to 1.20, were obtained for males and females for each of three outcomes (total mortality, cardiovascular mortality and major coronary events, i.e. coronary death/non-fatal myocardial infarction), and patterns of risk examined. Coronary revascularisation and angina were not included as endpoints. Hazard ratios for low versus normal ABI categorised into four groups for the three outcomes (total mortality, cardiovascular mortality and major coronary event) were obtained from a proportional hazards model stratified by gender and study, both unadjusted and adjusted for FRS (categorised into five strata for males and four for females). These hazard ratios were then pooled using a random effects model and summarised using forest plots (Review Manager, Version 4.2.9, The Cochrane Collaboration 2006 ©).
Kaplan–Meier estimates and standard errors for outcome rates (total mortality, cardiovascular mortality and major coronary event) at ten years were obtained for each study stratified by gender and FRS / ABI categories (grouped as described above). Outcome rates for studies within strata were combined to provide overall summaries using random effects pooling.18 Area under receiver operating characteristic (AUROC) curves were calculated for the prediction of events using the FRS alone and with addition of the ABI.
Results
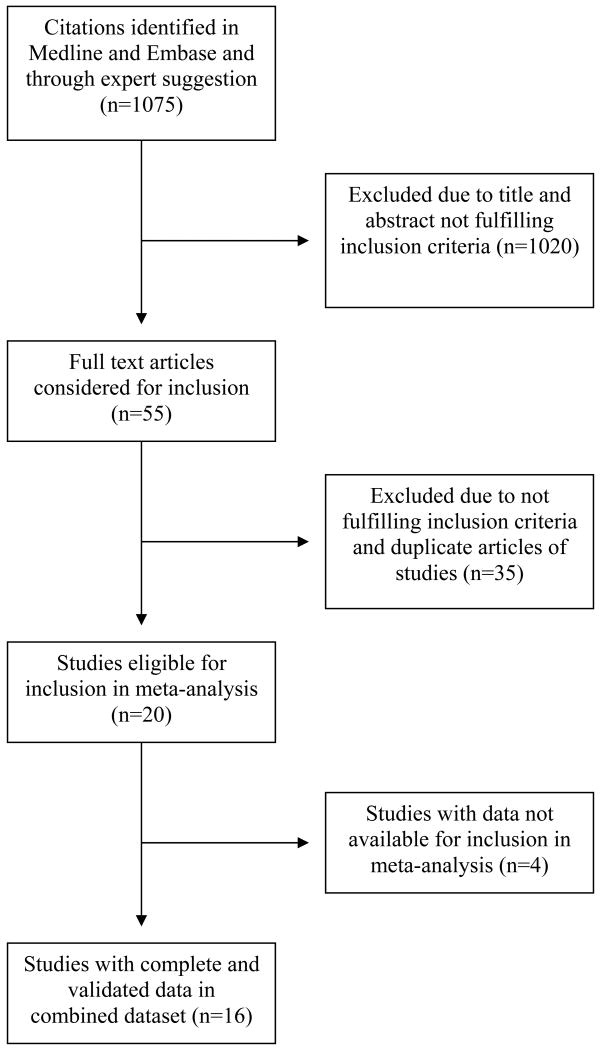
The literature search and information from experts identified 1075 citations from which 20 studies which fulfilled the inclusion criteria were identified (Figure 1). Selected investigators of 16 of these studies9-17, 19-25 agreed to participate in the ABI Collaboration and provided data prior to the analysis. The participating studies and investigators are shown in Appendix 1. The studies were based in Australia, Belgium, Italy, Netherlands, Sweden, UK and USA and comprised predominantly white populations except for the Honolulu Heart Program (Japanese Americans)11 and the Strong Heart Study (American Indians). 12 The populations in the Cardiovascular Health Study10 and the Atherosclerosis Risk in Communities9 (ARIC) study comprised 15% and 26% blacks respectively. In the San Luis Valley Diabetes Study24 the included healthy non-diabetic population was 42% Hispanic. Eleven studies included both sexes, four only men and one only women.
Figure 1.
Flow diagram of selection of studies for inclusion in meta-analysis.
The characteristics of the subjects in the studies at baseline when the ABI was measured are shown in Table 1. A total of 24,955 men and 23,339 women without a history of coronary heart disease were included. They were late middle aged to elderly with a mean age in the studies ranging from 47 to 78 years. The 10-year incidence of coronary heart disease (SD) predicted by the FRS at baseline varied across studies from 11.0 (6.1)% to 31.6 (14.1)% in men and from 7.1 (6.1)% to 14.5 (10.1)% in women. Mean ABI (SD) was greater than 1.00 in all studies and ranged from 1.02 (0.13) to 1.21 0.13) in men and 1.01 (0.16) to 1.15 (0.17) in women; most of the studies comprising both sexes had higher mean values in men than in women, as previously reported.24
Table 1. Baseline characteristics of subjects in studies in the ABI collaboration.
| Study | Number of subjects1 | Age in years Mean (SD) |
Framingham Risk Score2 % Mean (SD) |
Ankle Brachial Index Mean (SD) |
|||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| ARIC Study | 6105 | 8004 | 54 (5.7) | 12.8 (7.6) | 7.3 (6.0) | 1.17 (0.13) | 1.12 (0.13) |
| Belgian Physical Fitness Study | 2068 | 0 | 47 (4.4) | 11.0 (6.1) | - | 1.21 (0.13) | - |
| Cardiovascular Health Study | 1846 | 2779 | 73 (5.5) | 25.4 (12.5) | 8.0 (5.3) | 1.10 (0.19) | 1.06 (0.15) |
| Edinburgh Artery Study | 690 | 702 | 64 (5.7) | 26.2 (13.0) | 11.5 (6.2) | 1.07 (0.19) | 1.01 (0.16) |
| Framingham Offspring Study | 1423 | 1703 | 58 (9.6) | 15.3 (10.3) | 7.5 (5.9) | 1.16 (0.12) | 1.10 (0.10) |
| Health in Men Study | 2771 | 0 | 72 (4.4) | 29.4 (9.6) | - | 1.07 (0.17) | - |
| Honolulu Heart Program | 3123 | 0 | 78 (4.6) | 31.6 (14.1) | - | 1.05 (0.17) | - |
| Hoorn Study | 270 | 284 | 63 (7.2) | 26.8 (13.9) | 14.5 (10.1) | 1.03 (0.14) | 1.02 (0.12) |
| InCHIANTI Study | 481 | 569 | 67 (15.5) | 24.8 (15.4) | 8.0 (5.8) | 1.04 (0.16) | 1.05 (0.14) |
| Limburg PAOD Study | 1031 | 1320 | 57 (9.4) | 20.2 (10.6) | 11.7 (5.8) | 1.08 (0.16) | 1.07 (0.13) |
| Men born in 1914 Study | 391 | 0 | 69 (0.5) | 31.5 (10.5) | - | 1.02 (0.13) | - |
| Rotterdam Study | 2134 | 3515 | 69 (9.2) | 29.6 (15.6) | 10.2 (7.2) | 1.10 (0.21) | 1.05 (0.21) |
| San Diego Study | 244 | 314 | 66 (10.4) | 21.6 (12.9) | 7.8 (5.1) | 1.08 (0.19) | 1.02 (0.12) |
| San Luis Valley Diabetes Study | 674 | 838 | 53 (12.1) | 15.6 (12.0) | 9.1 (9.4) | 1.16 (0.15) | 1.10 (0.14) |
| Strong Heart Study | 1704 | 2622 | 56 (8.0) | 15.5 (9.6) | 10.8 (7.3) | 1.15 (0.14) | 1.15 (0.17) |
| Women's Health & Aging Study | 0 | 689 | 78 (8.1) | - | 7.1 (6.1) | - | 1.05 (0.21) |
| TOTAL | 24955 | 23339 | |||||
- no history of coronary heart disease, including myocardial infarction, angina, revascularisation, as defined in each study;
- ABI available at baseline;
- follow-up data available.
