SUMMARY
Background
Neuropsychiatric symptoms are nearly universal in dementia, yet little is known about their longitudinal course in the community.
Objective
To estimate point and 5-year period prevalence of neuropsychiatric symptoms in an incident sample of 408 dementia participants from the Cache County Study.
Methods
The Neuropsychiatric Inventory assessed symptoms at baseline and at 1.5 years, 3.0 years, 4.1 years, and 5.3 years. Point prevalence, period prevalence and mean symptom severity at each time point were estimated.
Results
Point prevalence for delusions was 18% at baseline and 34–38% during the last three visits; hallucinations, 10% at baseline and 19–24% subsequently; agitation/aggression fluctuated between 13% and 24%; depression 29% at baseline and 41–47% subsequently; apathy increased from 20% at baseline to 51% at 5.3 years; elation never rose above 1%; anxiety 14% at baseline and 24–32% subsequently; disinhibition fluctuated between 2% and 15%; irritability between 17% and 27%; aberrant motor behavior gradually increased from 7% at baseline to 29% at 5.3 years. Point prevalence for any symptom was 56% at baseline and 76–87% subsequently. Five-year period prevalence was greatest for depression (77%), apathy (71%), and anxiety (62%); lowest for elation (6%), and disinhibition (31%). Ninety-seven percent experienced at least one symptom. Symptom severity was consistently highest for apathy.
Conclusions
Participants were most likely to develop depression, apathy, or anxiety, and least likely to develop elation or disinhibition. Give converging evidence that syndromal definitions may more accurately capture neuropsychiatric co-morbidity in dementia, future efforts to validate such syndromes are warranted.
Keywords: dementia, neuropsychiatric, prevalence
INTRODUCTION
Neuropsychiatric symptoms affect nearly all dementia patients over the course of their illness (Tariot et al., 1995; Steinberg et al., 2004; Aalten et al., 2005). They have been associated with patient and caregiver distress (Rabins et al., 1999; Tan et al., 2005; Shin et al., 2005; Steele et al., 1990). In the prevalent dementia sampling of the Cache County Study on Memory and Health in Aging (CCSMHA), prevalence of any one neuropsychiatric symptom was 61% (Lyketsos et al., 2000), and 69% who were symptom-free at baseline had at least one symptom when followed up 18 months later (Steinberg et al., 2003).
No studies to our knowledge have examined the longitudinal course of neuropsychiatric symptoms of dementia over several years at the population level in the community setting. Population-based studies avoid referral biases inherent in clinical samples, which may overestimate symptom frequency and severity. The Cache County Study on Memory in Aging (CCSMHA) and Dementia Progression Study (DPS) provide the opportunity for such longitudinal observation. The Cache County studies have followed a cohort of dementia participants for up to 5 years. Those diagnosed with incident dementia in the CCSMHA completed further longitudinal assessment in the DPS, at this time up to a mean (and median) of 5 years post dementia identification. Thus, the point and period prevalence of neuropsychiatric symptoms, as well as symptom severity, was determined at each assessment visit in this population cohort.
METHODS
Sampling and screening
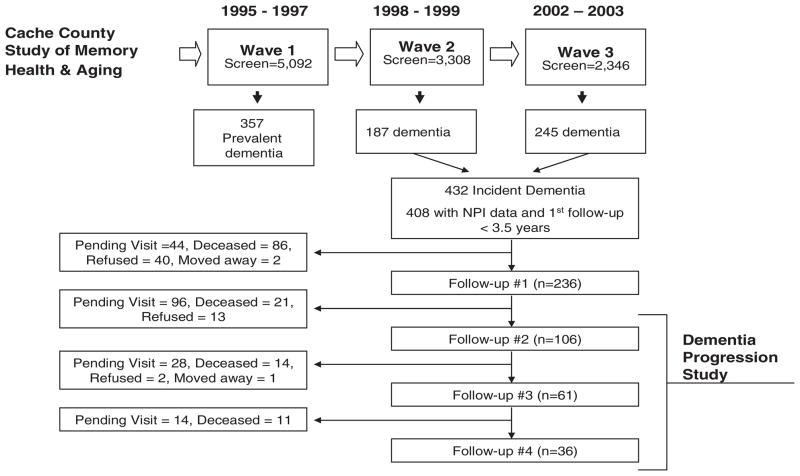
The methods of the CCSMHA are reported elsewhere (Breitner et al., 1999; Lyketsos et al., 2000) and illustrated in Figure 1. Briefly, we approached all permanent residents of Cache County, Utah, who were 65 years old or older in January 1995 (n = 5,677). We enrolled 5,092 of these (90%), all of whom were screened for dementia. Participants whose screening score suggested suspected or probable dementia underwent a comprehensive assessment in the presence of a collateral informant. After careful case ascertainment, we identified 357 prevalent cases of dementia. The remaining 3,308 participants without dementia at baseline were re-assessed over the course of two waves 3–4 years apart. We thus identified 432 participants with new-onset (i.e. incident) dementia in the CCSMHA. Twenty-four participants were excluded due to lack of neuropsychiatric data or first follow-up longer than 3.5 years after diagnosis. The remaining 408 participants were followed past 36 months in the DPS.
