Abstract
In order to investigate the contributions of visual experience vs. preprogrammed mechanisms on visual development, the current study compared contrast sensitivity in preterm vs. fullterm infants. If development is tied to time since conception, preterm infants should match the developmental trajectories of fullterm infants when plotted in postterm age. By contrast, if development is influenced by visual experience, preterm and fullterm infants should match when plotted in postnatal age. Luminance (light/dark) and chromatic (red/green) contrast sensitivities (CS) were measured in 25 preterm (born, on average, 6.6 weeks early) and 77 fullterm infants, between 1 and 6 months postterm. In the first few months, luminance CS was found to be predicted by postterm age, suggesting that preprogrammed development is sufficient to account for luminance CS. By contrast, chromatic CS exceeded that predicted by postterm age, which suggests that time since birth confers a benefit on chromatic CS. The preterms’ 6.6 weeks of additional time since birth is roughly equivalent to 3.7 weeks of development in chromatic CS. In sum, these results suggest that chromatic CS is more influenced by early postnatal visual experience than luminance CS, which may have implications for development of parvocellular and magnocellular pathways.
Keywords: infant vision, preterm, premature, contrast sensitivity, luminance, chromatic
Introduction
In the field of visual development, one of the most commonly asked questions is whether visual experience is necessary for visual development to proceed normally, with the assumption that if it is, preprogrammed mechanisms are not sufficient. This question has typically been addressed in animals by dark rearing (Hendrickson & Boothe, 1976; Regal, Boothe, Teller, & Sackett, 1976), lid suturing (Blakemore & Vital-Durand, 1983; Harwerth, Smith, Boltz, Crawford, & von Noorden, 1983) or atropine after birth (Movshon et al., 1987). In humans, study of individuals who experienced abnormal visual input since birth (e.g., due to congenital cataracts) allows the necessity question to be addressed. The results of these animal and human studies reveal deficits, with varying severity, depending on the function studied and the length of deprivation (for reviews, see Boothe, Dobson, & Teller, 1985; Maurer & Lewis, 1993; Maurer, Lewis, & Mondloch, 2005). Most relevant to the current study, contrast sensitivity is quite abnormal for medium to high spatial frequencies following early and prolonged visual deprivation in humans (Birch, Stager, Leffler, & Weakley, 1998; Ellemberg, Lewis, Maurer, & Brent, 2000; Ellemberg, Lewis, Maurer, Lui, & Brent, 1999; Maurer & Lewis, 1993; Tytla, Maurer, Lewis, & Brent, 1988). In non-human primates, neuroscientific studies have shown that visual deprivation results in massive physiological and morphological changes in the primary visual cortex (e.g., Hendrickson, Movshon, Eggers, Gizzi, Boothe, & Kiorpes, 1987; Kiorpes, Boothe, Hendrickson, Movshon, Eggers, & Gizzi, 1987; Kiorpes & Movshon, 2004). By contrast, in the lateral geniculate nucleus (LGN), deprivation results in mainly morphological changes (including a recent report of changes in gene expression; see Cheng, Kaminski, Gong, Zhou, Hatala, Howell, Zhou, & Mustari, 2008), while visual response properties appear normal (Blakemore & Vital-Durand, 1986; Hendrickson et al., 1987; Levitt, Schumer, Sherman, Spear, & Movshon, 2001; but see Hess, Thompson, Gole, & Mullen, 2009 for recent human fMRI results showing overall reduced LGN activity after early abnormal visual experience). In sum, human and animal studies demonstrate that visual experience is necessary, and by extension, that preprogrammed mechanisms are not sufficient, for normal visual development.
Another way to address the influence of visual experience is to measure the effects of enriched visual environments. In animal studies (rats, cats, and primates), it has been shown that such environments alter neural development of visual areas (subcortical and cortical), most notably, by leading to increased myelin density (Black, Sirevaag, & Greenough, 1987; Gyllensten & Malmfors, 1963; Sanchez, Hearn, Do, Rilling, & Herndon, 1998; Sirevaag, Black, Shafron, & Greenough, 1988; Sirevaag & Greenough, 1991). In humans, one of the best ways to address the influence of enriched (or additional) visual experience is to study development in preterm infants. Here, the question is whether visual developmental trajectories of preterm infants are tied to their amount of time in the world after birth, reflected in their postnatal age, or by preprogrammed mechanisms that are timed to the point of conception, reflected in their postconceptional or postterm age.1 For example, a preterm infant born 1 month before term, at 3 months postbirth would be considered 2 months postterm. With these definitions in mind, if preprogrammed mechanisms are sufficient to account for visual development, preterm infants are expected to show the same developmental trajectories as fullterm infants when plotted with respect to postterm age. This scenario suggests that the preterms’ additional time since birth has no influence on development. In addition, given that earlier birth affords more visual experience, this scenario suggests that additional visual experience does not influence visual development. Conversely, if visual development is plotted with respect to postterm age and the developmental trajectories of preterms exceed those of fullterm infants, this suggests that the additional visual experience of preterms does influence their visual development, presumably by guiding biological mechanisms. Whether this guidance is permissive or instructive is a much-debated and interesting issue, which is outside the scope of the current study (see Crair, 1999; Feller & Scanziani, 2005; Kiorpes & Movshon, 2004; Movshon & Van Sluyters, 1981).
Before further conjecturing on the probabilities of these different scenarios, it is important to address whether preterm infants can even receive, and respond to, visual input before term age.2 Previous studies have shown that preterm infants open their eyes by 26 weeks postconception (Robinson, Moseley, Thompson, & Fielder, 1989), allowing them to at least receive visual input. By 31 weeks, they exhibit pupillary reactions (Robinson, 1966). In terms of subcortical responses, by 31 weeks, wavelet components from flash visual evoked potential (VEP) can be recorded (Chin, Taylor, Menzies, & Whyte, 1985; Hrbeck, Karlberg, & Olsson, 1973; Kurtzberg & Vaughan, 1985; Leaf, Green, Esack, Costeloe, & Prior, 1995; Taylor, Menzies, MacMillan, & Whyte, 1987; Watanabe, Iwase, & Hara, 1972). In terms of cortical responses, by 30 weeks, pattern reversal VEPs can be recorded (Grose, Harding, Wilton, & Bissenden, 1989; Harding, Grose, Wilton, & Bissenden, 1989), and steady-state pattern VEP can be recorded by 35 weeks (Birch, Birch, Hoffman, & Uauy, 1992; Birch, Birch, Petrig, & Uauy, 1990). In terms of behavioral responses, preferential looking behavior with highly visible stimuli can be demonstrated by 34–35 weeks, although pursuit and spontaneous scanning do not emerge until later (Brown & Yamamoto, 1986; Hack, Mostow, & Miranda, 1976; Hack, Muszynski, & Miranda, 1981; Robinson, 1966). In sum, the results from these previous studies indicate that infants receive, and respond to, visual input before term age, and thus there is the potential for visual experience to shape development during this period. Moreover, because so many aspects of the retina, optic nerve, LGN, and cortex are actively developing before term age (especially between 30 and 40 weeks gestation, reviewed in Birch & Bosworth, 2004; Birch & O’Connor, 2001), visual experience may have particularly strong effects during this period.
Although there have been many studies of visual development in preterm infants, predominantly investigating spatial acuity (see Discussion section), the results from such studies are mixed. Some studies have reported that development of acuity in preterms is tied to postterm age, in line with preprogrammed development being sufficient (FPL: Dobson, Mayer, & Lee, 1980; Fantz & Fagan, 1975; Getz, Dobson, & Luna, 1994; Ipata, Cioni, Boldrini, Bottai, & van Hof-van Duin, 1992, OKN: Weinacht, Kind, Monting, & Gottlob, 1999, and VEP: Harding et al., 1989; Kos-Pietro, Towle, Cakmur, & Spire, 1997; Mirabella, Kjaer, Norcia, Good, & Madan, 2006; Oliveira, Costa, de Souza, & Ventura, 2004). By contrast, other studies have reported that preterm acuity exceeds that predicted by postterm age, in line with an influential role of visual experience (FPL: van Hof-van Duin & Mohn, 1986, OKN: Roy, Lachapelle, & Lepore, 1989, VEP: Norcia, Tyler, Piecuch, Clyman, & Grobstein, 1987; Oliveira et al., 2004; Roy, Barsoum-Homsy, Orquin, & Benoit, 1995; Sokol & Jones, 1979; Taylor et al., 1987; Tsuneishi & Casaer, 2000, and VEP amplitude: Mirabella et al., 2006). There are several potential reasons for discrepancies across studies (see Discussion section), one of which lies in the inclusion of premature infants who meet a criterion of “very low birth weight” (under 1,500 g and are generally born ≤30 weeks gestation), a population with significant risk for brain abnormalities (Inder, Warfield, Wang, Huppi, & Volpe, 2005; Maalouf, Duggan, Rutherford, Counsell, Fletcher, Battin, Cowan, & Edwards, 1999; Rezaie & Dean, 2002) and ocular impairments (O’Connor, Spencer, & Birch, 2007; O’Connor, Stephenson, Johnson, Tobin, Ratib, Moseley, & Fielder, 2004; O’Connor, Wilson, & Fielder, 2007). Although many of the above-mentioned studies used neonatal cranial ultrasound with an attempt to exclude or separately analyze infants who were not neurologically normal (e.g., Atkinson, Anker, Rae, Weeks, Braddick, & Rennie, 2002; Downie, Jakobson, Frisk, & Ushycky, 2003; Hammarrenger, Roy, Ellemberg, Labrosse, Orquin, Lippe, & Lepore, 2007; Jackson, Ong, McIndoe, & Ripley, 2003; Jakobson, Frisk, & Downie, 2006; MacKay, Jakobson, Ellemberg, Lewis, Maurer, & Casiro, 2005; Mirabella et al., 2006), recent studies employing a combination of MRI and ultrasound have shown that cranial ultrasounds often do not detect neurological insult (Maalouf et al., 1999). For this reason, studies that fail to find that preterm trajectories exceed performance predicted by postterm age (i.e., suggesting that preprogrammed mechanisms are sufficient) could potentially be explained by undetected brain lesions that counteract positive effects of visual experience.