Predicted 10-year % incidence of coronary heart disease, including coronary death, myocardial infarction and angina.
Tables 2 and 3 show the total mortality, cardiovascular mortality and major coronary events occurring during follow up in each of the studies for men and women respectively. Median duration of follow up ranged from 3.0 to 16.7 years, with 9 of the 16 studies having greater than 10 years of follow-up. Overall, 9,924 deaths occurred during 480,325 person years of follow up with around one quarter of deaths due to coronary heart disease or stroke in both men and women. The annual rates of deaths and events varied considerably between the studies. For example, in men in the Belgian Physical Fitness Study with mean age (SD) 47 (4.4) years the annual mortality (95% CI) was 0.37 (0.29 to 0.45)% whereas in men in the Honolulu Heart Program with mean age (SD) 78 (4.6) years, the annual mortality (95% CI) was 4.91 (4.59 to 5.22)% (Table 2). Likewise, in women annual mortality (95% CI) varied between 0.55 (0.42 to 0.68)% in the Framingham Offspring Study to 7.34 (6.39 to 8.29)% in the Women's Health and Aging Study (Table 3).
Table 2. Total mortality, cardiovascular mortality and major coronary events for men in studies in the ABI Collaboration.
| Total Mortality | Cardiovascular Mortality1 | Major Coronary Events2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Median (Q1,Q3) years of follow-up | Person years of follow-up | Number of deaths | Annual mortality % (95% CI) | Person years of follow-up | Number of deaths | Annual mortality % (95% CI) | Person years of follow-up | Number of events | Annual events % (95% CI) |
| ARIC Study | 13.1 (12.4, 13.9) | 76497 | 903 | 1.18 (1.10 to 1.26) | 76497 | 170 | 0.22 (0.19 to 0.26) | 73991 | 571 | 0.77 (0.71 to 0.83) |
| Belgian Physical Fitness Study | 10.9 (10.5, 11.4) | 22292 | 83 | 0.37 (0.29 to 0.45) | 22292 | 13 | 0.06 (0.03 to 0.09) | 22136 | 98 | 0.44 (0.36 to 0.53) |
| Cardiovascular Health Study | 11.0 (7.2, 11.6) | 16583 | 839 | 5.06 (4.73 to 5.39) | 16583 | 263 | 1.59 (1.40 to 1.78) | 15542 | 432 | 2.78 (2.52 to 3.04) |
| Edinburgh Artery Study | 15.5 (9.0, 15.9) | 8667 | 295 | 3.40 (3.02 to 3.79) | 8667 | 84 | 0.97 (0.76 to 1.18) | 8090 | 113 | 1.40 (1.14 to 1.65) |
| Framingham Offspring Study | 7.4 (6.6, 8.2) | 10182 | 113 | 1.11 (0.91 to 1.31) | 10182 | 20 | 0.20 (0.11 to 0.28) | 10052 | 56 | 0.56 (0.41 to 0.70) |
| Health in Men Study | 6.3 (5.9, 6.5) | 16446 | 402 | 2.44 (2.21 to 2.68) | 16446 | 114 | 0.69 (0.57 to 0.82) | - | - | - |
| Honolulu Heart Program | 6.2 (5.5, 6.9) | 17976 | 882 | 4.91 (4.59 to 5.22) | 17976 | 231 | 1.29 (1.12 to 1.45) | 17703 | 205 | 1.16 (1.00 to 1.32) |
| Hoorn Study | 12.5 (9.8, 13.1) | 2969 | 88 | 2.96 (2.35 to 3.57) | 2969 | 26 | 0.88 (0.54 to 1.21) | - | - | - |
| InCHIANTI Study | 3.0 (2.9, 3.1) | 1427 | 30 | 2.10 (1.36 to 2.85) | 1427 | 11 | 0.77 (0.32 to 1.22) | - | - | - |
| Limburg PAOD Study | 7.1 (6.6, 7.7) | 7088 | 148 | 2.09 (1.76 to 2.42) | 7088 | 34 | 0.48 (0.32 to 0.64) | 6864 | 82 | 1.19 (0.94 to 1.45) |
| Men born in 1914 Study | 13.3 (8.1, 13.9) | 4248 | 182 | 4.28 (3.68 to 4.89) | 4248 | 70 | 1.65 (1.26 to 2.03) | 4028 | 92 | 2.28 (1.82 to 2.75) |
| Rotterdam Study | 10.9 (8.2, 11.8) | 20538 | 813 | 3.96 (3.70 to 4.23) | 20538 | 221 | 1.08 (0.94 to 1.23) | 19805 | 260 | 1.31 (1.15 to 1.47) |
| San Diego Study | 16.7 (10.4, 22.3) | 3843 | 156 | 4.06 (3.44 to 4.68) | 3843 | 77 | 2.00 (1.56 to 2.45) | 3581 | 80 | 2.23 (1.75 to 2.72) |
| San Luis Valley Diabetes Study | 15.6 (14.4, 16.9) | 9765 | 167 | 1.71 (1.45 to 1.97) | 9765 | 51 | 0.52 (0.38 to 0.67) | 9265 | 82 | 0.89 (0.69 to 1.08) |
| Strong Heart Study | 9.7 (8.9, 10.4) | 14935 | 481 | 3.22 (2.94 to 3.50) | 14935 | 122 | 0.82 (0.67 to 0.96) | 14573 | 184 | 1.27 (1.09 to 1.46) |
| TOTAL | 233457 | 5582 | 233457 | 1507 | 205628 | 2255 | ||||
Q1,Q3 = lower and upper quartiles.
Cardiovascular mortality is death due to coronary heart disease or stroke.
Major coronary events are myocardial infarction or deaths from coronary heart disease. Data not available for 3 studies.
Table 3. Total mortality, cardiovascular mortality and major coronary events for women in studies in the ABI Collaboration.