Figure 1.
Sampling methods of CCSMHA and DPS.
The studies were approved by the institutional review boards at Duke University Medical Center, Johns Hopkins University, and Utah State University, and all participants and their next of kin signed an informed consent document for each stage of the study.
Procedure
The Neuropsychiatric Inventory (NPI) (Cummings et al., 1994) was administered at the time of case ascertainment to the 408 participants with new-onset (e.g. incident) dementia identified in the CCSMHA. Administration of the NPI was repeated at the following mean time intervals on all participants who were alive and available for follow-up: 1.5 years (SD = 0.41 years, range = 0.8–3.3 years) (n = 236), 3.0 years (SD = 1.2 years, range = 1.4–7.0 years) (n = 106), 4.1 years (SD = 1.1 years, range = 2.2–7.3 years) (n = 61), 5.3 years (SD = 0.7 years, range = 3.8–8.2 years) (n = 36). This is illustrated in Figure 1. Among the 372 ‘attritions’, 182 (49%) remain enrolled in the DPS, but their next scheduled visit was not completed at the time of these analyses. This reflects the nature of DPS as an ongoing longitudinal study. The cohort studied represents a collection of incident (new-onset) dementia cases collected over a five year period. Follow-up data is thus available over a variable amount of time depending on when new-onset dementia was first ascertained. Additional reasons for attrition during each follow-up interval are summarized in Figure 1.
At baseline, we reviewed data to classify study participants into distinct dementia groups by dementia diagnosis. A Clinical Dementia Rating (CDR) was assigned to each participant (Morris, 1994). A General Medical Health Rating (GMHR) (Lyketsos et al., 1999) was also assigned. The GMHR, developed at Johns Hopkins University, is a four-point global rating of seriousness of non-cognitive medical co-morbidity in persons with cognitive disorders.
Of the 408 participants of interest, 255 (63.0%) were diagnosed with Alzheimer’s disease (AD) and 44 (10.8%) with vascular dementia (VaD). Twenty-seven (6.6%) were diagnosed with mixed AD/VaD. Eighty-two (20.1%) were diagnosed with other dementias: Dementia of Undetermined etiology (58), Dementia secondary to Parkinson’s disease (14). The remaining 10(*) consisted of Frontotemporal dementia, possible Dementia with Lewy Bodies, Progressive Supranuclear Palsy, dementia due to head injury, and dementia due to hypoperfusion and other neuropathological findings (*numerical counts suppressed due to low frequencies, in compliance with the policies of the Centers for Medicare and Medicaid Services to protect participant confidentiality).
Assessment of neuropsychiatric symptoms
The NPI version used in the CCSMHA (Cummings et al., 1994) assessed ten categories of neuropsychiatric symptoms that have been reported in dementia: delusions, hallucinations, agitation/aggression, depression, apathy, elation, anxiety, disinhibition, irritability, and aberrant motor behavior (such as wandering or pacing). The NPI examines whether symptoms have occurred over the past month. Each operationally defined symptom is ascertained by a trained examiner, through a structured interview with the caregiver. The informant is asked about the frequency of symptoms in the domain on a four-point scale from 1 (occasionally; less than once a week) to 4 (very frequently; more than once a day). The informant is also asked to rate the severity (disruptiveness, burden) of the behavior on a three-point scale (mild, moderate, or severe). By multiplying the severity and frequency scores, the NPI yields a domain rating with a range of 1–12.