In the current study, we investigated developmental trajectories in preterm infants but attempted to circumvent potential confounds of neurological insult by testing only healthy “late” preterm infants who were born no more than 9 weeks premature, a population that has less than a 1% incidence of neurological abnormalities (Harris, Palacio, Ginzel, Richardson, & Swischuk, 2007). This moderate-to-late preterm range currently accounts for more than 70% of all preterm births and is the fastest growing population of birth rates in the United States over the past two decades (Davidoff, Dias, Damus, Russell, Bettegowda, Dolan, Schwarz, Green, & Petrini, 2006). Our visual measure was contrast sensitivity (CS), and we tested both luminance (light/dark) and chromatic (red/green) CS. In the Discussion section, we address the possibility that these two different types of CS are mediated by the parvocellular and magnocellular pathways, as there is speculation that the two pathways may be differentially affected by visual experience and/or differentially susceptible in various developmental disorders (see Braddick, Atkinson, & Wattam-Bell, 2003).
Methods
Subjects
Subject populations
Preterm and fullterm infants were recruited by mass mailings to new parents residing in San Diego County. At the time of enrollment, parents provided their infant’s birth date and due date. Parent report has been shown to be quite accurate for due date when the information is obtained soon after birth (Seidman, Slater, Ever-Hadani, & Gale, 1987), which is the case in the current study. Because we employed red/green stimuli, we excluded infants with a greater than 50% chance of colorblindness, for example, male infants whose paternal grandfather was known to be colorblind. To further ensure that all our infants were generally healthy, inclusion criteria for all infants (preterms and fullterms) included: (1) at the time of birth, no indication of hypoxia or fetal stress, a weight of at least 1,500 g and an appropriate weight for their gestational age (according to growth norms by Usher & McLean, 1969), (2) less than 2 days of assisted ventilation in the NICU after birth, and (3) between birth and while enrolled in our study, no history of surgery, hospitalizations, retinopathy of prematurity, convulsions, neurological abnormalities or brain lesions.
The inclusion criterion for fullterms was that the length of their gestational period was between 39 and 41 weeks. For preterms, gestational length was between 31 and 35 weeks. We chose this criterion for preterms because previous studies have shown that they are likely to be healthy (see Introduction section). Gestational length was calculated from the difference between an infant’s birth date and due date. Note that there will be some variability in our gestational length measure due to error in predicted due date, the latter derived based on ultrasound dating, typically within the first trimester (86% of our sample) or last menstrual period (13% of our sample). This error is on the order of ±2 days (see Dobkins, Bosworth, & McCleery, 2009 for discussion) and should be inconsequential for our purposes as our main goal is to compare our preterm and fullterm samples, which differ substantially in their gestational length.
A total of 29 preterm and 89 fullterm infants was tested, with 4 (14%) preterm and 12 fullterm (14%) infants excluded due to not meeting our minimum number of trials criterion (>50 total trials) due to fussiness or sleepiness. The final sample included in our analyses consisted of 25 preterm infants (13 males, 12 females) with gestational length that ranged from 31.5 to 35.4 weeks (i.e., born 4.6 to 8.5 weeks early) and a mean of 33.5 (±1.1) weeks, and 77 fullterm infants (36 males, 41 females) with gestational length that ranged from 38.1 to 41.0 weeks and a mean of 39.9 (±0.6) weeks.
Total number of data points
The total number of data points, which reflects the number of infants tested at each age, is presented in Table 1. Although the current study was set up as a longitudinal design, i.e., we asked parents to bring their infants to the laboratory at each monthly birthday between 2 and 7 months postnatal age, not all infants were tested at all ages. This is because some infants enrolled past 2 months, and because parents could not always make every time point. Thus, the final data set consisted of subjects who came in between 1 and 5 time points, with the particular time point(s) tested varying from subject to subject. Of the total sample of 102 infants, 70, 19, 9, 2, and 2 infants provided data for 1, 2, 3, 4, and 5 time points, respectively. This yielded 153 total data points (53 data points from preterms, and 100 data points from fullterms).
Table 1.
Number of data points in each postterm and postnatal group, for preterm and fullterm infants.
| Age (month) | Preterm |
Fullterm |
||
|---|---|---|---|---|
| Postterm | Postnatal | Postterm | Postnatal | |
| 1 | 5 | 0 | 0 | 0 |
| 2 | 7 | 2 | 24 | 25 |
| 3 | 11 | 6 | 17 | 16 |
| 4 | 15 | 8 | 21 | 20 |
| 5 | 9 | 15 | 15 | 15 |
| 6 | 6 | 14 | 16 | 16 |
| 7 | 0 | 8 | 7 | 8 |
| Total data points | 53 | 53 | 100 | 100 |
Postterm age and postnatal age
Mean postterm and postnatal ages, along with other birth statistics, are presented in Table 2. The postterm calculation (based on the due date) was performed for fullterms, as well as preterms, since our fullterm sample included infants born ±1 week from their due dates. For the purpose of plotting data and conducting statistical analyses, age groups were created by, first, determining the exact postterm and postnatal ages in months of each infant, which was calculated by dividing age by 30.4 days. Infants were then binned into age groups by monthly intervals, by rounding to the nearest whole digit month, i.e., 1.499 to 2.499 was binned as age category “2 months”, and this value was used as a categorical factor in the ANOVA. For Figures 1 and 2, which plot contrast sensitivity vs. age, we used the average of the exact age of the infants as the X-axis value. As expected, these averages of exact values were very close (although not identical) to the categorical values.
Table 2.
Means and standard deviations of postterm age, postnatal age, gestational length, birth weight and birth height. Data are divided into different postterm age groups, separately for preterm and fullterm infants.
| Age category (months) | Data points (#) | Postterm age at test (months) | Postnatal age at test (months) | Gestation length (weeks) | Birth weight (lb) | Birth height (in.) |
|---|---|---|---|---|---|---|
| Preterm infants | ||||||
| 1 | 5 | 1.1 (0.3) | 2.6 (0.6) | 33.5 (1.4) | 4.9 (1.4) | 17.9 (1.9) |
| 2 | 7 | 2.1 (0.3) | 3.7 (0.5) | 32.9 (1.3) | 4.6 (0.6) | 18.7 (1.5) |
| 3 | 11 | 3.1 (0.3) | 4.7 (0.4) | 33.3 (1.0) | 4.7 (0.7) | 17.9 (0.8) |
| 4 | 15 | 4.0 (0.3) | 5.4 (0.4) | 33.7 (1.1) | 5.0 (0.7) | 18.8 (1.2) |
| 5 | 9 | 5.0 (0.2) | 6.5 (0.4) | 33.7 (1.2) | 4.8 (0.5) | 17.9 (0.9) |
| 6 | 6 | 5.9 (0.1) | 7.3 (0.1) | 34.0 (1.0) | 5.1 (0.7) | 17.9 (1.2) |
| Fullterm infants | ||||||
| 2 | 24 | 2.1 (0.2) | 2.1 (0.1) | 39.8 (0.7) | 7.6 (1.0) | 19.8 (1.0) |
| 3 | 17 | 3.0 (0.3) | 3.1 (0.3) | 40.0 (0.6) | 7.9 (1.2) | 19.9 (1.9) |
| 4 | 21 | 4.1 (0.2) | 4.1 (0.2) | 40.0 (0.5) | 8.1 (1.0) | 20.6 (1.2) |
| 5 | 15 | 5.1 (0.2) | 5.1 (0.2) | 40.0 (0.6) | 7.8 (0.7) | 20.6 (0.9) |
| 6 | 16 | 6.0 (0.2) | 6.1 (0.2) | 39.8 (0.6) | 7.8 (1.4) | 20.3 (1.6) |
| 7 | 7 | 6.9 (0.3) | 7.0 (0.2) | 39.8 (0.9) | 8.0 (1.1) | 20.6 (0.4) |
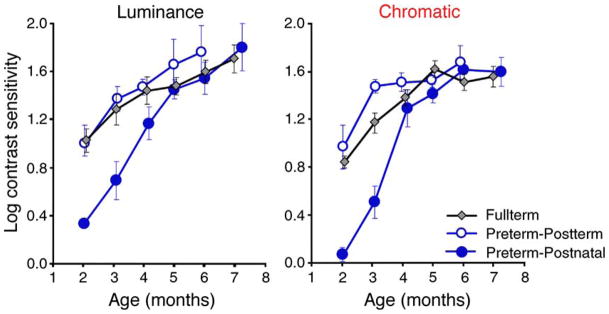
Figure 1.