| Total Mortality | Cardiovascular Mortality1 | Major Coronary Events2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Median (Q1,Q3) years of follow-up | Person years of follow-up | Number of deaths | Annual mortality % (95% CI) | Person years of follow-up | Number of deaths | Annual mortality % (95% CI) | Person years of follow-up | Number of events | Annual events % (95% CI) |
| ARIC Study | 13.2 (12.4, 13.9) | 102458 | 773 | 0.75 (0.70 to 0.81) | 102458 | 133 | 0.13 (0.11 to 0.15) | 101121 | 362 | 0.36 (0.32 to 0.39) |
| Cardiovascular Health Study | 11.2 (8.3, 11.6) | 27447 | 851 | 3.10 (2.90 to 3.31) | 27447 | 262 | 0.95 (0.84 to 1.07) | 26652 | 374 | 1.40 (1.26 to 1.54) |
| Edinburgh Artery Study | 15.8 (14.2, 16.1) | 9836 | 200 | 2.03 (1.75 to 2.31) | 9836 | 41 | 0.42 (0.29 to 0.54) | 9602 | 57 | 0.59 (0.44 to 0.75) |
| Framingham Offspring Study | 7.4 (6.6, 8.3) | 12344 | 68 | 0.55 (0.42 to 0.68) | 12344 | 5 | 0.04 (0.01 to 0.08) | 12272 | 24 | 0.20 (0.12 to 0.27) |
| Hoorn Study | 12.6 (10.6, 13.2) | 3212 | 76 | 2.37 (1.84 to 2.89) | 3212 | 23 | 0.72 (0.42 to 1.01) | - | - | - |
| InCHIANTI Study | 3.0 (2.9, 3.2) | 1711 | 26 | 1.52 (0.94 to 2.10) | 1711 | 12 | 0.70 (0.31 to 1.10) | - | - | - |
| Limburg PAOD Study | 7.1 (6.7, 7.6) | 9273 | 114 | 1.23 (1.01 to 1.45) | 9273 | 26 | 0.28 (0.17 to 0.39) | 9168 | 53 | 0.58 (0.42 to 0.73) |
| Rotterdam Study | 11.1 (9.3, 12.1) | 35407 | 1131 | 3.19 (3.01 to 3.38) | 35407 | 352 | 0.99 (0.89 to 1.10) | 34968 | 283 | 0.81 (0.72 to 0.90) |
| San Diego Study | 19.6 (13.0, 22.6) | 5443 | 177 | 3.25 (2.78 to 3.72) | 5443 | 76 | 1.40 (1.08 to 1.71) | 5361 | 65 | 1.21 (0.92 to 1.51) |
| San Luis Valley Diabetes Study | 15.8 (14.6, 17.1) | 12542 | 163 | 1.30 (1.10 to 1.50) | 12542 | 53 | 0.42 (0.31 to 0.54) | 12293 | 58 | 0.47 (0.35 to 0.59) |
| Strong Heart Study | 9.9 (9.1, 10.7) | 24305 | 551 | 2.27 (2.08 to 2.45) | 24305 | 137 | 0.56 (0.47 to 0.66) | 24010 | 183 | 0.76 (0.65 to 0.87) |
| Women's Health & Aging Study | 5.0 (3.8, 5.1) | 2890 | 212 | 7.34 (6.39 to 8.29) | 2890 | 91 | 3.15 (2.51 to 3.79) | 2620 | 170 | 6.49 (5.55 to 7.43) |
| TOTAL | 246868 | 4342 | 246868 | 1211 | 238066 | 1629 | ||||
Q1,Q3 = lower and upper quartiles.
Cardiovascular mortality is death due to coronary heart disease or stroke.
Major coronary events are myocardial infarction or deaths from coronary heart disease. Data not available for 3 studies.
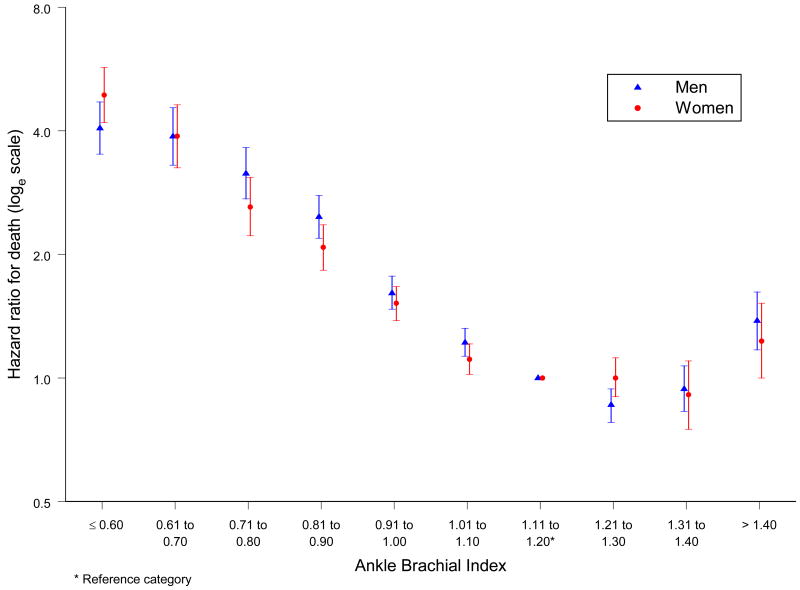
The hazard ratios (HRs) of death for different levels of ABI compared with a reference ABI of 1.11 to 1.20 in all studies combined formed a reverse J shaped curve for both men and women (Figure 2). For levels of ABI below 1.11 the HRs increased consistently with decreasing ABI. For an ABI >1.40 the HRs were also increased: 1.38 (95% CI 1.17 to 1.62) for men and 1.23 (95% CI 1.00 to 1.52) for women. For levels of ABI between 1.11 and 1.40 only small and mostly non significant differences in HRs were found. Tables 4 and 5 show the HRs for total and cardiovascular mortality and major coronary events by ABI in men and women, respectively. The patterns of risk for cardiovascular mortality and major coronary events were similar to that for total mortality; for levels of ABI below 1.11, the HRs for cardiovascular mortality were consistently higher than for total mortality.
Figure 2.
Hazard ratios of total mortality in men and women by ankle brachial index at baseline for all studies combined in the ABI collaboration.
(Hazard ratios are not adjusted for age or cardiovascular risk factors)
Table 4. Hazard ratios of total mortality, cardiovascular mortality and major coronary events by ankle brachial index at baseline for men in all studies combined in the ABI Collaboration.
| Ankle Brachial Index: | ≤ 0.60 | 0.61 to 0.70 | 0.71 to 0.80 | 0.81 to 0.90 | 0.91 to 1.00 | 1.01 to 1.10 | 1.11 to 1.20 | 1.21 to 1.30 | 1.31 to 1.40 | >1.40 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n=24955) |
360 | 279 | 428 | 774 | 2438 | 5775 | 7576 | 4936 | 1681 | 708 |
| Total Mortality | ||||||||||
| Number of deaths (n=5582) |
215 | 170 | 217 | 355 | 741 | 1338 | 1364 | 745 | 270 | 167 |
| Hazard ratio 95% CI |
4.06 3.51 to 4.70 |
3.88 3.30 to 4.55 |
3.15 2.73 to 3.64 |
2.47 2.19 to 2.78 |
1.61 1.47 to 1.77 |
1.22 1.13 to 1.32 |
1 (reference) |
0.86 0.78 to 0.94 |
0.94 0.83 to 1.07 |
1.38 1.17 to 1.62 |
| Cardiovascular Mortality1 | ||||||||||
| Number of deaths (n=1507) |
80 | 54 | 81 | 116 | 208 | 352 | 341 | 179 | 62 | 34 |
| Hazard ratio 95% CI |
5.58 4.36 to 7.15 |
4.60 3.44 to 6.14 |
4.49 3.51 to 5.74 |
3.03 2.45 to 3.75 |
1.68 1.40 to 2.00 |
1.24 1.07 to 1.44 |
1 (reference) |
0.85 0.71 to 1.02 |
0.93 0.71 to 1.22 |
1.14 0.80 to 1.63 |
| Major Coronary Events2 | ||||||||||
| Number of events (n=2255) |
70 | 48 | 74 | 119 | 252 | 516 | 642 | 353 | 125 | 56 |
| Hazard ratio 95% CI |
3.45 2.68 to 4.43 |
2.71 2.01 to 3.64 |
2.76 2.16 to 3.52 |
2.15 1.76 to 2.63 |
1.43 1.23 to 1.66 |
1.12 1.00 to 1.26 |
1 (reference) |
0.78 0.68 to 0.88 |
0.78 0.64 to 0.95 |
0.90 0.68 to 1.18 |
Cardiovascular mortality is death due to coronary heart disease or stroke.