Analysis
Our primary goal was to estimate at the population level the point and cumulative prevalence of each neuropsychiatric symptom (NPI score >0 in a given domain) and of any neuropsychiatric symptom (total NPI score >0). For point prevalence, we estimated the proportion of participants with each symptom at baseline and at each of the four follow-up points. For period prevalence, we estimated the proportion of participants with each symptom at the given assessment or at any assessment prior. The mean severity score for each symptom and for total NPI was also estimated at baseline and at each follow-up.
RESULTS
The baseline characteristics of participants at the time of case ascertainment are shown in Table 1. Approximately 60% were diagnosed with AD. Twenty-six percent were being treated with psychotropics and 7% of the sample were being treated with cholinesterase inhibitors. These treatment percentages remained stable over the five years of follow-up; at no assessment point were more than 28% treated with psychotropics, or more than 9% treated with cholinesterase inhibitors. Compared to those who completed at least one follow-up visit, those who did not were older and had more severe dementia. They also had higher frequencies of delusions, hallucinations, agitation/aggression, anxiety, disinhibition and irritability (Table 2).
Table 1.
The baseline characteristics of 408 Cache County Study participants
| Characteristic | No follow-up after t-0 (n = 172) (mean, SD) | At least one follow-up after t–0 (n = 236) (mean, SD) | t(df) | p |
|---|---|---|---|---|
| Mean age (yrs) | 84.9 (6.6) | 81.8 (6.6) | 4.4 (406) | <0.01 |
| Mean years of education | 13.1 (2.9) | 13.2 (3.0) | 0.2 (405) | 0.84 |
| Mean duration of dementia (yrs) | 1.9 (1.3) | 2.0 (1.3) | −0.9 (406) | 0.39 |
| Characteristic | n (%) | n (%) | Chi square (df) | p |
| Male gender | 60 (34.9) | 94 (39.8) | 1.0 (1) | 0.31 |
| One or more APOE 4 allele(s) | 77 (45.6) | 94 (39.8) | 1.3 (1) | 0.25 |
| Alzheimer’s disease | 99 (57.6) | 156 (66.1) | 3.1 (1) | 0.08 |
| Psychotropic treatment | 39 (23.1) | 67 (28.4) | 1.4 (1) | 0.23 |
| Cholinesterase inhibitor treatment | 8 (4.7) | 19 (8.1) | 1.7 (1) | 0.19 |
| Clinical Dementia Rating | ||||
| 0.5–1 (mild) | 125 (74.0) | 197 (85.6) | ||
| 2 (moderate) | 24 (14.2) | 25 (10.9) | ||
| 3–5 (severe) | 20 (11.8) | 8 (3.5) | 12.2 (2) | <0.01 |
| GMHR | ||||
| 1–2 (fair or poor)* | 82 (47.7) | 93 (39.4) | ||
| 3 (good) | 73 (42.4) | 124 (52.5) | ||
| 4 (excellent) | 17 (9.9) | 19 (8.1) | 4.1 (2) | 0.13 |
Note: Bold entries indicate clinical significance (e.g. p <0.05).
Table 2.
The baseline neuropsychiatric symptom frequency of 408 Cache County Study participants
| Neuropsychiatric symptom | No follow-up after t-0 (n = 172) n (%) | At least one follow-up after t–0 (n = 236) n (%) | Chi square (df) | p |
|---|---|---|---|---|
| Delusions | 40 (23.3) | 33 (14.0) | 5.8 (1) | 0.02 |
| Hallucinations | 24 (14.1) | 17 (7.2) | 5.2 (1) | 0.02 |
| Agitation/aggression | 34 (19.9) | 24 (10.2) | 7.7 (1) | 0.01 |
| Depression | 54 (31.4) | 64 (27.1) | 0.9 (1) | 0.35 |
| Apathy | 33 (19.2) | 50 (21.2) | 0.3 (1) | 0.62 |
| Elation | 1 (0.6) | 1 (0.6) | 0.1 (1) | 0.82 |
| Anxiety | 35 (20.3) | 22 (9.3) | 10.1 (1) | <0.01 |
| Disinhibition | 17 (9.9) | 11 (4.7) | 4.2 (1) | 0.04 |
| Irritability | 43 (25.0) | 38 (16.1) | 5.0 (1) | 0.03 |
| Aberrant Motor Behavior | 16 (9.3) | 12 (5.1) | 2.8 (1) | 0.10 |
Note: Bold entries indicate clinical significance (e.g. p <0.05).