Group mean log luminance (left) and chromatic (right) cone contrast sensitivity. The two preterm functions are the same infants, one plotted with respect to postterm age (open circles), and the other with respect to postnatal age (filled circles). For fullterm infants (diamonds), data are plotted with respect to postnatal age, but note that the mean postnatal and postterm ages are nearly identical for this subject group. Error bars denote standard errors of the mean.
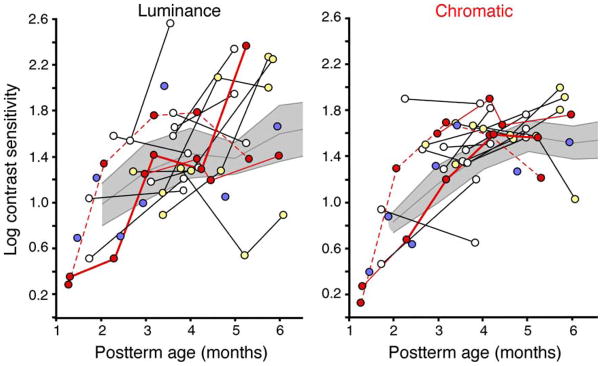
Figure 2.
Preterm infants’ individual log luminance (left) and chromatic (right) cone contrast sensitivity values plotted with respect to postterm age. Lines connect multiple time points. Blue, white, yellow, and red dots represent infants with 1, 2, 3, and 4/5 time points, respectively. The shaded area represents the 95% confidence intervals around the means from fullterm infants (central gray line).
Apparatus and stimuli
Luminance (light/dark) and chromatic (red/green) stimuli were presented on an Iiyama Vision Master Pro 510 monitor (1024 × 768 pixels, 100 Hz) powered by a Dell Dimension computer, and viewed at a distance of 38 cm. Stimuli were horizontally oriented sinusoidal gratings (moving upward or downward) with a spatial frequency of 0.27 cycles/degree and a temporal frequency of 4.2 Hz. These parameters were chosen because they are near the peak of the contrast sensitivity functions for young infants (e.g., Atkinson, Braddick, & Moar, 1977; Banks & Salapatek, 1978; Dobkins, Anderson, & Lia, 1999; Hartmann & Banks, 1992; Rasengane, Allen, & Manny, 1997). The stimuli subtended 11° by 11° and were centered 15° to the left or right of the middle of the video monitor. The mean chromaticity of the gratings and the background was CIE = 0.486, 0.442. The mean luminance of gratings and the background was 20 cd/m2. Contrasts of stimuli are described in terms of cone contrast, i.e., the amount of response modulation produced in the long-wavelength-selective (L) and medium-wavelength-selective (M) cones in the eye (see Dobkins et al., 1999 or Gunther & Dobkins, 2002 for methodological details).
Determining red/green isoluminance
The red/green chromatic stimulus in the main experiment was presented at the mean isoluminance value obtained from 22 adults, using standard motion photometry (Dobkins & Teller, 1996b; Rydberg, Jonsson, Roder, & Melander, 1994; Teller & Lindsey, 1993). In the motion photometry, adults fixated on a small dot in the center of a moving red/green grating and adjusted the luminance contrast in the grating until the percept of motion was least salient. Each adult subject’s isoluminance point was determined from the mean of 25 trials. The stimulus conditions for the motion photometry procedure were identical to those employed in the main experiments (i.e., same size, orientation, spatiotemporal frequency). As previously discussed (e.g., Dobkins & Teller, 1996b), the justification for using the adult mean isoluminance value in our infant experiments is based on previous experiments demonstrating that infant and adult mean isoluminance points are highly similar for red/green stimuli (Bieber, Volbrecht, & Werner, 1995; Brown, Lindsey, McSweeney, & Walters, 1995; Dobkins, Anderson, & Kelly, 2001; Maurer, Lewis, Cavanagh, & Anstis, 1989; Morrone, Burr, & Fiorentini, 1993; Pereverzeva, Hui-Lin Chien, Palmer, & Teller, 2002; Teller & Lindsey, 1989). Moreover, Brown et al. argue quantitatively that the variability of isoluminance points across infant subjects is comparable to the variability across adult subjects, when measurement error is taken into account. In previous studies, we have calculated that the amount of luminance error likely to exist in our red/green stimuli is below luminance contrast threshold for infants (see Dobkins & Teller, 1996b).
Obtaining contrast sensitivities
For each infant, a luminance and chromatic contrast threshold was obtained using forced-choice preferential looking (FPL), which relies on the fact infants prefer to look at a patterned stimulus on one side of a display rather than a blank, homogeneous field on the opposite side (Teller, 1979; see Dobkins & Teller, 1996a, 1996b for details). An adult experimenter held the infant 38 cm away from the front of the stimulus monitor in the view of a video camera aimed at the infant’s face. On each trial, a grating stimulus appeared on the left or right side of the video monitor, and the experimenter used cues such as the infant’s head turning and gazing behavior to judge the left vs. right location of the stimulus. Typically, five contrast values (1.25–25% cone contrast) were presented for each luminance and chromatic conditions, with these conditions and contrast levels randomized across trials. Stimuli remained present on the video monitor until the experimenter made the left/right judgment, which was typically less than 2 s. The experimenter’s answer was entered into the computer by pressing keys on the keyboard and computer beeps provided feedback as to whether the experimenter was correct. Because the mean luminance and chromaticity of the stimulus is the same as that of the background, when the contrast in the stimulus is at or below “contrast threshold”, it blends into the background and cannot be seen. Data from each infant were obtained over the course of 2 or 3 days within a 1-week period. (The infant’s age was calculated as the average of the first and last visits.) On average, 75 (±29) and 90 (±29) trials were obtained for preterm and fullterm infants, respectively, for each chromatic and luminance condition.
For each infant, a psychometric curve was fit to chromatic and luminance data using Weibull functions and maximum likelihood analysis (Watson, 1979; Weibull, 1951). Threshold was defined as the contrast yielding 75% correct performance. Slopes were unfixed for all infants, with the exception of two young infants, whose performance was very poor even at the highest contrast presented; for these infants their thresholds were obtained with fixed slopes of 1.0 (after Dobkins et al., 1999). Contrast sensitivity (CS) was computed as the inverse of threshold * 100, and then logged since log, but not linear, sensitivity data conform to normal distributions (Graham, 1989).
Because the current study predicts contrast sensitivity differences between preterm and fullterm infants, we believe it is important to show that group differences are not a result of preterms being less engaged in the FPL task. For example, one might suppose that preterm infants are less attentive/focused and more distractible than fullterm infants, which if were the case would lead to lower performance (i.e., lower estimates of CS) in preterms, without necessarily reflecting lower neural sensitivity. To address this possibility, we compared psychometric slopes between groups, with the notion that preterm infants disengaging on a proportion of trials would result in them showing shallower psychometric slopes (see Huang & Dobkins, 2005 for discussion). Statistical analyses on slope values revealed that this was not the case as there was no main effect of subject group (F(1,140) = 0.34; p = 0.56), nor an interaction between subject group and stimulus condition (F(1,140) = 0.35; p = 0.56). Mean slopes for preterm and fullterm infants were, for the luminance condition, 0.9 (±0.5) and 0.8 (±0.5) and, for the chromatic condition, 1.3 (±1.9) and 1.4 (±1.0), respectively. Thus, we can say with some certainty that differences in CS between subject groups reflects group differences in neural sensitivity rather than group differences in engagement on the FPL task.
Data analyses
For the purpose of plotting data, mean log luminance and chromatic contrast sensitivities were obtained by averaging log CS across infants. This was performed separately for the two subject groups (preterm and fullterm infants) and for each age group, with age defined both in terms of postterm and postnatal ages. Statistical analyses consisted of two-factor analyses of variance (ANOVAs) conducted on log CS values, with subject group (preterms vs. fullterms) and age group being the factors. These ANOVAs were conducted separately for luminance and chromatic CS, using postterm age. Note that age group was treated as a between-subjects factor, since repeated-subjects ANOVA was not an option due to some infants coming in for a single time point or mismatches across infants in the time points tested. To justify our use of a between-subjects analysis using all data points, we conducted an ANOVA using only a single time point’s data from each infant (i.e., infants who came in only once or the first time point of an infant who came in for multiple time points). In this analysis, where the age group variable was truly between-subjects (102 total subjects), the results were identical to when all data points were included. Note that our between-subjects design using all data points is a more conservative test, and the inclusion of all data provides a better representation of the sample populations and effects of age. Two-tailed Student’s t-tests were employed to compare fullterm vs. preterm infants at specific ages. Normality of data, using Kolmogorov–Smirnov tests, and homogeneity of variance, using Levene’s test, for each luminance and chromatic CS and each subject group, were verified before statistical analyses.