Major coronary events are myocardial infarction or deaths from coronary heart disease. (Total sample size 21433).
Hazard ratios are not adjusted for age or cardiovascular risk factors.
Table 5. Hazard ratios of total mortality, cardiovascular mortality and major coronary events by ankle brachial index at baseline for women in all studies combined in the ABI Collaboration.
| Ankle Brachial Index: | ≤ 0.60 | 0.61 to 0.70 | 0.71 to 0.80 | 0.81 to 0.90 | 0.91 to 1.00 | 1.01 to 1.10 | 1.11 to 1.20 | 1.21 to 1.30 | 1.31 to 1.40 | >1.40 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n=23339) |
314 | 251 | 403 | 933 | 3186 | 6586 | 6862 | 3363 | 932 | 509 |
| Total Mortality | ||||||||||
| Number of deaths (n=4342) |
199 | 145 | 174 | 326 | 707 | 1078 | 999 | 489 | 125 | 100 |
| Hazard ratio 95% CI |
4.89 4.19 to 5.71 |
3.88 3.25 to 4.63 |
2.61 2.22 to 3.08 |
2.08 1.83 to 2.36 |
1.52 1.38 to 1.67 |
1.11 1.02 to 1.21 |
1 (reference) |
1.00 0.90 to 1.12 |
0.91 0.75 to 1.10 |
1.23 1.00 to 1.52 |
| Cardiovascular Mortality1 | ||||||||||
| Number of deaths (n=1211) |
79 | 51 | 66 | 114 | 218 | 271 | 241 | 119 | 24 | 28 |
| Hazard ratio 95% CI |
7.04 5.43 to 9.12 |
5.06 3.72 to 6.87 |
3.65 2.77 to 4.81 |
2.77 2.21 to 3.47 |
1.84 1.53 to 2.22 |
1.14 0.95 to 1.36 |
1 (reference) |
1.04 0.83 to 1.29 |
0.74 0.49 to 1.13 |
1.48 1.00 to 2.21 |
| Major Coronary Events2 | ||||||||||
| Number of events (n=1629) |
79 | 54 | 64 | 119 | 260 | 412 | 387 | 174 | 47 | 33 |
| Hazard ratio 95% CI |
5.43 4.24 to 6.94 |
3.82 2.86 to 5.11 |
2.58 1.97 to 3.37 |
2.06 1.67 to 2.53 |
1.53 1.30 to 1.79 |
1.11 0.97 to 1.28 |
1 (reference) |
0.91 0.76 to 1.09 |
0.86 0.64 to 1.17 |
1.11 0.77 to 1.58 |
Cardiovascular mortality is death due to coronary heart disease or stroke.
Major coronary events are myocardial infarction or deaths from coronary heart disease (Total sample size 22486).
Hazard ratios are not adjusted for age or cardiovascular risk factors.
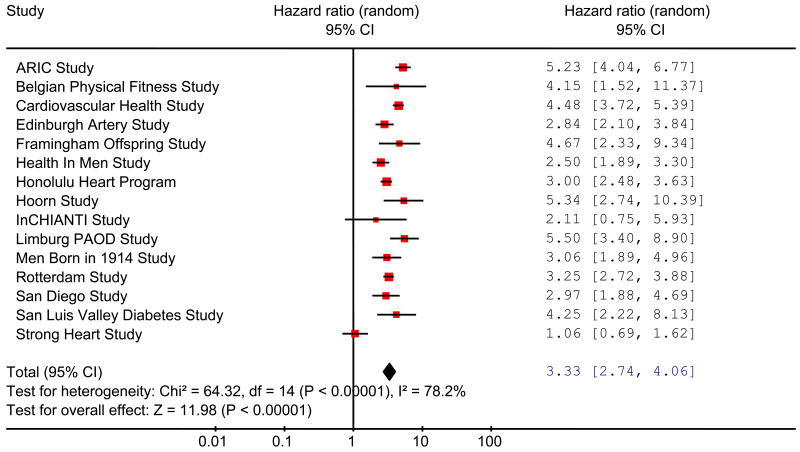
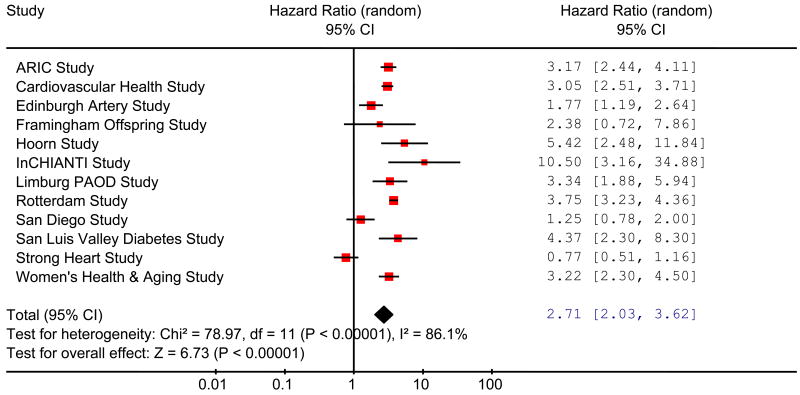
Values of the ABI less than 0.90 have been taken traditionally as a measure of increased risk. In nearly all the studies in men (Fig 3), the HRs for total mortality were statistically significantly higher in individuals with an ABI ≤0.90 than in individuals with normal ABI values of 1.11 to 1.40, with a total HR (95% CI) of 3.33 (2.74 to 4.06). In women, the results were more heterogeneous (Fig 4) but the total HR of 2.71 (2.03 to 3.62) was comparable to that in men. Likewise, significantly increased total HRs (95%CI) were found in men and in women both for cardiovascular mortality, men 4.21 (3.29 to 5.39), women 3.46 (2.36 to 5.08), and for major coronary events, men 2.97 (2.33 to 3.78), women 3.05 (2.25 to 4.15). Adjustment of the HRs of ABI ≤0.90 relative to 1.11 to 1.40 for FRS reduced the HRs but they were still elevated substantially and significantly. In men adjusted HRs (95% CI) for total mortality, cardiovascular mortality and major coronary events were 2.34 (1.97 to 2.78), 2.92 (2.31 to 3.70) and 2.16 (1.76 to 2.66), respectively, and in women were 2.35 (1.76 to 3.13), 2.97 (2.02 to 4.35) and 2.49 (1.84 to 3.36), respectively.
Figure 3.
Hazard ratios of total mortality for low (≤0.90) compared with normal (1.11-1.40) ankle brachial index in men in studies in the ABI collaboration.
(Hazard ratios are not adjusted for age or cardiovascular risk factors) (Area of each square is proportional to weight of the study in the meta-analysis).
(Total deaths/population for ≤ 0.90 group is 957/1841 and for 1.11 to 1.40 group is 2379/14,193).
Figure 4.
Hazard ratios of total mortality for low (≤0.90) compared with normal (1.11-1.40) ankle brachial index in women in studies in the ABI collaboration.
(Hazard ratios are not adjusted for age or cardiovascular risk factors) (Area of each square is proportional to weight of the study in the meta-analysis).
(Total deaths/population for ≤ 0.90 group is 844/1901 and for 1.11 to 1.40 group is 1613/11,157).