Figure 2 shows the point prevalence of neuropsychiatric symptoms at baseline and at each of the four follow-up intervals. Point prevalence for delusions was 18% at baseline and 34–38% during the last three visits; hallucinations 10% at baseline and 19–24% at all subsequent visits; agitation fluctuated between 13 and 24%; depression 29% at baseline and 40–47% subsequently; apathy gradually increased from 20% at baseline to 51% at 5.3 years; elation never rose above 1%; anxiety 14% at baseline and 24–32% subsequently; irritability fluctuated between 17% and 27%; disinhibition fluctuated between 2% and 15%; aberrant motor behavior gradually increased from 7% at baseline to 29% at 5.3 years. Point prevalence for any symptom (total NPI >0) rose from 56% at baseline to 81–87% at the last three visits.
Figure 2.
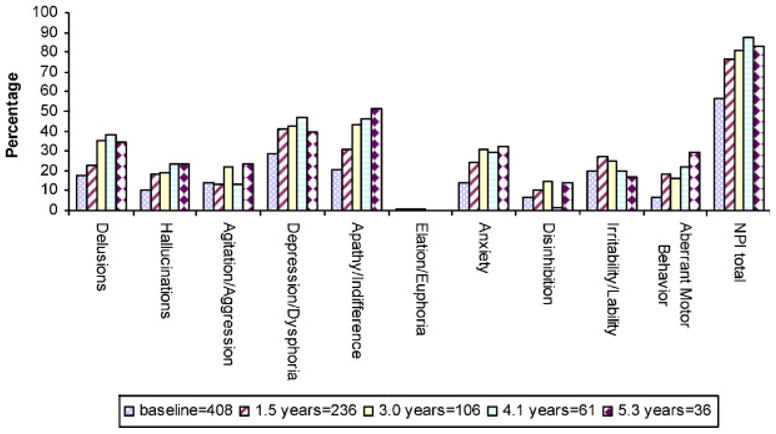
Point prevalence of NPI symptoms (NPI > 0)
Figure 3 shows the period prevalence of neuropsychiatric symptoms at baseline and at each of the four follow-up intervals (i.e. baseline prevalence, 1.5-year period prevalence, 3.0-year period prevalence, 4.1-year period prevalence, and 5.3-year period prevalence). Over 5 years, period prevalence was greatest for depression (77%), apathy (71%), and anxiety (62%); lowest for elation (6%) and disinhibition (31%). Ninety-seven percent experienced at least one neuropsychiatric symptom.
Figure 3.
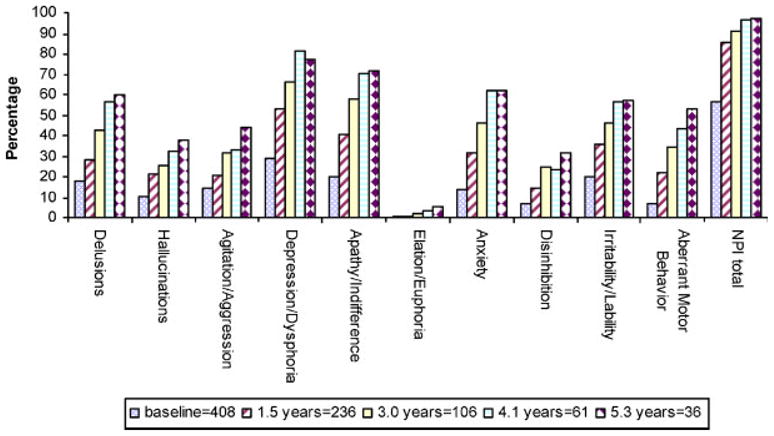
Five-year period prevalence of NPI symptoms (NPI >0).
The mean symptom severity among participants with an NPI score >0 in each domain is presented in Table 3. The highest severity score at each assessment point was consistently for apathy. For most other items, mean severity was in the 2.0 to 4.0 range at most assessment points.