Results
Figure 1 presents group mean log contrast sensitivities (CS) plotted as a function of both postterm and postnatal ages, separately for luminance and chromatic stimuli. Figure 2 presents each preterm infant’s individual data plotted as a function of postterm age, with lines connecting multiple time points. The shaded area represents the 95% confidence intervals around the means (central gray line) obtained from fullterm infants.
As seen in Figure 1, for luminance CS, when preterm data are plotted with respect to postterm age (open circles), there is clear overlap between preterms and fullterms (filled diamonds), especially within the first 3 months. This is supported statistically by the results of a two-factor ANOVA (subject group × postterm age), which showed neither a main effect of subject group (F(1,131) = 1.27; p = 0.26) nor an interaction between subject group and age (F(4,131) < 1). Although both Figures 1 and 2 suggest a slight superiority in preterms at 5 and 6 months postterm, this pattern is not supported by post hoc tests at these ages (5 months: t(22) = 1.32; p = 0.20, and 6 months: t(20) = 0.64; p = 0.53). Still, it is interesting to note that when the preterm data are plotted with respect to postnatal age, they align quite well with the fullterm infants, suggesting that preterms “catch up” to fullterms by 5 months postnatal age.
A different pattern is seen for chromatic CS. Here, Figure 1 shows that the chromatic function of preterms plotted with respect to postterm age (filled circles) is clearly above that of fullterms (filled diamonds), especially obvious during the first three postterm months. This is supported by a two-factor ANOVA, which revealed a main effect of subject group (F(1,131) = 6.80; p < 0.01). Post hoc t-tests at each age revealed a significant group difference at 3 months (t(28) = 2.85; p = 0.005) and 6 months (t(34) = 2.29, p = 0.03) postterm age. In sum, for chromatic contrast sensitivity, our results reveal that preterm infants outperform fullterm infants when plotted as postterm age. One way to describe the outperformance is in terms of how many “effective” weeks of chromatic CS development were afforded by preterm infants’ early birth, by estimating the horizontal shift needed to align the preterm (in postterm age) function with the fullterm function. Using data from postterm months one to three, we calculated this amount to be 3.7 weeks. Since these preterm infants were born, on average, 6.6 (±1.1) weeks early, one can conjecture that the preterms’ 6.6 weeks of additional time since birth (a proxy for 6.6 weeks of additional visual experience) is equivalent to 3.7 weeks of development in chromatic CS. Thus, early additional experience does exert an influence, albeit incomplete, on chromatic CS, and by about 4 to 5 months postnatal, both luminance and chromatic CS of preterms “catch up” to those of fullterms, presumably because the rate of contrast sensitivity development slows down at this time.
Discussion
The current study of preterm infants was designed to ask whether early visual development of luminance and chromatic contrast sensitivity (CS) is tied primarily to preprogrammed development (i.e., not requiring visual experience) or visual experience. The results of the current study show that luminance and chromatic CS differ in the extent to which they are predicted by postterm age. For luminance CS, the developmental trajectory of preterms is indistinguishable from that of fullterms in the first few postterm months, suggesting that preprogrammed mechanisms are sufficient for the development of luminance CS (i.e., with no effects of additional time since birth, which can be considered a proxy for additional visual experience). By contrast, for chromatic CS, preterms show greater sensitivity than fullterms when plotted in terms of postterm age. This result suggests that preprogrammed mechanisms are not sufficient for the development of chromatic CS, and that preterms’ additional time since birth (i.e., additional visual experience) has a significant impact on development of chromatic CS. Interestingly, the conclusions of the current study are similar to those of our recent study (Dobkins et al., 2009), which used multiple regression analysis in a large sample of fullterm 2-month-old infants to ask whether luminance and chromatic CS are predicted by a variety of factors, including gestational length, postnatal age, gender, and birth order. In line with the theme of the current study, the premise of the previous study was that effects of gestational length on CS are likely tied to preprogrammed development, as there is no visual experience in utero (although we discuss the possibility that gestational length could be tied to other non-visual types of prenatal experience). By contrast, effects of postnatal age can be tied to both amount of visual experience and preprogrammed development. The results of our previous study showed that for 2-month-old infants, gestational length, which ranged from 38 to 42 weeks, predicted luminance CS, but not chromatic CS. Conversely, postnatal age, which ranged from 8 to 11 weeks, was a better predictor of chromatic, than luminance, CS. In sum, both our previous and current studies suggest that development of luminance CS may be more tied to preprogrammed mechanisms, whereas development of chromatic CS may be more tied to visual experience.
Note that in both the current and previous studies, because we did not control the visual experience of our subjects, we cannot know whether the presumed effects of visual experience are “permissive” (i.e., simply allowing preprogrammed mechanisms to proceed normally) or “instructive” (i.e., shaping development in a way that is meaningful based on the statistics of the environment). In addition note that these studies tested only a single spatiotemporal frequency, and thus it is yet determined whether the observed effects generalize across a broad range of stimulus parameters. Future studies manipulating infants’ visual experience and parameters of the test stimuli will be required to investigate these questions.
Differential effects on magnocellular vs. parvocellular pathway development
The current study measured luminance and chromatic CS with the notion that they are differentially related to the magnocellular (M) and parvocellular (P) subcortical pathways, respectively. Specifically, M neurons are more sensitive than P neurons to luminance contrast, and conversely, P neurons are more sensitive than M neurons to red/green chromatic contrast (Lee, Pokorny, Smith, Martin, & Valberg, 1990; Shapley, 1990; Smith, Pokorny, Davis, & Yeh, 1995). However, it is important to note that although the P pathway may be the sole mediator of chromatic CS, the M pathway is unlikely to be the sole mediator of luminance CS, for two main reasons (see Lennie & D’Zmura, 1988; Merigan & Maunsell, 1993; Skottun, 2000, for reviews). First, there are about eight times more P than M neurons, and thus while each individual P neuron may have lower luminance CS than each M neuron, probability summation across neurons may give the P pathway the upper hand on luminance CS. Second, lesion studies have shown that both M and P pathway lesions impair luminance CS (e.g., Merigan & Eskin, 1986; Merigan, Katz, & Maunsell, 1991; Merigan & Maunsell, 1990; Schiller, Logothetis, & Charles, 1990). Thus, while the P pathway probably largely mediates chromatic CS, both the M and P pathways are likely to mediate luminance CS. As such, the results of the current study can be interpreted as suggesting that early development of chromatic CS within the P pathway is more influenced by visual experience than is development of luminance CS within the M and P pathways.
The M vs. P pathway differentiation implicated in the current study is generally in line with results from previous studies that investigated the effects of abnormal early visual experience on M and P pathway development. In humans, the bulk of the data reports greater deficits in aspects of vision thought to be mediated by the P pathway (deficits for high spatial frequency stimuli: Bradley & Freeman, 1981; Hess & Howell, 1977; Levi & Harwerth, 1977, deficits for red/green chromatic stimuli: Davis, Sloper, Neveu, Hogg, Morgan, & Holder, 2006; Demirci, Gezer, Sezen, Ovali, Demiralp, & Isoglu-Alkoc, 2002; but see Zele, Pokorny, Lee, & Ireland, 2007). Corroborating the human results, studies of visually deprived animals have reported that morphological changes are greater within the P layers, compared to the M layers, of the LGN (Hendrickson et al., 1987; LeVay, Wiesel, & Hubel, 1980 and see von Noorden, Crawford, & Levacy, 1983 for greater P disruption in the LGN of a single human). In addition, within primary visual cortex, greater effects of deprivation have been noted within the P-pathway recipient (4C-beta) lamina than the M-pathway recipient (4C-alpha) lamina (Hendrickson et al., 1987).
In sum, studies of humans and animals who experienced early visual deprivation are generally consistent with the notion that P pathway development, more so than the M pathway development, requires normal visual experience. On the flip side of the coin, the results of the current study suggest that development of chromatic CS within the P pathway is affected by visual experience. Because contrast sensitivity is thought to be determined by the sensitivities of neurons at or before the level of primary visual cortex (Boynton, Demb, Glover, & Heeger, 1999; Hawken & Parker, 1990; Palmer, Cheng, & Seidemann, 2007, although this may not be the case early in development, see Stavros & Kiorpes, 2008), this suggests that the locus of visual experience effects could likewise be at or before the level of primary visual cortex. Accordingly, the change could be at the level of subcortical P neurons themselves or on the P representation at the level of visual cortex.3 On a final note, observed differences in effects of visual experience on the M vs. P pathways is interesting in light of other studies showing that the P pathway may mature more slowly than the M pathway, particularly in the first couple of months of life. This is based on psychophysically derived contrast thresholds for luminance and chromatic stimuli (Dobkins et al., 2001, 1999), VEP responses to luminance vs. chromatic stimuli (Crognale, Kelly, Weiss, & Teller, 1998; Hammarrenger, Lepore, Lippe, Labrosse, Guillemot, & Roy, 2003; Kelly, Borchert, & Teller, 1997; Madrid & Crognale, 2000; Morrone, Fiorentini, & Burr, 1996, but see Allen, Banks, & Norcia, 1993), and developmental neuroanatomical data from monkeys (Distler, Bachevalier, Kennedy, Mishkin, & Ungerleider, 1996; Florence & Casagrande, 1990; Lachica & Casagrande, 1988; Lund & Harper, 1991; Lund & Holbach, 1991; Mates & Lund, 1983 but see Headon, Sloper, Hiorns, & Powell, 1981) and humans (Burkhalter, Bernardo, & Charles, 1993, but see Hickey, 1977). Thus, it may be that by virtue of its relatively delayed maturation, the P pathway is more affected by visual experience.