Tables 6 and 7 show the impact of inclusion of an ABI measurement on the apparent risk of 10-year total mortality, cardiovascular mortality and major coronary events over the range of FRSs in men and women. Compared with the overall rates without ABI included, an ABI ≤0.90 was associated with a greatly increased risk of mortality (total and cardiovascular) and major coronary events across all FRS categories in both men and women, more so in the lower than higher categories. Women had especially high mortality and event rates in the lowest FRS category. Men and women with an ABI 0.91 to 1.10 also had higher mortality and event rates than those with a normal ABI (1.11 to 1.40) but the magnitudes of the increase were much less than for those with ABI ≤0.90. Those with an ABI >1.40 also had higher rates across most FRS categories.
Table 6. 10-year total mortality, cardiovascular mortality and major coronary event rates in men by Framingham Risk Category and Ankle Brachial Index at baseline for all studies combined in the ABI Collaboration.
| Ankle Brachial Index: | ≤ 0.90 | 0.91 to 1.10 | 1.11 to 1.40 | >1.40 | Overall |
|---|---|---|---|---|---|
| Total mortality % (95% CI) | |||||
| Framingham Risk Category1 | |||||
| 1. Lowest (n=5,746) | 27.1 (16.0 to 38.2) | 11.4 (5.9 to 16.8) | 8.3 (5.4 to 11.2) | 14.1 (4.2 to 24.0) | 10.4 (6.9 to 13.9) |
| 2. (n=4,319) | 37.3 (17.8 to 56.9) | 15.8 (10.6 to 21.0) | 11.3 (8.2 to 14.5) | 19.9 (7.5 to 32.4) | 13.8 (9.9 to 17.7) |
| 3. (n=3,544) | 37.6 (26.1 to 49.1) | 19.7 (13.6 to 25.9) | 14.2 (9.9 to 18.5) | 23.5 (9.5 to 37.6) | 17.6 (13.1 to 22.2) |
| 4. (n=5,814) | 38.1 (28.5 to 47.8) | 23.6 (16.9 to 30.4) | 19.2 (14.8 to 23.5) | 38.4 (12.3 to 64.6) | 23.1 (17.6 to 28.6) |
| 5. Highest (n=5,532) | 57.1 (45.4 to 68.9) | 36.4 (29.1 to 43.7) | 31.0 (25.2 to 36.7) | 43.6 (28.1 to 59.1) | 38.0 (30.9 to 45.0) |
| Overall (n=24,955) | 46.3 (36.1 to 56.6) | 23.0 (15.8 to 30.2) | 16.7 (12.4 to 21.0) | 29.2 (18.9 to 39.5) | |
| Cardiovascular mortality % (95% CI) | |||||
| 1. Lowest (n=5,746) | 4.6 (0.0 to 10.8) | 3.1 (0.0 to 6.5) | 1.3 (0.5 to 2.0) | 2.7 (0.0 to 6.8) | 1.6 (0.8 to 2.4) |
| 2. (n=4,319) | 17.5 (6.6 to 28.3) | 3.5 (1.5 to 5.5) | 1.5 (0.7 to 2.3) | 8.2 (0.0 to 18.8) | 2.3 (1.3 to 3.4) |
| 3. (n=3,544) | 11.5 (2.4 to 20.6) | 5.1 (3.1 to 7.2) | 3.6 (1.9 to 5.2) | 8.3 (0.3 to 16.2) | 4.4 (2.8 to 6.0) |
| 4. (n=5,814) | 14.2 (10.2 to 18.2) | 8.0 (5.2 to 10.8) | 4.8 (3.3 to 6.4) | 5.6 (0.0 to 12.2) | 7.3 (5.2 to 9.3) |
| 5. Highest (n=5,532) | 27.9 (20.7 to 35.1) | 12.5 (8.9 to 16.1) | 9.9 (6.8 to 13.1) | 10.7 (2.0 to 19.4) | 14.0 (10.6 to 17.4) |
| Overall (n=24,955) | 18.7 (13.3 to 24.1) | 7.3 (5.0 to 9.6) | 4.4 (3.2 to 5.7) | 6.9 (2.8 to 11.0) | |
| Major coronary events2 % (95% CI) | |||||
| 1. Lowest (n=5,643) | 5.8 (0.0 to 12.7) | 3.7 (1.4 to 6.0) | 3.4 (2.5 to 4.3) | 4.0 (1.1 to 6.8) | 3.5 (2.4 to 4.6) |
| 2. (n=4.151) | 20.0 (9.6 to 30.4) | 5.9 (3.6 to 8.1) | 6.8 (5.7 to 8.0) | 5.0 (0.7 to 9.3) | 7.1 (5.5 to 8.8) |
| 3. (n=3,241) | 20.2 (8.0 to 32.3) | 10.0 (6.2 to 13.8) | 8.7 (6.4 to 11.0) | 12.9 (0.0 to 27.8) | 10.1 (7.5 to 12.6) |
| 4. (n=4,179) | 27.5 (18.5 to 36.6) | 14.8 (9.9 to 19.7) | 12.6 (9.6 to 15.7) | 9.7 (0.0 to 19.7) | 15.3 (11.5 to 19.1) |
| 5. Highest (n=4,219) | 31.4 (21.9 to 40.8) | 20.0 (14.4 to 25.5) | 17.6 (12.2 to 23.0) | 12.0 (3.6 to 20.3) | 21.5 (16.7 to 26.3) |
| Overall (n=21,433) | 26.8 (19.5 to 34.1) | 12.9 (9.2 to 16.7) | 9.4 (7.4 to 11.4) | 7.2 (4.3 to 10.1) | |
Categories of predicted 10-year % incidence of coronary heart disease, including coronary death, myocardial infarction and angina, are based on whole number cutpoints for scores; category 1 = <10%, 2 = 10-14%, 3 = 15-19%, 4 = 20-29%, 5 = ≥ 30%
Excluding Health in Men, Hoorn and InCHIANTI studies.
Analysis based on random effects pooling of Kaplan-Meier estimates from the individual studies.
Table 7. 10-year total mortality, cardiovascular mortality and major coronary event rates in women by Framingham Risk Category and Ankle Brachial Index at baseline for all studies combined in the ABI Collaboration.