Table 3.
Mean NPI symptom severity
| Observation Period | Neuropsychiatric Symptom Mean (SD) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Delusions | Hallucinations | Agitation/Aggression | Depression | Apathy | Anxiety | Irritability | Disinhibition | Aberrant Motor Behavior | |
| baseline | 3.2 (2.5) | 2.1 (1.5) | 3.4 (2.6) | 3.7 (3.1) | 5.1 (3.4) | 3.6 (2.7) | 3.9 (2.9) | 3.0 (2.6) | 4.3 (3.1) |
| 1.5 years | 2.7 (2.1) | 2.6 (2.7) | 3.1 (2.4) | 3.0 (2.4) | 5.8 (2.9) | 2.6 (1.9) | 3.2 (2.2) | 2.0 (1.4) | 2.6 (1.8) |
| 3.0 years | 3.9 (2.1) | 3.8 (2.4) | 3.8 (2.6) | 3.5 (2.3) | 6.1 (2.6) | 3.3 (2.1) | 3.3 (2.3) | 3.0 (2.0) | 4.5 (2.5) |
| 4.1 years | 3.8 (1.9) | 3.3 (2.0) | 3.2 (2.1) | 2.8 (1.7) | 6.1 (2.7) | 3.4 (2.0) | 3.5 (2.8) | 8.0 (n/a)* | 3.8 (1.6) |
| 5.3 years | 2.9 (1.3) | 2.4 (1.2) | 2.5 (2.3) | 3.4 (3.0) | 6.1 (3.0) | 2.7 (1.1) | 4.3 (3.9) | 1.6 (0.9) | 3.4 (1.3) |
cannot be calculated due to small n.
DISCUSSION
In this study of the longitudinal course of neuropsychiatric symptoms in dementia over a mean (and median) of 5 years, we found a trend for the point prevalence of nearly all symptoms to increase over time. Nevertheless, prevalence varied among individual symptoms. Apathy, depression, delusions overall were most common, while disinhibition and elation were less so. Elation never occurred in more than 1% of participants during any visit. In this regard, our finding of the most and least commonly occurring symptoms in dementia are similar to those of our earlier studies of prevalence, incidence and persistence (Lyketos et al., 2000; Steinberg et al., 2003; Steinberg et al., 2004) as well as to those of the MAAstrict Study of BEhavior in Dementia (MAASBED) study (Aalten et al., 2005). Symptom severity was consistently highest for apathy. This may reflect the tendency of this symptom to be persistent (e.g. present more days than not over the interval assessed) as opposed to the degree that it is disruptive or burdensome.
In our study of cumulative 5-year period prevalence, 97% of participants experienced at least one neuropsychiatric symptom. This is consistent with studies of even shorter duration which suggest such symptoms in dementia are nearly universal (Tariot et al., 1995; Steinberg et al., 2003; Aalten et al., 2005). Similar to our findings of point prevalence, cumulative prevalence was greatest for depression (77%) and apathy (71%). In most prior studies using the NPI, apathy has been the most common symptom (Lyketsos et al., 2000; Mirakhur et al., 2004; Aalten et al., 2005).
Anxiety, despite modest point prevalences (14–32%), had the third highest five-year period prevalence at 62%. Meanwhile, lower five-year period prevalences were seen for elation (6%), and disinhibition (31%). This suggests that the relative rarity of these symptoms in our prior point prevalence and 18-month incidence and persistence studies (Lyketsos et al., 2000; Steinberg et al., 2003; Steinberg et al., 2004) is indeed a valid finding.
The rare use (less than 9%) of cholinesterase inhibitors, suggests undertreatment of cognitive symptoms of dementia in the community. Psychotropic use was more common. At baseline 26% of participants were treated with psychotropics, and 14–28% were treated with them at subsequent visits. Thus an overall increase over time in neuropsychiatric co-morbidity was not reflected by an increase in psychotropic use in this cohort. This may reflect under-recognition of psychiatric co-morbidity as dementia advances. Alternatively, clinicians may have assessed symptoms as not warranting psychotropic use, or stopped psychotropics due to lack of efficacy.