Intermediate effects of visual experience
Our finding that chromatic CS of preterms exceeds that predicted by postterm age suggests that preprogrammed development is not sufficient, and, in turn, that preterms’ additional visual experience has a significant effect. We are then in a position to ask whether chromatic CS is well predicted by additional visual experience, which can be tested by asking whether preterm and fullterm trajectories overlap when plotted with respect to postnatal age. The results of our analyses clearly show that this is not the case; within the first few postnatal months, chromatic CS in preterms is lower than that of fullterms. Together, these two sets of results (for postterm and postnatal ages) suggest that while visual experience plays a role in chromatic CS development, it is not the full account. Why, then, in the first few postnatal months are the effects of visual experience on chromatic CS intermediate? One simple possibility is that preterm infants may receive less frequent (or less rich) visual input than fullterm infants matched in postnatal age. An obvious reason for this could be that their eyes are open less frequently (because they sleep longer hours and/or because they shut their eyes more in waking hours). A second possibility is that effects of visual experience in preterms may not be fully actualized if there are limitations placed by immature biological factors. For example, early in development, biological factors involved in Hebbian mechanisms (such as functionality of NMDA receptors, e.g., Rauschecker, Egert, & Kossel, 1990) may not be mature or malleable enough to be affected by visual input. Perhaps later on in development of preterms, when these biological factors are “ready”, visual experience might accelerate the rate of development, allowing preterms to “catch-up” to fullterms, which is, in fact, observed in the current study. It is also possible that preterms catch up simply because the rate of development slows down later on (see Results section).
Discrepancies across studies
As described in the Introduction section, previous studies of visual development in preterm infants have yielded mixed results with regard to the question of whether or not preterm performance exceeds that predicted by postterm age (with negative vs. positive results consistent with no effect vs. effects of visual experience, respectively). The bulk of these studies tested visual acuity, using forced-choice preferential looking (FPL), optokinetic nystagmus (OKN), or visually evoked potentials (VEPs). There are several reasons for the discrepancies across studies. First, many previous studies tested preterms who were premature enough (>9 weeks early) to have some degree of ocular or neural abnormality. Thus, studies that failed to find effects of visual experience could potentially be explained by undetected neural abnormalities that counteract positive effects of visual experience. However, it should be said that it is unlikely that a substantial proportion of preterm infants in these previous studies had neural abnormalities, because such a scenario would predict preterm performance falling below fullterm when plotted in postterm age, which has not been reported in the literature. Second, and along a similar line, discrepancies across studies may be due to variations in the severity of prematurity across studies. In fact, even within single studies, there is sometimes a considerable range in the severity of prematurity (larger than that in the current study), with preterm infants having various lengths of gestational period all averaged together, which is not factored into the analyses. Third, the question of whether or not preterm performance exceeds that predicted by postterm age is likely to depend heavily on the particular postterm/postnatal ages tested, with studies testing older ages more likely to reveal that preterm performance exceeds that predicted by postterm age (i.e., consistent with effects of visual experience). A dependency on postterm/postnatal age was, in fact, observed in the current study.
A final possibility is that discrepancies across studies may be due to variation in the particular visual measure tested as well as the technique used to obtain the measure, both of which can affect the level of visual system processing being tapped. With regard to techniques, OKN may be biased toward tapping subcortical mechanisms involved in involuntary eye movements (especially in the first couple of months of life, see Dobkins, Fine, Hsueh, & Vitten, 2004; Mason, Braddick, & Wattam-Bell, 2003 for discussion), VEP is believed to tap cortical responses (e.g., Berninger, Arden, Hogg, & Frumkes, 1989; Dobson & Teller, 1978; Norcia, 2004; Norcia & Tyler, 1985), and FPL is believed to represent a more integrated response of the visual system (see Banks & Salapatek, 1983; Dobson & Teller, 1978; Mason et al., 2003; Teller, 1997 for reviews). With regard to the measure, spatial acuity is thought to be largely dependent on the density of the photoreceptor mosaic of the retina (see Banks & Bennett, 1988; Blakemore & Vital-Durand, 1981; Boothe et al., 1985; Kiorpes & Movshon, 2004). Because visual deprivation is believed to have only mild effects on retinal development (Sherman & Spear, 1982; Sherman & Stone, 1973), it has been suggested that development of spatial acuity may rely less on visual experience than other aspects of visual development (Kiorpes & Movshon, 2004). Tasks that are more limited by retinal factors are probably less likely to change due to visual experience, as compared to other tasks such as position discrimination or vernier acuity that depend on higher cortical function (Geisler, 1984; Levi & Klein, 1985; Levi, Klein, & Aitsebaomo, 1985; Skoczenski & Norcia, 1999; Wilson, 1986). It is also interesting to consider the possibility that tests of spatial acuity in preterms (which is the most commonly used measure) may minimize the chance to reveal effects of their added visual experience, since higher spatial frequencies are not readily experienced by infants (because of optical and neural immaturities), which may be especially true in preterm infants. Thus, one could argue that if visual experience is instructive, it might not be surprising that acuity in preterms does not benefit from their additional time in the world, since they received only minimal experience with high spatial frequencies. By contrast, studies that investigate the potential effects of additional visual experience in preterms might be more likely to reveal positive results if low spatial frequencies (which are readily experienced) are tested, as in the current study.
Acknowledgments
We would like to thank Fiona Yeh and Marie Chuldzhyan for assistance with data collection and Vanitha Sampath and Jeff Judson for assistance in programming. We are also very grateful to all parents and infants who participated. This work was supported by NIH Grants R01-EY12153 (KRD) and R01-EY19035 (RGB/KRD).
Footnotes
Commercial relationships: none.
Postconceptional and postterm age are equivalent descriptions, with the former being used to emphasize the length of the gestational period and the latter being used to emphasize the “adjusted” postnatal age, or the age the preterm infant would be if they were born at term (i.e., at 40 weeks gestation).
There is variability across these studies in both the postconceptional and postnatal age tested, making it difficult to know whether a given visual ability is present because the preterm infant has passed a critical post-conceptional age at the time of birth or because the preterm infant has received some degree, even a few hours or days, of postnatal visual experience. To simplify, the data from studies we describe here are presented with respect to infants’ postconceptional age, without regard for their postnatal age.
At the cortical level, past layer 4 of V1, P cell signals certainly mingle with M pathway signals (see Dobkins & Albright, 2004 for review), however, the mingling is not entirely complete, and thus it seems reasonable to propose a “P pathway representation” in the cortex.