| Ankle Brachial Index: | ≤ 0.90 | 0.91 to 1.10 | 1.11 to 1.40 | >1.40 | Overall |
|---|---|---|---|---|---|
| Total mortality % (95% CI) | |||||
| Framingham Risk Category1 | |||||
| 1. Lowest (n=5,507) | 44.2 (7.5 to 80.9) | 21.3 (12.5 to 30.1) | 14.1 (9.1 to 19.1) | 27.4 (14.6 to 40.2) | 18.2 (10.6 to 25.8) |
| 2. (n=6,016) | 28.2 (9.2 to 47.2) | 13.3 (7.7 to 18.9) | 10.3 (6.3 to 14.3) | 8.1 (1.9 to 14.3) | 12.2 (7.0 to 17.4) |
| 3. (n=5,581) | 27.1 (16.0 to 38.1) | 15.2 (11.0 to 19.4) | 10.9 (7.5 to 14.2) | 20.6 (11.7 to 29.5) | 15.7 (11.2 to 20.2) |
| 4. Highest (n=6,235) | 31.4 (23.2 to 39.7) | 17.6 (13.3 to 21.9) | 16.2 (12.2 to 20.3) | 20.9 (0.0 to 48.2) | 19.8 (16.6 to 23.0) |
| Overall (n=23,339) | 30.1 (18.0 to 42.1) | 16.6 (10.9 to 22.3) | 13.1 (8.5 to 17.6) | 26.6 (9.7 to 43.4) | |
| Cardiovascular mortality % (95% CI) | |||||
| 1. Lowest (n=5,507) | 45.5 (29.7 to 61.4) | 4.5 (1.9 to 7.0) | 4.0 (1.6 to 6.4) | 14.1 (0.0 to 32.3) | 4.8 (3.2 to 6.4) |
| 2. (n=6,016) | 15.1 (1.5 to 28.7) | 4.1 (1.6 to 6.6) | 2.9 (0.9 to 4.9) | 4.3 (0.0 to 12.7) | 3.5 (1.6 to 5.4) |
| 3. (n=5,581) | 9.7 (5.1 to 14.3) | 4.4 (2.5 to 6.3) | 3.2 (1.5 to 4.8) | 14.7 (0.0 to 45.6) | 4.8 (3.0 to 6.6) |
| 4. Highest (n=6,235) | 15.7 (9.5 to 22.0) | 5.1 (3.4 to 6.9) | 5.5 (3.5 to 7.6) | 15.5 (8.4 to 22.5) | 6.8 (4.5 to 9.2) |
| Overall (n=23,339) | 12.6 (6.2 to 19.0) | 4.7 (3.0 to 6.3) | 4.1 (2.2 to 6.1) | 6.9 (4.0 to 9.7) | |
| Major coronary events2 % (95% CI) | |||||
| 1. Lowest (n=5,355) | 29.9 (9.0 to 50.8) | 3.9 (1.7 to 6.1) | 5.3 (2.4 to 8.2) | 10.7 (0.0 to 24.3) | 5.8 (3.9 to 7.7) |
| 2. (n=5,842) | 16.9 (6.8 to 27.1) | 5.1 (2.4 to 7.7) | 3.7 (2.0 to 5.5) | 2.1 (0.0 to 6.3) | 4.7 (2.6 to 6.7) |
| 3. (n=5,334) | 15.3 (8.0 to 22.6) | 7.5 (4.5 to 10.4) | 5.2 (3.5 to 6.9) | 14.1 (0.0 to 47.9) | 6.7 (4.3 to 9.1) |
| 4. Highest (n=5,955) | 23.3 (14.5 to 32.0) | 9.8 (7.4 to 12.2) | 9.4 (6.7 to 12.0) | 14.9 (8.8 to 21.1) | 11.9 (9.3 to 14.5) |
| Overall (n=22,486) | 18.9 (11.6 to 26.2) | 7.3 (5.0 to 9.6) | 6.1 (4.1 to 8.1) | 5.5 (0.7 to 10.3) | |
Categories of predicted 10-year % incidence of coronary heart disease, including coronary death, myocardial infarction and angina, are based on whole number cutpoints for scores; category 1 = ≤ 4%, 2 = 5-7%, 3 = 8-11%, 4 = ≥ 12%
Excluding Hoorn and InCHIANTI studies.
Analysis based on random effects pooling of Kaplan-Meier estimates from the individual studies.
Inclusion of the ABI had an overall effect on the prediction of events, especially in women. In men the area under the ROC curve, (95% CI) when predicting major coronary events using only the FRS was 0.646 (0.643 to 0.657) and with the addition of the ABI was 0.655 (0.643 to 0.666). In women the area (95% CI) increased from 0.605 (0.590 to 0.619) to 0.658 (0.644 to 0.672).
The FRS is mostly used to predict risk of total coronary heart disease (CHD) (including coronary death, myocardial infarction and angina) and Table 8 shows the impact of including the ABI on this prediction. The calibration of the FRS categories was reasonable because the overall CHD rate in each FRS category was within the range predicted, except for low risk women in which the overall CHD rate of 11% was higher than predicted. Likewise, the ability of the FRS to discriminate between risk categories was good, except that the overall CHD rate in women in the low risk group was only slightly lower than those in the intermediate risk group (11% v 13%). In each category of FRS in both men and women a low ABI (≤0.90) was associated with an increased risk of future CHD. Normal levels of the ABI (1.11 to 1.40) were associated with a slightly reduced risk from the overall rates but levels >1.40 did not differ consistently from the overall rates, although this may have been influenced by the relatively low numbers of subjects.
Table 8. 10-year total coronary heart disease rates in men and women by Framingham Risk Category and Ankle Brachial Index at baseline for all studies1 combined in the ABI Collaboration.
| Ankle Brachial Index: | ≤ 0.90 | 0.91 to 1.10 | 1.11 to 1.40 | >1.40 | Overall |
|---|---|---|---|---|---|
| Total Coronary Heart Disease3 % (n) | |||||
| Men | |||||
| Framingham Risk Category2 | |||||
| Low <10% | 8 (76) | 5 (1076) | 4 (4255) | 5 (236) | 5 (5643) |
| Intermediate 10-19% | 16 (245) | 12 (2069) | 12 (4815) | 8 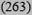
|
13 (7392) |
| High ≥20% | 40 (1149) | 21 (3406) | 18 
|
14 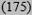
|
23 (8398) |
| Women | |||||
| Framingham Risk Category2 | |||||
| Low <10% | 21 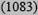
|
10 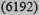
|
9 (7909) | 11 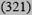
|
11 (15505) |
| Intermediate 10-19% | 25 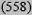
|
12 (2429) | 11 (2433) | 13 (143) | 13 (5563) |
| High ≥20% | 44 (200) | 21 (598) | 22 (577) | 34 (43) | 27 (1418) |
Excluding Health in Men, Hoorn and InCHIANTI studies, in which non-fatal events unavailable.
Categories of predicted 10-year % incidence of coronary heart disease, including coronary death, myocardial infarction and angina.
Total coronary heart disease, including coronary death, myocardial infarction and angina. Rates are approximate based on observed major coronary events (coronary death or myocardial infarction) adjusted by established conversion factors.26
The n between the brackets are the total number of subjects in each cell with the specified Framingham Risk Category and ABI level, irrespective of whether they did or did not have coronary heart disease.
Shaded numbers indicate subjects who would change between low (<10%), intermediate (10-19%) and high (≥20%) risk categories from that predicted by Framingham Risk Score when ABI included.
Analysis based on random effects pooling of Kaplan-Meier estimates from the individual studies
The results in Table 8 also indicate in which categories of FRS the ABI is likely to change individuals' clinical risk levels i.e. between <10%, 10-19%, and ≥20%. In men, the greatest impact would be in high risk individuals (≥20%) with a normal ABI (1.11 to 1.40) in whom the risk level would be reduced to intermediate (10-19%). All men with a low ABI (≤0.90) had a relatively high risk but their clinical risk level would not change from that predicted overall by the FRS. In women the main impact of the ABI would be to change all women in the low FRS category (<10%) with an abnormal ABI (≤0.90 or 0.91 to 1.10 or >1.40) to a higher risk level. Also women in the intermediate FRS category (10-19%) with a low ABI (≤0.90) would become high risk (≥20%). Table 8 also shows that the number of men changing risk category (shaded numbers) would be 4,106 out of a total of 21, 433 (19%) and in women would be 8,154 out of a total of 22,486 (36%).
Discussion
Predicting future coronary heart disease and mortality accurately in individuals in the community who have no prior history of cardiovascular disease has proven difficult when based solely on traditional risk factors and scoring systems. In a recent systematic review of 27 studies using the Framingham risk equation, the predicted to observed ratios ranged from an underprediction of 0.43 in a high risk population to an overprediction of 2.87 in a low risk population.4 We found that the ABI provided independent risk information as compared with the FRS and, when combined with the FRS, a low ABI (≤ 0.9) approximately doubled the risk of total mortality, cardiovascular mortality and major coronary events across all Framingham risk categories.