Most studies of treatment for neuropsychiatric co-morbidity in dementia report findings for individual symptoms (e.g. depression, apathy), while converging evidence suggests that this co-morbidity may be more accurately captured by syndromal definitions (Hope et al., 1997; Frisoni et al., 1999; Fuh et al., 2001; Lyketsos et al., 2001a, 2001b; Aalten et al., 2003, Robert et al., 2005; Steinberg and Lyketsos, 2005). Recently, several definitions have been proposed for an affective (Lyketsos et al., 2001b; Olin et al., 2002) and a psychotic (Jeste and Finkel, 2000; Lyketos et al., 2001b) syndrome in AD.
Several limitations of our study deserve discussion. First, as we have noted before, we studied a population that is older and less ethnically diverse than the rest of the United States. Second the principal method of assessment of neuropsychiatric symptoms, the NPI, relied on informants and not on direct examination of participants. Third, the attrition rate at each assessment point was high. At 5 years, less than 10% of the baseline sample was available for follow-up. Nearly half of these subjects remain enrolled in the DPS, but their next scheduled visit was not completed at the time of these analyses, reflecting the nature of the DPS as an ongoing longitudinal study. Most of the other losses were due to death, an inevitable consequence of following participants with progressive dementia over extended periods. Those who were not followed past baseline were older, and had more severe dementia and higher frequencies of six out of ten neuropsychiatric symptoms assessed. Thus, the longitudinal prevalences reported here may be underestimates. Fourth, the wide range in the time elapsed between follow-up visits limits our ability to draw precise estimates about prevalence, although this does not compromise our study’s central finding of the overall high point and period prevalence of neuropsychiatric symptoms. Finally, the ten-item NPI did not assess sleep and appetite disturbances (subsequent study waves have used the 12-item NPI, which includes these items).
In summary, we found a trend for most neuropsychiatric symptoms to increase in both point and cumulative prevalence over 5 years. Given converging evidence that syndromal definitions may more accurately capture neuropsychiatric co-morbidity in dementia, efforts to validate such syndromes through the study of risk factors, genetics, clinical-pathological studies, and the effect of treatment on course are warranted.
Acknowledgments
Supported by National Institute of Aging grant AG-11380 for the Cache County Study on Memory and Aging and grant AG-21136 for the Dementia Progression Study.
We wish to thank Kathleen Hayden for her thoughtful review of this manuscript. We are grateful to the neurogenetics laboratory of the Bryan Alzheimer’s Disease Research Center at Duke University for the APOE genotyping, and to Cara Brewer, BA, Tony Calvert, BSC, Michelle McCart, BA, Tiffany Newman, BA, Roxane Pfister, MA, Nancy Sassano, PhD, and Joslin Werstack, BA for expert technical assistance. Other Cache County Study of Memory, Health, and Aging Investigators include: James Anthony, PhD, Erin Bigler, PhD, John Breitner, MD, MPH, Ron Brookmeyer, PhD, James Burke, MD, PhD, Eric Christopher, MD, Chris Corcoran, PhD, Jane Gagliardi, MD, Robert Green, MD, Kathleen Hayden, PhD, Michael Helms, Christine Hulette, MD, Liz Klein, MPH, Carol Leslie, MS, Constantine Lyketsos, MD, MHS, Lawrence Mayer, MD, John Morris, MD, Ron Munger, PhD, MPH, Maria Norton, PhD, Chiadi Onyike, MD, MHS, Truls Ostbye, MD, PhD, MPH, Ron Petersen, MD, Kathy Piercy, PhD, Carl Pieper, DrPH, Brenda Plassman, PhD, Peter Rabins, MD, Pritham Raj, MD, Russell Ray, MS, Linda Sanders, MPH, Ingmar Skoog, MD, David Steffens, MD, MHS, Martin Steinberg, MD, Marty Toohill, PhD, Leslie Toone, MS, Jeannette Townsend, MD, JoAnn Tschanz, PhD, Lauren Warren, Kathleen Welsh-Bohmer, PhD, Heidi Wengreen, PhD, Michael Williams, MD, Bonita Wyse, PhD, and Peter Zandi, PhD.