Contributor Information
Rain G. Bosworth, Department of Psychology, University of California, San Diego, La Jolla, CA, USA
Karen R. Dobkins, Department of Psychology, University of California, San Diego, La Jolla, CA, USA
References
- Allen D, Banks MS, Norcia AM. Does chromatic sensitivity develop more slowly than luminance sensitivity? Vision Research. 1993;33:2553–2562. doi: 10.1016/0042-6989(93)90134-i. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Anker S, Rae S, Weeks F, Braddick O, Rennie J. Cortical visual evoked potentials in very low birthweight premature infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2002;86:F28–F31. doi: 10.1136/fn.86.1.F28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Moar K. Contrast sensitivity of the human infant for moving and static patterns. Vision Research. 1977;17:1045–1047. doi: 10.1016/0042-6989(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Banks MS, Bennett PJ. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:2059–2079. doi: 10.1364/josaa.5.002059. [DOI] [PubMed] [Google Scholar]
- Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Investigative Ophthalmology & Visual Science. 1978;17:361–365. [PubMed] [Google Scholar]
- Banks MS, Salapatek P. Infant visual perception. In: Haith M, Campos J, editors. Handbook of child psychology: Biology and infancy. New York: Wiley; 1983. pp. 435–571. [Google Scholar]
- Berninger TA, Arden GB, Hogg CR, Frumkes T. Separable evoked retinal and cortical potentials from each major visual pathway: Preliminary results. British Journal of Ophthalmology. 1989;73:502–511. doi: 10.1136/bjo.73.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber ML, Volbrecht VJ, Werner JS. Spectral efficiency measured by heterochromatic flicker photometry is similar in human infants and adults. Vision Research. 1995;35:1385–1392. doi: 10.1016/0042-6989(95)98718-o. [DOI] [PubMed] [Google Scholar]
- Birch DG, Birch EE, Hoffman DR, Uauy RD. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Investigative Ophthalmology & Visual Science. 1992;33:2365–2376. [PubMed] [Google Scholar]
- Birch EE, Birch DG, Petrig B, Uauy RD. Retinal and cortical function of very low birthweight infants at 36 and 57 weeks postconception. Clinical Vision Research. 1990;5:363–373. [Google Scholar]
- Birch EE, Bosworth RG. Visual evoked potentials in infants and children. In: Aminoff MJ, editor. Electrodiagnosis in clinical neurology. New York: Churchill-Livingstone; 2004. pp. 439–450. [Google Scholar]
- Birch EE, O’Connor AR. Preterm birth and visual development. Seminars in Neonatology. 2001;6:487–497. doi: 10.1053/siny.2001.0077. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Investigative Ophthalmology & Visual Science. 1998;39:1560–1566. [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neuroscience Letters. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Vital-Durand F. Postnatal development of the monkey’s visual system. Ciba Foundation Symposium. 1981;86:152–171. doi: 10.1002/9780470720684.ch7. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Vital-Durand F. Visual deprivation prevents the postnatal maturation of spatial resolution and contrast sensitivity for neurones of the monkey’s striate cortex. The Journal of Physiology. 1983;345:40P. [Google Scholar]
- Blakemore C, Vital-Durand F. Effects of visual deprivation on the development of the monkey’s lateral geniculate nucleus. The Journal of Physiology. 1986;380:493–511. doi: 10.1113/jphysiol.1986.sp016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and non-human primates. Annual Review of Neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Research. 1999;39:257–269. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: Motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21:467–476. [PubMed] [Google Scholar]
- Brown AM, Lindsey DT, McSweeney EM, Walters MM. Infant luminance and chromatic contrast sensitivity: Optokinetic nystagmus data on 3-month-olds. Vision Research. 1995;35:3145–3160. doi: 10.1016/0042-6989(95)00017-t. [DOI] [PubMed] [Google Scholar]
- Brown AM, Yamamoto M. Visual acuity in newborn and preterm infants measured with grating acuity cards. American Journal of Ophthalmology. 1986;102:245–253. doi: 10.1016/0002-9394(86)90153-4. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. Journal of Neuroscience. 1993;13:1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kaminski HJ, Gong B, Zhou L, Hatala D, Howell SJ, Zhou X, et al. Monocular visual deprivation in macaque monkeys: A profile in the gene expression of lateral geniculate nucleus by laser capture microdissection. Molecular Vision. 2008;14:1401–1413. [PMC free article] [PubMed] [Google Scholar]
- Chin KC, Taylor MJ, Menzies R, Whyte H. Development of visual evoked potentials in neonates. A study using light emitting diode goggles. Archives of Disease in Childhood. 1985;60:1166–1168. doi: 10.1136/adc.60.12.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: Permissive or instructive? Current Opinion in Neurobiology. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Crognale MA, Kelly JP, Weiss AH, Teller DY. Development of the spatio-chromatic visual evoked potential (VEP): A longitudinal study. Vision Research. 1998;38:3283–3292. doi: 10.1016/s0042-6989(98)00074-1. [DOI] [PubMed] [Google Scholar]
- Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: Impact on rates of late preterm birth, 1992 to 2002. Seminars in Perinatology. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes of magnocellular and parvocellular visual function in early- and late-onset strabismic amblyopia. Investigative Ophthalmology & Visual Science. 2006;47:4836–4841. doi: 10.1167/iovs.06-0382. [DOI] [PubMed] [Google Scholar]
- Demirci H, Gezer A, Sezen F, Ovali T, Demiralp T, Isoglu-Alkoc U. Evaluation of the functions of the parvocellular and magnocellular pathways in strabismic amblyopia. Journal of Pediatric Ophthalmology and Strabismus. 2002;39:215–221. doi: 10.3928/0191-3913-20020701-09. [DOI] [PubMed] [Google Scholar]
- Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: A 14C-2-deoxyglucose study. Cerebral Cortex. 1996;6:184–195. doi: 10.1093/cercor/6.2.184. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. Merging processing streams: Color cues for motion detection and interpretation. In: Chalupa L, Werner J, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 1217–1228. [Google Scholar]
- Dobkins KR, Anderson CM, Kelly JP. Development of psychophysically-derived detection contours in L- and M-cone contrast space. Vision Research. 2001;41:1791–1807. doi: 10.1016/s0042-6989(01)00070-0. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: Evidence for precocious magnocellular development? Vision Research. 1999;39:3223–3239. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Bosworth RG, McCleery JP. Effects of gestational length, gender, postnatal age, and birth order on visual contrast sensitivity in infants. Journal of Vision. 2009;9(10):19, 1–21. doi: 10.1167/9.10.19. http://journalofvision.org/9/10/19/ [DOI] [PMC free article] [PubMed]
- Dobkins KR, Fine I, Hsueh AC, Vitten C. Pattern motion integration in infants. Journal of Vision. 2004;4(3):2, 144–155. doi: 10.1167/4.3.2. http://journalofvision.org/4/3/2/ [DOI] [PubMed]
- Dobkins KR, Teller DY. Infant contrast detectors are selective for direction of motion. Vision Research. 1996a;36:281–294. doi: 10.1016/0042-6989(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Teller DY. Infant motion: Detection (M:D) ratios for chromatically defined and luminance-defined moving stimuli. Vision Research. 1996b;36:3293–3310. doi: 10.1016/0042-6989(96)00069-7. [DOI] [PubMed] [Google Scholar]
- Dobson V, Mayer DL, Lee CP. Visual acuity screening of preterm infants. Investigative Ophthalmology & Visual Science. 1980;19:1498–1505. [PubMed] [Google Scholar]
- Dobson V, Teller DY. Visual acuity in human infants: A review and comparison of behavioral and electrophysiological studies. Vision Research. 1978;18:1469–1483. doi: 10.1016/0042-6989(78)90001-9. [DOI] [PubMed] [Google Scholar]
- Downie AL, Jakobson LS, Frisk V, Ushycky I. Periventricular brain injury, visual motion processing, and reading and spelling abilities in children who were extremely low birthweight. Journal of the International Neuropsychological Society. 2003;9:440–449. doi: 10.1017/S1355617703930098. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brent HP. Influence of monocular deprivation during infancy on the later development of spatial and temporal vision. Vision Research. 2000;40:3283–3295. doi: 10.1016/s0042-6989(00)00165-6. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Lui CH, Brent HP. Spatial and temporal vision in patients treated for bilateral congenital cataracts. Vision Research. 1999;39:3480–3489. doi: 10.1016/s0042-6989(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Fantz RL, Fagan JF., 3rd Visual attention to size and number of pattern details by term and preterm infants during the first six months. Child Development. 1975;46:3–18. [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Current Opinion in Neurobiology. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Florence SL, Casagrande VA. Development of geniculocortical axon arbors in a primate. Visual Neuroscience. 1990;5:291–309. doi: 10.1017/s0952523800000365. [DOI] [PubMed] [Google Scholar]
- Geisler WS. Physical limits of acuity and hyperacuity. Journal of the Optical Society of America A, Optics and Image Science. 1984;1:775–782. doi: 10.1364/josaa.1.000775. [DOI] [PubMed] [Google Scholar]
- Getz L, Dobson V, Luna B. Development of grating acuity, letter acuity, and visual fields in small-for-gestational-age preterm infants. Early Human Development. 1994;40:59–71. doi: 10.1016/0378-3782(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Graham NVS. Visual pattern analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Grose J, Harding G, Wilton A, Bissenden J. The maturation of the pattern reversal VEP and flash ERG in preterm infants. Clinical Vision Science. 1989;4:239–246. [Google Scholar]