In predicting the 10-year risk of total CHD, our results (Table 8) indicate that approximately 1 out of 5 men would have their broad category of risk (<10, 10-19, ≥20%) changed from that predicted by FRS alone to that found on inclusion of the ABI. These changes from higher to lower categories of risk would be likely to have an effect on decisions to commence preventive treatment, such as lipid lowering therapy, as recommended in the Adult Treatment Panel (ATP) III guidelines.27 In contrast, the main effect in women of inclusion of the ABI would be that many at low FRS risk (<10%) would change to a higher risk level. In total around 1 in 3 women would be affected. It should be recognised, however, that the proportion of men and women affected by inclusion of the ABI is approximate due to the method of estimating total CHD endpoints and possible residual confounding within FRS groups.
Our results also confirm the recent findings of the Strong Heart Study,12 Cardiovascular Health Study28 and Multi-ethnic Study of Atherosclerosis29 that the relationship between ABI and cardiovascular disease is non linear and varies across the range of ABI. High values (>1.40) could be related to poor arterial compressibility resulting from stiffness and calcification which may occur more commonly in those with diabetes,29,30 and may be one explanation why those with an ABI >1.40 are at increased risk. The differences in risk between ABI values of 1.11 and 1.40 in both men and women were so small that, for practical purposes, an ABI within this range could be considered normal. Subjects with an ABI between 0.91 and 1.10 were at slightly increased risk. These results would suggest that the widely accepted high risk cutpoint of 0.90 is reasonable but, for values 0.91 to 1.10 and > 1.40, individuals might be advised that their risk may be slightly higher than normal levels.
The ABI Collaboration includes 16 international cohort studies. The consistency of results, especially in men (Figure 3), across a diverse spectrum of populations strengthens the validity of our findings. This consistency was also apparent despite some differences in methods of measuring the ABI and in ascertaining outcome events. We did not recalibrate the FRS, as has been suggested in populations very different from that in Framingham,31 because in our collaboration there was no evidence that particular studies had substantially worse calibration than others and also the FRS when used in routine clinical practice is not usually calibrated to the local population. Although the area under receiver operating characteristic (AUROC) curves examining the added effect of the ABI are presented, from a clinical perspective, the added value of the ABI is the extent to which its inclusion re-classifies patient risk at an individual level.32
Other indicators of asymptomatic atherosclerosis, notably coronary artery calcium (CAC) score and carotid intima media thickness (IMT) have been evaluated as incremental risk predictors to the FRS. Population studies of apparently healthy subjects have suggested that CAC score may provide added value,33,34 particularly in discriminating high and low risk individuals in those with an intermediate FRS (predicted 10-year coronary event risk between 10 and 20%).35 In the ARIC study,36 inclusion of carotid IMT had a modest effect on the area under the ROC curve for prediction of coronary heart disease using traditional risk factors. Likewise, in patients with dyslipidaemia37 and diabetes38 a combination of carotid IMT and FRS improved prediction compared with FRS alone. We are not aware however of reports of any direct comparisons in the same study of the additional values which different measures of asymptomatic atherosclerosis (for example CAC versus IMT) make to FRS prediction in the general population.
The ABI is potentially a useful tool for prediction of cardiovascular risk. In contrast to measurement of CAC and IMT, it has the advantage of ease of use in the primary care physician's office and in community settings. The equipment is inexpensive – a hand held Doppler costing less than $600. The procedure is simple, takes less than 10-15 minutes39,40 and can be carried out by a suitably trained nurse or other health care professional. Technological advances to make the test quicker and easier to apply are being investigated, including automatic pressure measurement at the ankle.41 Given the non-invasiveness of the test and minimal discomfort, patient acceptability is high. Variability is comparable to that of routine blood pressure42,43 and individuals with borderline results may benefit from a repeat measure at a different visit.43
Although widely used in specialist vascular clinics, the ABI is rarely applied in routine clinical practice. There are probably several barriers to its use: firstly, most clinicians are not aware that a low ABI is a marker of cardiovascular risk; secondly, it is perceived as a specialist test for use only by vascular surgeons and physicians; and thirdly, most clinicians would not know how to perform the test. Clearly, physician education would be essential in promoting use of the ABI in practice. Furthermore in a survey of physicians primed to use the ABI in one program in the USA, time constraints, lack of reimbursement, and staff availability were barriers to use of the ABI, each reported by around half the physicians.40
The yield of a screening test is also important. Our results indicate that a proportion of men and women having an ABI test would be placed in a different risk category. However, this proportion may vary considerably by age because the prevalence of a low ABI is known to increase substantially with age. For example, in the United States in 2000, the prevalence of an ABI < 0.9 in non Hispanic white men aged 40-49 years was 1.4% increasing to 22.6% in those aged 80 years and older.44 Significantly higher prevalences were found in African Americans. In Scotland in 12,300 men free of cardiovascular disease in the general population, the prevalence of an ABI ≤ 0.9 in those aged 50-54 years was 3.7% increasing to 12.7% in those aged 75 years and older.45 While recognising that most risk factors also increase with age, it is likely that the added yield of a low ABI is age related.
Recently published guidelines by the American Heart Association (AHA)/American College of Cardiology (ACC),46 Transatlantic Inter-Society Consensus (TASC II) Working Group47 and Fourth Joint European Task Force48 have suggested that the ABI should be considered for the purposes of cardiovascular risk assessment. The results of our study indicate that, when using FRS, this may indeed be justified in order to improve prediction of cardiovascular risk and provision of advice on ways to reduce that risk. A new risk equation incorporating the ABI and relevant Framingham risk variables could more accurately predict risk and our intention is to develop and validate such a model in our combined dataset. Cost effectiveness modelling of the impact of using the ABI on long term clinical outcomes would also be of interest, as has been recommended recently by an AHA expert working group on screening for atherosclerotic peripheral vascular disease (Michael H Criqui, MD, University of California San Diego: written communication, January 08). A cost-effectiveness analysis would also be useful because successful implementation of the ABI in programmes for assessment of cardiovascular risk would require a change in reimbursement regulations in some countries.
Supplementary Material
Acknowledgments
The authors are grateful to Sanofi Aventis/BMS for an unrestricted educational grant for data processing and initial statistical analysis. They had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Contributions to individual studies in the ABI Collaboration are listed in the papers referenced.9-17, 19-25 In addition, the Framingham Offspring Study is supported by NIH grant NOI-HC-25195 and the InCHIANTI study and Women's Health and Aging Study by the Intramural Research Program, National Institute on Aging, NIH.