Neuropsychological testing and clinical assessment procedures were developed by Dr Welsh-Bohmer and Dr Breitner. Dr Tschanz provided training and oversight of all field staff and reviewed all individual neuropsychological test results to render professional diagnoses. The board-certified or board-eligible psychiatrists or neurologists who examined the study members included Drs Steinberg, Breitner, Steffens, Lyketsos, and Green. Dr. Williams also examined several subjects and provided expert neurologic consultation. Autopsy examinations were conducted by Dr. Townsend. Ms. Leslie coordinated the autopsy enrollment program. Diagnosticians at the expert consensus conferences included Drs Breitner, Burke, Lyketsos, Plassman, Steffens, Steinberg, Tschanz, and Welsh-Bohmer.
Footnotes
CONFLICT OF INTEREST
The authors have no financial or personal relationships which might bias this work.
References
- Aalten P, de Vugt ME, Louisberg R, et al. Behavioural problems in dementia: a factor analysis of the Neuropsychiatric Inventory. Dement Geriatr Cogn Disord. 2003;15:99–105. doi: 10.1159/000067972. [DOI] [PubMed] [Google Scholar]
- Aalten P, de Vugt ME, Jaspers N, et al. The course of neuropsychiatric symptoms in dementia. Part 1: findings from the two year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;15:523–530. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- Breitner JCS, Wyse BW, Anthony JC, et al. APOE-epsilon 4 count predicts age when prevalence of Alzheimer’s disease increases- then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Rozzini L, Gozzetti A. Behavioral syndromes in Alzheimer’s disease: description and correlates. Dement Geriatr Cogn Dis. 1999;10:130–138. doi: 10.1159/000017113. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Liu CK, Mega MS, et al. Behavioral disorders and caregivers’ reaction in Taiwaese patients with Alzheimer’s disease. Int Psychogeriatr. 2001;13:121–128. doi: 10.1017/s1041610201007517. [DOI] [PubMed] [Google Scholar]
- Hope T, Keene J, Fairburn C, et al. Behaviour changes in dementia. 2: are there behavioural syndromes? Int J Geriatr Psychiatry. 1997;12:1074–1078. doi: 10.1002/(sici)1099-1166(199711)12:11<1074::aid-gps696>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias. Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Galik E, Steele C, et al. The general medical health rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- Lyketsos C, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JE, Steinberg M, et al. Neuropsychiatric symptoms in Alzheimer’s disease clusters into three groups: the Cache County Study. 2001a. Int J Geriatr Psychiatry. 2001a;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Breitner JCS, Rabins PV. An evidence-based proposal for the classification of neuropsychiatric disturbances in Alzheimer’s disease. Int J Geriatr Psychiatry. 2001b;16:1037–1042. doi: 10.1002/gps.440. [DOI] [PubMed] [Google Scholar]
- Mirakhur A, Craig D, Hart DJ, et al. Behavioural and psychological syndromes in Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19:1035–1039. doi: 10.1002/gps.1203. [DOI] [PubMed] [Google Scholar]
- Morris J. Clinical Dementia Rating Scale. Washington University; St Louis: 1994. [Google Scholar]
- Olin JT, Schneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:125–128. [PubMed] [Google Scholar]
- Rabins PV, Lyketsos CG, Steele CD. Practical Dementia Care. Oxford University Press; New York: 1999. [Google Scholar]
- Robert PH, Verhey FRJ, Byrne EJ, et al. Grouping for behavioral and psychological symptoms in dementia: clinical and biological aspect. Consensus paper of the European Alzheimer disease consortium. Eur Psychiatry. 2005;20:490–496. doi: 10.1016/j.eurpsy.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Shin IS, Carter M, Masterman D, et al. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry. 1990;147:1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Sheppard JM, Tschanz JT, et al. The incidence of mental and behavioral disturbances in dementia: the Cache County Study. J Neuropsychiatry Clin Neurosci. 2003;15:340–345. doi: 10.1176/jnp.15.3.340. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004;19:19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Lyketsos CG. Pharmacological treatment of neuropsychiatric symptoms in dementia (letter) JAMA. 2005;11:2211–2212. doi: 10.1001/jama.293.18.2211-b. [DOI] [PubMed] [Google Scholar]
- Tan LL, Wong HB, Allen H. The impact of neuropsychiatric symptoms of dementia in family and professional caregivers in Singapore. Int Psychogeriatr. 2005;17:253–263. doi: 10.1017/s1041610205001523. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]