- Gunther KL, Dobkins KR. Individual differences in chromatic (red/green) contrast sensitivity are constrained by the relative number of L- versus M-cones in the eye. Vision Research. 2002;42:1367–1378. doi: 10.1016/s0042-6989(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function—A quantitative investigation in mice. Journal of Embryology and Experimental Morphology. 1963;11:255–266. [PubMed] [Google Scholar]
- Hack M, Mostow A, Miranda SB. Development of attention in preterm infants. Pediatrics. 1976;58:669–674. [PubMed] [Google Scholar]
- Hack M, Muszynski SY, Miranda SB. State of awakeness during visual fixation in preterm infants. Pediatrics. 1981;68:87–92. [PubMed] [Google Scholar]
- Hammarrenger B, Lepore F, Lippe S, Labrosse M, Guillemot JP, Roy MS. Magnocellular and parvocellular developmental course in infants during the first year of life. Documenta Ophthalmologica. 2003;107:225–233. doi: 10.1023/b:doop.0000005331.66114.05. [DOI] [PubMed] [Google Scholar]
- Hammarrenger B, Roy MS, Ellemberg D, Labrosse M, Orquin J, Lippe S, et al. Developmental delay and magnocellular visual pathway function in very-low-birthweight preterm infants. Developmental Medicine and Child Neurology. 2007;49:28–33. doi: 10.1017/s0012162207000084.x. [DOI] [PubMed] [Google Scholar]
- Harding GF, Grose J, Wilton A, Bissenden JG. The pattern reversal VEP in short-gestation infants. Electroencephalography and Clinical Neurophysiology. 1989;74:76–80. doi: 10.1016/0168-5597(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Harris NJ, Palacio D, Ginzel A, Richardson CJ, Swischuk L. Are routine cranial ultrasounds necessary in premature infants greater than 30 weeks gestation? American Journal of Perinatology. 2007;24:17–21. doi: 10.1055/s-2006-954960. [DOI] [PubMed] [Google Scholar]
- Hartmann EE, Banks MS. Temporal contrast sensitivity in human infants. Vision Research. 1992;32:1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: Temporal modulation sensitivity. Vision Research. 1983;23:1511–1517. doi: 10.1016/0042-6989(83)90163-3. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Parker AJ. Detection and discrimination mechanisms in the striate cortex of the old-world monkey. In: Blakemore C, editor. Vision: Coding and efficiency. Cambridge, UK: Cambridge University Press; 1990. pp. 103–116. [Google Scholar]
- Headon MP, Sloper JJ, Hiorns RW, Powell TP. Cell sizes in the lateral geniculate nucleus of normal infant and adult rhesus monkeys. Brain Research. 1981;229:183–186. doi: 10.1016/0006-8993(81)90754-x. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Boothe R. Morphology of the retina and dorsal lateral geniculate nucleus in dark-reared monkeys (Macaca nemestrina) Vision Research. 1976;16:517–521. doi: 10.1016/0042-6989(76)90033-x. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS, Boothe RG, Kiorpes L. Effects of early unilateral blur on the macaque’s visual system. II. Anatomical observations. Journal of Neuroscience. 1987;7:1327–1339. doi: 10.1523/JNEUROSCI.07-05-01327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: Evidence for a two type classification. Vision Research. 1977;17:1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Gole G, Mullen KT. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. European Journal of Neuroscience. 2009;29:1064–1070. doi: 10.1111/j.1460-9568.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey TL. Postnatal development of the human lateral geniculate nucleus: Relationship to a critical period for the visual system. Science. 1977;198:836–838. doi: 10.1126/science.918665. [DOI] [PubMed] [Google Scholar]
- Hrbeck A, Karlberg P, Olsson T. Development of visual and somatosensory evoked responses in preterm newborn infants. Electroencephalography and Clinical Neurophysiology. 1973;34:225–232. doi: 10.1016/0013-4694(73)90249-6. [DOI] [PubMed] [Google Scholar]
- Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: Evidence for both contrast gain and response gain. Vision Research. 2005;45:1201–1212. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Cioni G, Boldrini A, Bottai P, van Hof-van Duin J. Visual acuity of low- and high-risk neonates and acuity development during the first year. Behavioral Brain Research. 1992;49:107–114. doi: 10.1016/s0166-4328(05)80200-1. [DOI] [PubMed] [Google Scholar]
- Jackson TL, Ong GL, McIndoe MA, Ripley LG. Monocular chromatic contrast threshold and achromatic contrast sensitivity in children born prematurely. American Journal of Ophthalmology. 2003;136:710–719. doi: 10.1016/s0002-9394(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Jakobson LS, Frisk V, Downie AL. Motion-defined form processing in extremely premature children. Neuropsychologia. 2006;44:1777–1786. doi: 10.1016/j.neuropsychologia.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Borchert K, Teller DY. The development of chromatic and achromatic contrast sensitivity in infancy as tested with the sweep VEP. Vision Research. 1997;37:2057–2072. doi: 10.1016/s0042-6989(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Boothe RG, Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS. Effects of early unilateral blur on the macaque’s visual system. I. Behavioral observations. Journal of Neuroscience. 1987;7:1318–1326. doi: 10.1523/JNEUROSCI.07-05-01318.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa L, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 159–173. [Google Scholar]
- Kos-Pietro S, Towle VL, Cakmur R, Spire JP. Maturation of human visual evoked potentials: 27 weeks conceptional age to 2 years. Neuropediatrics. 1997;28:318–323. doi: 10.1055/s-2007-973723. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG., Jr Electrophysiologic assessment of auditory and visual function in the newborn. Clinical Perinatology. 1985;12:277–299. [PubMed] [Google Scholar]
- Lachica EA, Casagrande VA. Development of primate retinogeniculate axon arbors. Visual Neuroscience. 1988;1:103–123. doi: 10.1017/s095252380000105x. [DOI] [PubMed] [Google Scholar]
- Leaf AA, Green CR, Esack A, Costeloe KL, Prior PF. Maturation of electroretinograms and visual evoked potentials in preterm infants. Developmental Medicine and Child Neurology. 1995;37:814–826. doi: 10.1111/j.1469-8749.1995.tb12065.x. [DOI] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. Journal of Optical Society of America A, Optics and Image Science. 1990;7:2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Lennie P, D’Zmura M. Mechanisms of color vision. Critical Reviews in Neurobiology. 1988;3:333–400. [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. Journal of Comparative Neurology. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Research. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Levi M, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology and Visual Science. 1977;16:90–95. [PubMed] [Google Scholar]
- Levitt JB, Schumer RA, Sherman SM, Spear PD, Movshon JA. Visual response properties of neurons in the LGN of normally reared and visually deprived macaque monkeys. Journal of Neurophysiology. 2001;85:2111–2129. doi: 10.1152/jn.2001.85.5.2111. [DOI] [PubMed] [Google Scholar]
- Lund JS, Harper TR. Postnatal development of thalamic recipient neurons in the monkey striate cortex: III. Somatic inhibitory synapse acquisition by spiny stellate neurons of layer 4C. Journal of Comparative Neurology. 1991;309:141–149. doi: 10.1002/cne.903090110. [DOI] [PubMed] [Google Scholar]
- Lund JS, Holbach SM. Postnatal development of thalamic recipient neurons in the monkey striate cortex: I. Comparison of spine acquisition and dendritic growth of layer 4C alpha and beta spiny stellate neurons. Journal of Comparative Neurology. 1991;309:115–128. doi: 10.1002/cne.903090108. [DOI] [PubMed] [Google Scholar]
- Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. Journal of Pediatrics. 1999;135:351–357. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- MacKay TL, Jakobson LS, Ellemberg D, Lewis TL, Maurer D, Casiro O. Deficits in the processing of local and global motion in very low birthweight children. Neuropsychologia. 2005;43:1738–1748. doi: 10.1016/j.neuropsychologia.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Madrid M, Crognale MA. Long-term maturation of visual pathways. Visual Neuroscience. 2000;17:831–837. doi: 10.1017/s0952523800176023. [DOI] [PubMed] [Google Scholar]
- Mason AJ, Braddick OJ, Wattam-Bell J. Motion coherence thresholds in infants—Different tasks identify at least two distinct motion systems. Vision Research. 2003;43:1149–1157. doi: 10.1016/s0042-6989(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Mates SL, Lund JS. Developmental changes in the relationship between type 2 synapses and spiny neurons in the monkey visual cortex. Journal of Comparative Neurology. 1983;221:98–105. doi: 10.1002/cne.902210108. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL. Visual outcomes after infantile cataract. In: Simons K, editor. Early visual development: Normal and abnormal. New York: Oxford University Press; 1993. pp. 454–484. [Google Scholar]