Appendix 1: Studies and investigators participating in Ankle Brachial Index Collaboration
| Co-ordinating Centre: | FGR Fowkes, GD Murray, I Butcher, CL Heald, RJ Lee |
| Atherosclerosis Risk in Communities (ARIC) Study9: | LE Chambless, AR Folsom, AT Hirsch |
| Belgian Physical Fitness Study17: | M Dramaix, G deBacker, J-C Wautrecht, M Kornitzer |
| Cardiovascular Health Study10: | AB Newman, M Cushman, K Sutton-Tyrrell |
| Edinburgh Artery Study13: | FGR Fowkes, AJ Lee, JF Price |
| Framingham Offspring Study19: | RB d'Agostino, JM Murabito |
| Health in Men Study20: | PE Norman, K Jamrozik |
| Honolulu Heart Program11: | JD Curb, KH Masaki, BL Rodríguez |
| Hoorn Study21: | JM Dekker, LM Bouter, RJ Heine, G Nijpels, CDA Stehouwer |
| InCHIANTI Study22: | L Ferrucci, MM McDermott |
| Limburg PAOD Study14: | HE Stoffers, JD Hooi, JA Knottnerus |
| Men Born in 1914 Study15: | M Ogren, L Janzon*, B Hedblad |
| Rotterdam Study16: | JC Witteman, MMB Breteler, MGM Hunink, A Hofman |
| San Diego Study23: | MH Criqui, RD Langer, A Fronek |
| San Luis Valley Diabetes Study24: | WR Hiatt, R Hamman |
| Strong Heart Study12: | HE Resnick |
| Women's Health and Aging Study25: | J Guralnik, MM McDermott |
Appendix 2: Electronic Search Strategy to Identify Population Cohort Studies of Ankle Brachial Index
| Databases: | Ovid MEDLINE ® 1950 to February Week 4 2008 EMBASE 1980 to February Week 4 2008 |
ABPI.tw.
ABI.tw.
AAI.tw.
ankle brachial pressure index $.tw.
ankle brachial pressure$.tw.
ankle brachial index$.tw.(or ankle brachial index/)
ankle arm index$.tw.
ankle arm blood pressure$.tw.
ankle arm blood pressure index$.tw.
ankle blood pressure$.tw.
or/1-10
follow up stud$.tw.
follow up studies/ or follow up/
epidemiological stud$.tw.
epidemiological studies/ or epidemiology/
cohort$.tw.
cohort analysis/ or cohort studies/
or/12-17
11 and 18
Footnotes
FGR Fowkes and GD Murray had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
deceased
Disclosures: Several authors have received honoraria, consulting fees or research grants from Sanofi Aventis/BMS for purposes other than this research. In addition Dr McDermott has received consulting fees from Hutchison Technology and educational honoraria from Otsuka Pharmaceuticals, and Dr Ogren is an employee of AstraZeneca R + D. Otherwise, the authors have no potential conflict of interest, including special financial interests and relationships and affiliations, relevant to the subject of this manuscript.
References
- 1.Greenland P, Smith SC, Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–7. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Brindle PM, Beswick AD, Fahey T, Ebrahim SB. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–9. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–50. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the Cardiovascular Health Study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(Suppl):C19–31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 9.Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, Rosamond WD. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord. 2007;7:3. doi: 10.1186/1471-2261-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 11.Abbott RD, Petrovitch H, Rodriguez BL, Yano K, Schatz IJ, Popper JS, Masaki KH, Ross GW, Curb JD. Ankle/brachial blood pressure in men >70 years of age and the risk of coronary heart disease. Am J Cardiol. 2000;86:280–4. doi: 10.1016/s0002-9149(00)00914-0. [DOI] [PubMed] [Google Scholar]
- 12.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 13.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–4. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol. 2004;57:294–300. doi: 10.1016/j.jclinepi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Ogren M, Hedblad B, Isacsson SO, Janzon L, Jungquist G, Lindell SE. Non-invasively detected carotid stenosis and ischaemic heart disease in men with leg arteriosclerosis. Lancet. 1993;342:1138–41. doi: 10.1016/0140-6736(93)92123-b. [DOI] [PubMed] [Google Scholar]
- 16.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109:1089–94. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 17.Kornitzer M, Dramaix M, Sobolski J, Degre S, De Backer G. Ankle/arm pressure index in asymptomatic middle-aged males: an independent predictor of ten-year coronary heart disease mortality. Angiology. 1995;46:211–9. doi: 10.1177/000331979504600304. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead A. Meta-Analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons Ltd; 2002. [Google Scholar]
- 19.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–5. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 20.Fowler B, Jamrozik K, Norman P, Allen Y. Prevalence of peripheral arterial disease: persistence of excess risk in former smokers. Aust N Z J Public Health. 2002;26:219–24. doi: 10.1111/j.1467-842x.2002.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 21.Jager A, Kostense PJ, Ruhe HG, Heine RJ, Nijpels G, Dekker JM, Bouter LM, Stehouwer CD. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:617–24. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–10. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 23.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–9. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101:1007–12. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Pasternak R, Greenland P, Smith S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations. A statement for health care professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 1999;34:1348–59. doi: 10.1016/s0735-1097(99)00387-3. [DOI] [PubMed] [Google Scholar]
- 27.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum. Results from the Cardiovascular Health Study. Circulation. 2006;113:388–93. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle brachial index and subclinical cardiac and carotid disease. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 30.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988:958–65. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 31.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 32.Cook NR. Use and misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 33.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham Score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 34.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 35.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Vogel RA, Wesley DJ, American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography); Society of Atherosclerosis Imaging and Prevention; Society of Cardiovascular Computed Tomography ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–26. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 36.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–90. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 37.Baldassarre D, Amato M, Pustina L, Castelnuovo S, Sanvito S, Gerosa L, Veglia F, Keidar S, Tremoli E, Sirtori CR. Measurement of carotid artery intima-media thickness in dyslipidemic patients increases the power of traditional risk factors to predict cardiovascular events. Atherosclerosis. 2007;191:403–8. doi: 10.1016/j.atherosclerosis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Bernard S, Serusclat A, Targe F, Charriere S, Roth O, Beaune J, Berthezene F, Moulin P. Incremental predictive value of carotid ultrasonography in the assessment of coronary risk in a cohort of asymptomatic type 2 diabetic subjects. Diabetes Care. 2005;28:1158–62. doi: 10.2337/diacare.28.5.1158. [DOI] [PubMed] [Google Scholar]
- 39.Farkouh ME, Oddone EZ, Simed DL. Improving the clinical examination for a low ankle-brachial index. Int J Angiol. 2002;11:41–45. [Google Scholar]
- 40.Mohler ER, III, Treat-Jacobson D, Reilly MP, Cunningham KE, Miami M, Criqui MH, Hiatt WR, Hirsch AT. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;9:253–60. doi: 10.1191/1358863x04vm559oa. [DOI] [PubMed] [Google Scholar]
- 41.Jönsson B, Laurent C, Eneling M, Skau T, Lindberg LG. Automatic ankle pressure measurements using PPG in ankle-brachial pressure index determination. Eur J Vasc Endovasc Surg. 2005;30:395–401. doi: 10.1016/j.ejvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Baker JD, Dix DE. Variability of Doppler ankle pressures with arterial occlusive disease: an evaluation of ankle index and brachial-ankle pressure gradient. Surgery. 1981;89:134–7. [PubMed] [Google Scholar]
- 43.Fowkes FGR, Housley E, Macintyre CCA, Prescott RJ, Ruckley CV. Variability of ankle and brachial systolic pressures in the measurement of atherosclerotic peripheral arterial disease. J Epidemiol Commun Health. 1988;42:128–33. doi: 10.1136/jech.42.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Price JF, Stewart MCW, Douglas A, Murray GD, Fowkes FGR. Frequency of a low ankle brachial index in the general population by age, sex and deprivation: cross sectional survey of 28,980 men and women. Eur J Cardiovasc Prev Rehab. doi: 10.1097/HJR.0b013e3282f8b36a. in press. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 47.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, Rutherford RB. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33 1:S1–S70. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]; J Vasc Surg. 2007;45:1S–68S. [Google Scholar]
- 48.Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European guidelines on cardiovascular disease prevention in clinical practice. Eur J of Cardiovasc Prev Rehab. 2007;14 2:S1–S113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.