- Maurer D, Lewis TL, Cavanagh P, Anstis S. A new test of luminous efficiency for babies. Investigative Ophthalmology and Visual Science. 1989;30:297–303. [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Mondloch CJ. Missing sights: Consequences for visual cognitive development. Trends in Cognitive Science. 2005;9:144–151. doi: 10.1016/j.tics.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Eskin TA. Spatio-temporal vision of macaques with severe loss of P beta retinal ganglion cells. Vision Research. 1986;26:1751–1761. doi: 10.1016/0042-6989(86)90125-2. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Katz LM, Maunsell JH. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. Journal of Neuroscience. 1991;11:994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. Macaque vision after magnocellular lateral geniculate lesions. Visual Neuroscience. 1990;5:347–352. doi: 10.1017/s0952523800000432. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Kjaer PK, Norcia AM, Good WV, Madan A. Visual development in very low birth weight infants. Pediatric Research. 2006;60:435–439. doi: 10.1203/01.pdr.0000238249.44088.2c. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Burr DC, Fiorentini A. Development of infant contrast sensitivity to chromatic stimuli. Vision Research. 1993;33:2535–2552. doi: 10.1016/0042-6989(93)90133-h. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Fiorentini A, Burr DC. Development of the temporal properties of visual evoked potentials to luminance- and colour-contrast in infants. Vision Research. 1996;36:3141–3156. doi: 10.1016/0042-6989(96)00050-8. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque’s visual system. III. Physiological observations. Journal of Neuroscience. 1987;7:1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Van Sluyters RC. Visual neural development. Annual Review of Psychology. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Norcia AM. Development of spatial selectivity and response timing in humans. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2004. pp. 174–188. [Google Scholar]
- Norcia AM, Tyler CW. Spatial frequency sweep VEP: Visual acuity during the first year of life. Vision Research. 1985;25:1399–1408. doi: 10.1016/0042-6989(85)90217-2. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW, Piecuch R, Clyman R, Grobstein J. Visual acuity development in normal and abnormal preterm human infants. Journal of Pediatric Ophthalmology Strabismus. 1987;24:70–74. doi: 10.3928/0191-3913-19870301-05. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Spencer R, Birch EE. Predicting long-term visual outcome in children with birth weight under 1001 g. Journal of AAPOS. 2007;11:541–545. doi: 10.1016/j.jaapos.2007.04.002. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Stephenson TJ, Johnson A, Tobin MJ, Ratib S, Moseley M, et al. Visual function in low birthweight children. British Journal of Ophthalmology. 2004;88:1149–1153. doi: 10.1136/bjo.2003.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Wilson CM, Fielder AR. Ophthalmological problems associated with preterm birth. Eye. 2007;21:1254–1260. doi: 10.1038/sj.eye.6702838. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Costa MF, de Souza JM, Ventura DF. Contrast sensitivity threshold measured by sweep-visual evoked potential in term and preterm infants at 3 and 10 months of age. Brazilian Journal of Medical Biology Research. 2004;37:1389–1396. doi: 10.1590/s0100-879x2004000900014. [DOI] [PubMed] [Google Scholar]
- Palmer C, Cheng SY, Seidemann E. Linking neuronal and behavioral performance in a reaction-time visual detection task. Journal of Neuroscience. 2007;27:8122–8137. doi: 10.1523/JNEUROSCI.1940-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereverzeva M, Hui-Lin Chien S, Palmer J, Teller DY. Infant photometry: Are mean adult isoluminance values a sufficient approximation to individual infant values? Vision Research. 2002;42:1639–1649. doi: 10.1016/s0042-6989(02)00089-5. [DOI] [PubMed] [Google Scholar]
- Rasengane TA, Allen D, Manny RE. Development of temporal contrast sensitivity in human infants. Vision Research. 1997;37:1747–1754. doi: 10.1016/s0042-6989(96)00300-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Egert U, Kossel A. Effects of NMDA antagonists on developmental plasticity in kitten visual cortex. International Journal of Developmental Neuroscience. 1990;8:425–435. doi: 10.1016/0736-5748(90)90075-d. [DOI] [PubMed] [Google Scholar]
- Regal DM, Boothe R, Teller DY, Sackett GP. Visual acuity and visual responsiveness in dark-reared monkeys (Macaca nemestrina) Vision Research. 1976;16:523–530. doi: 10.1016/0042-6989(76)90034-1. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Robinson J. Assessment of gestational age by neurological examination. Archives of Disease in Childhood. 1966;41:437–447. doi: 10.1136/adc.41.218.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Moseley MJ, Thompson JR, Fielder AR. Eyelid opening in preterm neonates. Archives of Disease in Childhood. 1989;64:943–948. doi: 10.1136/adc.64.7_spec_no.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MS, Barsoum-Homsy M, Orquin J, Benoit J. Maturation of binocular pattern visual evoked potentials in normal fullterm and preterm infants from 1 to 6 months of age. Pediatrics Research. 1995;37:140–144. doi: 10.1203/00006450-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Roy MS, Lachapelle P, Lepore F. Maturation of the optokinetic nystagmus as a function of the speed of stimulation in fullterm and preterm infants. Clinical Visual Science. 1989;4:357–366. [Google Scholar]
- Rydberg T, Jonsson A, Roder M, Melander A. Hypoglycemic activity of glyburide (gliben-clamide) metabolites in humans. Diabetes Care. 1994;17:1026–1030. doi: 10.2337/diacare.17.9.1026. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles ER. Role of the color-opponent and broad-band channels in vision. Visual Neuroscience. 1990;5:321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers’ recall of birthweight and gestational age. British Journal of Obstetrics and Gynaecology. 1987;94:731–735. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annual Review of Psychology. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiology Review. 1982;62:738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Stone J. Physiological normality of the retinal in visually deprived cats. Brain Research. 1973;60:224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Research. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Research. 1991;540:273–278. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- Skoczenski AM, Norcia AM. Development of VEP Vernier acuity and grating acuity in human infants. Investigative Ophthalmology and Visual Science. 1999;40:2411–2417. [PubMed] [Google Scholar]
- Skottun BC. The magnocellular deficit theory of dyslexia: The evidence from contrast sensitivity. Vision Research. 2000;40:111–127. doi: 10.1016/s0042-6989(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J, Davis M, Yeh T. Mechanisms subserving temporal modulation sensitivity in silent-cone substitution. Journal of Optical Society of America A, Optics, Image Science, and Vision. 1995;12:241–249. doi: 10.1364/josaa.12.000241. [DOI] [PubMed] [Google Scholar]
- Sokol S, Jones K. Implicit time of pattern evoked potentials in infants: An index of maturation of spatial vision. Vision Research. 1979;19:747–755. doi: 10.1016/0042-6989(79)90150-0. [DOI] [PubMed] [Google Scholar]
- Stavros KA, Kiorpes L. Behavioral measurement of temporal contrast sensitivity development in macaque monkeys (Macaca nemestrina) Vision Research. 2008;48:1335–1344. doi: 10.1016/j.visres.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Menzies R, MacMillan LJ, Whyte HE. VEPs in normal fullterm and premature neonates: Longitudinal versus cross-sectional data. Electroencephalography and Clinical Neurophysiology. 1987;68:20–27. doi: 10.1016/0168-5597(87)90066-9. [DOI] [PubMed] [Google Scholar]
- Teller DY. The forced-choice preferential looking procedure: A psychophysical technique for use with human infants. Infant Behavior & Development. 1979;2:135–153. [Google Scholar]
- Teller DY. First glances: The vision of infants. The Friedenwald lecture. Investigative Ophthalmology and Visual Science. 1997;38:2183–2203. [PubMed] [Google Scholar]
- Teller DY, Lindsey DT. Motion nulls for white versus isochromatic gratings in infants and adults. Journal of Optical Society of America A, Optics and Image Science. 1989;6:1945–1954. doi: 10.1364/josaa.6.001945. [DOI] [PubMed] [Google Scholar]
- Teller DY, Lindsey DT. Motion nulling techniques and infant color vision. In: Granrud C, editor. Visual perception and cognition in infancy. Hillsdale, NJ: Lawrence Erlbaum Associates; 1993. pp. 47–74. [Google Scholar]
- Tsuneishi S, Casaer P. Effects of preterm extrauterine visual experience on the development of the human visual system: A flash VEP study. Development Medicine & Children Neurology. 2000;42:663–668. doi: 10.1017/s0012162200001225. [DOI] [PubMed] [Google Scholar]
- Tytla ME, Maurer D, Lewis TL, Brent HP. Contrast sensitivity in children treated for congenital cataract. Clinical Vision Science. 1988;2:251–264. [Google Scholar]
- Usher R, McLean F. Intrauterine growth of live born Caucasian infants at sea level: Standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. Journal of Pediatrics. 1969;74:901–910. doi: 10.1016/s0022-3476(69)80224-6. [DOI] [PubMed] [Google Scholar]
- van Hof-van Duin J, Mohn G. The development of visual acuity in normal fullterm and preterm infants. Vision Research. 1986;26:909–916. doi: 10.1016/0042-6989(86)90149-5. [DOI] [PubMed] [Google Scholar]
- von Noorden GK, Crawford ML, Levacy RA. The lateral geniculate nucleus in human anisometropic amblyopia. Investigative Ophthalmology and Visual Science. 1983;24:788–790. [PubMed] [Google Scholar]
- Watanabe K, Iwase K, Hara K. Maturation of visual evoked responses in low-birthweight infants. Development Medicine & Children Neurology. 1972;14:425–435. doi: 10.1111/j.1469-8749.1972.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Watson AB. Probability summation over time. Vision Research. 1979;19:515–522. doi: 10.1016/0042-6989(79)90136-6. [DOI] [PubMed] [Google Scholar]
- Weibull W. A statistical distribution function of wide applicability. Journal of Applied Mechanics. 1951;18:292–297. [Google Scholar]
- Weinacht S, Kind C, Monting JS, Gottlob I. Visual development in preterm and fullterm infants: A prospective masked study. Investigative Ophthalmology and Visual Science. 1999;40:346–353. [PubMed] [Google Scholar]
- Wilson HR. Responses of spatial mechanisms can explain hyperacuity. Vision Research. 1986;26:453–469. doi: 10.1016/0042-6989(86)90188-4. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Pokorny J, Lee DY, Ireland D. Anisometropic amblyopia: Spatial contrast sensitivity deficits in inferred magnocellular and parvocellular vision. Investigative Ophthalmology and Visual Science. 2007;48:3622–3631. doi: 10.1167/iovs.06-1207. [DOI] [PubMed] [Google Scholar]